In the literature, oxidative stress has been the subject of several lines of research. Among these are the studies associated with diseases and metabolic disorders( Reference Moustafa, Sharma and Thornton 1 ), changes and damage to mitochondrial DNA( Reference Lee and Wei 2 ), effects of stress on autophagy( Reference Lee, Giordano and Zhang 3 ), on protein degradation( Reference Aiken, Kaake and Wang 4 ) and on DNA methylation( Reference Gu, Sun and Li 5 ), as well as the relationship between heat stress (HS) and oxidative stress( Reference Mujahid, Sato and Akiba 6 – Reference Yang, Tan and Fu 8 ).
HS causes damage to the performance and the yield of parts of chickens, which may be explained by physiological changes to the bird's body( Reference Geraert, Padilha and Guillaumin 9 , Reference Yunianto, Hayashi and Kaneda 10 ). These physiological changes might partly be due to oxidative stress that occurs in chickens exposed to HS. It is not entirely known how oxidative stress and HS are related; however, studies have shown that HS can induce mechanisms similar to those of oxidative stress such as increased lipid peroxidation( Reference Willemsen, Swennen and Everaert 11 ), decreased activity of the enzyme creatine kinase (CK)( Reference Del Vesco, Gasparino and Grieser 12 ), and increased protein oxidation( Reference Ronsein, Miyamoto and Bechara 13 ). HS is also associated with a decrease in the gene expression levels of uncoupling proteins (UCP)( Reference Mujahid, Sato and Akiba 6 , Reference Gasparino, Voltolini and Del Vesco 14 ). UCP are proteins that are found in the inner mitochondrial membrane, and their main function in mammals is related to heat production( Reference Ledesma, Lacoba and Rial 15 ). This uncoupling mechanism in ATP production has also been described as an agent that enables a reduction in the production of reactive oxygen species (ROS)( Reference Abe, Mujahid and Sato 16 ). Thus, lower production of UCP can contribute to the induction of oxidative stress.
Large amounts of ROS are present when there is an oxidative stress state; this occurs not only due to the overproduction of ROS, but also due to the deficiency in the antioxidant defence system( Reference Halliwell and Gutteridge 17 ). The antioxidant system of glutathione (GSH) is composed of the enzymes glutathione peroxidase (GPx) and GSH reductase; the effectiveness of the defence system depends on the coordinated activity of the whole( Reference Huber, Almeida and Fátima 18 ).
GSH biosynthesis occurs in most tissues based on three precursor amino acids. Among these is cysteine, which, during metabolism, can be synthesised from methionine by the trans-sulfuration pathway( Reference Shoveller, Stoll and Ball 19 ). Methionine is involved in homocysteine metabolism via two metabolic pathways: remethylation, in which homocysteine is converted to methionine through the enzyme methionine synthase or betaine–homocysteine methyltransferase (BHMT); trans-sulfuration, in which homocysteine is converted to cysteine by the action of two enzymes, cystathionine β-synthase (CBS) and cystathionine β-lyase. It has been estimated that approximately 50 % of GSH is generated from homocysteine, and that under conditions of oxidative stress, which requires higher amounts of GSH, the production rate increases through the stimulation of the trans-sulfuration pathway via the increased expression and activity of CBS ( Reference Mosharov, Cranford and Banerjee 20 ). Results confirm that the presence of free radicals can induce the overexpression of CBS and can also inhibit methionine synthase, thereby stimulating an increased production of cysteine and GSH( Reference Persa, Pierce and Ma 21 ).
The present study was developed under the hypothesis that HS induces oxidative stress, and that methionine supplementation may contribute to the production and action of antioxidant components, thereby reducing the damage caused by stress. We assumed that remethylation is the main alternative pathway when there is a deficiency of methionine, and that trans-sulfuration mediated by CBS is the main pathway when methionine is available for the synthesis of cellular components such as GSH. Thus, we aimed to evaluate the effects of HS and methionine supplementation on the markers of stress, on plasma homocysteine concentration, and on the expression levels of genes related to ROS production (UCP), genes involved in methionine metabolism (BHMT and CBS), and genes related to combating oxidative stress (glutathione synthetase (GSS) and GPx7) in broilers from 1 to 21 d and from 22 to 42 d of age.
Materials and methods
All procedures involving the birds used in the experiment were approved by the Committee on Animal Care of the Universidade Estadual de Maringá – Brazil.
Experimental design and animals
Expt 1: starter period (1–21 d old)
A total of 180 male broilers (Cobb 500, Gallus gallus) were used for the experiment conducted during the starter period. The broilers were divided into three treatment groups related to methionine supplementation: without methionine supplementation (MD, n 60); recommended level of methionine supplementation (DL1, n 60)( Reference Rostagno, Albino and Donzele 22 ); excess methionine supplementation (DL2, n 60) (Table 1). The broilers were distributed in a completely randomised design with four replications (pens) per treatment, and each replicate consisted of fifteen birds. Throughout the experimental period, the broilers had free access to food and water.
Table 1 Centesimal composition of the experimental diets (as-fed basis)

MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation; CP, crude protein; AME, apparent metabolisable energy.
* Supplied per kg of diet: retinyl acetate, 3·44 mg; cholecalciferol, 50 μg; dl-α-tocopherol, 15 mg; thiamin, 1·63 mg; riboflavin, 4·9 mg; pyridoxine, 3·26 mg; cyanocobalamin, 12 μg; d-pantothenic acid, 9·8 mg; d-biotin, 0·1 mg; menadione, 2·4 mg; folic acid, 0·82 mg; niacinamide, 35 mg; Se, 0·2 mg; Fe, 35 mg; Cu, 8 mg; Mn, 60 mg; Zn, 50 mg; I, 1 mg; choline, 650 mg; salinomycin, 60 mg; avilamycin, 5 mg; butyl hydroxy toluene, 80 mg.
† Feed formulations were made based on the total amino acids of maize and soyabean meal as analysed by Evonik Degussa. The digestibility coefficient suggested by Rostagno et al. ( Reference Rostagno, Albino and Donzele 22 ) was used to calculate amino acid digestibility. Amino acids, CP and DM were analysed by Evonik Degussa.
The 180 birds distributed among the treatment groups were raised in a climatised room at thermal comfort conditions (according to Cobb guidelines) until 20 d of age, after which ninety birds (thirty from each treatment group) were exposed to acute HS of 38°C for 24 h. During the stress period, the remaining ninety birds (thirty from each treatment group) were removed from the chamber and kept in a thermoneutral environment throughout the experiment. After 24 h of exposure to stress, the birds from both environments (thermal comfort and HS) were slaughtered by cervical dislocation at 21 d. Before slaughtering, rectal temperature was measured in birds maintained at thermal comfort conditions and in those exposed to HS.
To calculate the weight gain (WG) of broilers kept at thermal comfort conditions, the birds were weighed at days 20 and 21 of the thermal comfort period. To calculate the WG of broilers exposed to HS, the birds were weighed at the beginning (day 20) and the end (day 21) of the stress period. Feed intake (FI) was calculated as the difference between the amount of feed offered at day 20 and the feed residue at the end of the trial (day 21) in both environments. FI and WG were corrected for mortality.
Expt 2: grower period (22–42 d old)
A total of 180 male broilers (Cobb 500, G. gallus) were used for the experiment conducted during the grower period. The birds were raised conventionally until 21 d of age, and fed a balanced diet to meet their nutritional demands( Reference Rostagno, Albino and Donzele 22 ). After 21 d, the birds were divided into three treatment groups related to methionine supplementation: MD (n 60); DL1 (n 60)( Reference Rostagno, Albino and Donzele 22 ); DL2 (n 60) (Table 1). The birds were distributed in a completely randomised design with four replications (pens) per treatment, and each replicate consisted of fifteen birds. Throughout the experimental period, the birds had free access to food and water.
The 180 birds distributed among the treatment groups were raised in a climatised room at thermal comfort conditions (according to Cobb guidelines) until 41 d of age, after which ninety birds (thirty from each treatment group) were exposed to acute HS of 38°C for 24 h. During the stress period, the remaining ninety birds (thirty from each treatment group) were removed from the chamber and kept in a thermoneutral environment throughout the experiment. After 24 h of exposure to stress, the birds from both environments (thermal comfort and HS) were slaughtered by cervical dislocation at 42 d. Before slaughtering, rectal temperature was measured in birds maintained at thermal comfort conditions and in those exposed to HS.
To calculate the WG of broilers maintained at thermal comfort conditions, the birds were weighed at days 41 and 42 of the thermal comfort period. To calculate the WG of broilers exposed to stress conditions, the birds were weighed at the beginning (day 41) and the end (day 42) of the stress period. FI was calculated as the difference between the amount of feed offered at day 41 and the feed residue at the end of the trial (day 42) in both environments. FI and WG were corrected for mortality.
Plasma analyses
After exposure to HS, the broilers were slaughtered and blood was collected from five birds per treatment (starter or grower period) for the analyses of homocysteine and uric acid contents, plasma CK, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. Blood was drawn from the jugular vein into heparin tubes and was kept on ice. After centrifugation (3·024 g , 10 min, 4°C), plasma was collected and stored at − 20°C until further analysis.
Plasma homocysteine content was measured using the ADVIA Centaur (Siemens Healthcare Diagnostics) system by the method of chemiluminescence with a kit (09087913; Siemens Healthcare Diagnostics). Analyses of uric acid content, and ALT, AST and CK activities were carried out by colorimetric methods with the following kits, according to the manufacturer's recommendations (Gold Analisa): uric acid, MS 80022230171; ALT, MS 80022230086; AST, MS80022230083; total creatine kinase, MS 80022230088. The enzyme activities of ALT and AST in the sample were calculated based on the rate of decrease in absorbance at 340 nm when NADH becomes NAD+. One unit of CK activity was defined as the amount of enzyme needed to convert 1 mmol of creatine into creatine phosphate per min at 37°C, pH 9·0.
Gene expression
For the analysis of gene expression, samples of breast muscle (Pectoralis superficialis) were collected from five broilers per treatment (starter or grower period), and stored in RNA Holders (BioAgency Biotecnologia) at − 20°C until total RNA extraction.
Total RNA was extracted using TRIzol® (Invitrogen) according to the manufacturer's instructions (1 ml/100 mg tissue). All the materials used were previously treated with the RNase inhibitor RNase AWAY® (Invitrogen). The tissue and TRIzol mixture was triturated with a Polytron electric homogeniser until complete dissociation was achieved. Then, 200 μl chloroform was added to the sample, and the mixture was manually homogenised for 1 min. The samples were then centrifuged for 15 min at 12 000 rpm and 4°C. The aqueous phase was collected and transferred to a clean tube containing 500 μl isopropanol, and was again homogenised and centrifuged for 15 min at 12 000 rpm and 4°C. The supernatant was discarded, and the precipitate was washed with 1 ml of 75 % ethanol. The sample was again centrifuged at 12 000 rpm for 5 min, and the supernatant was discarded. The pellet was dried for 15 min and resuspended in ultrapure RNase-free water.
Total RNA concentration was measured using a spectrophotometer at a wavelength of 260 nm. RNA integrity was analysed using a 1 % agarose gel stained with 10 % ethidium bromide and visualised under UV light. The RNA samples were treated with DNase I (Invitrogen), according to the manufacturer's instructions, in order to remove possible genomic DNA contamination.
Complementary DNA was synthesised using a SuperScript™ III First-Strand Synthesis Super Mix (Invitrogen) kit, according to the manufacturer's instructions. For this reaction, 6 μl of total RNA, 1 μl of oligo dT (50 μm-oligo(dT)20) and 1 μl of annealing buffer were added to a sterile RNA-free tube. The reaction was then incubated for 5 min at 65°C and placed on ice for 1 min. Subsequently, 10 μl of 2 × First-Strand Reaction Mix and 2 μl of solution containing the SuperScript III Reverse Transcriptase enzyme and RNase inhibitor were added to the tubes. The solution was incubated for 50 min at 50°C for the synthesis of complementary DNA. Then, the reaction was incubated for 5 min at 85°C and was immediately placed on ice. The samples were stored at − 20°C until further use.
Real-time PCR were performed using the fluorescent dye SYBR Green (SYBR® Green PCR Master Mix; Applied Biosystems). All the reactions were analysed under the same conditions and normalised to the ROX reference dye (Invitrogen) in order to correct for fluctuations in the readings due to evaporation during the reaction.
The primers used in the amplification reactions of UCP, BHMT, CBS, GPx7 and GSS were designed based on gene sequences deposited at http://www.ncbi.nlm.nih.gov (accession no. AF433170.2, XM_414685.3, XM_416752.3, NM_001163245.1 and XM_425692.3, respectively) using the website http://www.idtdna.com (Table 2). For normalisation of mRNA expression, two endogenous controls, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were tested, and β-actin (accession no. L08165) was selected because its amplification was shown to be more efficient. All analyses were performed in duplicate, each in a volume of 25 μl.
Table 2 Primer sequences used for quantitative real-time PCR

UCP, uncoupling proteins; CBS, cystathionine β-synthase; BHMT, betaine–homocysteine methyltransferase; GSS, glutathione synthetase; GPx7, glutathione peroxidase 7.
Statistical analysis
Statistical analysis was performed separately for each experimental period. The
![]() $$2^{ - \Delta C _{T}} $$
method was used to analyse the changes in the relative expression of genes. Data for WG during the grower period (22–42 d of age) were analysed using the GENMOD procedure. Means were compared by contrasts. Data from other variables were analysed using the general linear model procedure, and means were compared by Tukey's test (P< 0·05) (SAS Institute, Inc.). Results are expressed as means and standard deviations.
$$2^{ - \Delta C _{T}} $$
method was used to analyse the changes in the relative expression of genes. Data for WG during the grower period (22–42 d of age) were analysed using the GENMOD procedure. Means were compared by contrasts. Data from other variables were analysed using the general linear model procedure, and means were compared by Tukey's test (P< 0·05) (SAS Institute, Inc.). Results are expressed as means and standard deviations.
Results
Performance
Regardless of the experimental period (1–21 or 22–42 d of age), we observed that acute HS (38°C for 24 h) was sufficient to increase the body temperature of broilers: 40·21 ± 0·30°C (thermal comfort) v. 41·99 ± 0·12°C (HS) (P< 0·0001) for birds in the starter period; 41·51 ± 0·33 v. 42·87 ± 0·21°C (P< 0·0001) for birds in the grower period.
The WG, FI and mortality of birds in the starter and grower periods are shown in Table 3. HS broilers in the starter period exhibited lower weight gain (P= 0·0018) and lower FI (P= 0·0016); regarding methionine supplementation, the broilers that received the DL1 diet had higher WG than those fed the MD diet (P= 0·0169).
Table 3 Weight gain (WG) and feed intake (FI) of broilers in the starter and grower periods (Mean values and standard deviations)

MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
In the grower period, the broilers kept at thermal comfort conditions exhibited higher WG (P< 0·0001) and higher FI (P< 0·0001) than those exposed to HS. Regarding methionine supplementation, the lowest FI was observed in broilers fed the DL2 diet. The effect of methionine supplementation on the WG of broilers was also observed. The differences between the means can be calculated through contrasts. The broilers fed the MD diet had lower WG than those fed the DL1 (P= 0·0499) and DL2 (P= 0·0182) diets. No difference in WG was observed between the DL1 and DL2 diets (P= 0·6172).
In the starter and grower periods, a higher mortality rate in broilers exposed to HS and fed the MD diet was also observed.
Gene expression
Table 4 presents the gene expression of birds recorded in the starter period for the three diets and two environments studied.
Table 4 Gene expression levels of uncoupling proteins (UCP), betaine–homocysteine methyltransferase (BHMT), cystathionine β-synthase (CBS), glutathione synthetase (GSS) and glutathione peroxidase 7 (GPx7) in the muscle of broilers in the starter period (Mean values and standard deviations)

a.u., Arbitrary units; MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Gene expression levels of UCP (P= 0·0095), BHMT (P< 0·0001) and GSS (P= 0·0012) in the muscle of broilers were influenced by the interaction between temperature and diet. The broilers maintained at thermal comfort conditions and fed the MD diet exhibited higher gene expression levels of UCP (3·25 arbitrary units (a.u.)) and BHMT (0·67 a.u.). Similarly, the broilers exposed to HS and fed the DL1 and DL2 diets exhibited a higher expression level of GSS.
The gene expression levels of CBS and GPx7 were influenced by both methionine supplementation (P= 0·0167 and 0·0042, respectively) and HS (P< 0·0001 and 0·0004, respectively). The gene expression levels of CBS and GPx7 were higher in broilers that received the DL1 and DL2 diets in comparison to those fed the MD diet, and also in broilers exposed to HS in comparison to those maintained at thermal comfort conditions.
Similar to the starter period, there was an interaction effect on almost all of the gene expression levels in the grower period. The gene expression levels of BHMT (P< 0·0001), CBS (P< 0·0001), GSS (P= 0·0036) and GPx7 were influenced by the interaction between temperature and methionine supplementation (Table 5).
Table 5 Gene expression levels of uncoupling proteins (UCP), betaine–homocysteine methyltransferase (BHMT), cystathionine β-synthase (CBS), glutathione synthetase (GSS) and glutathione peroxidase 7 (GPx7) in the muscle of broilers in the grower period (Mean values and standard deviations)

a.u., Arbitrary units; MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
The expression level of BHMT was higher in broilers kept at thermal comfort conditions and fed the MD diet (0·16 a.u.). No difference in the expression level of BHMT was observed among the other treatments.
HS broilers fed the DL1 and DL2 diets exhibited an increase in the gene expression level of CBS (6·57 and 7·12 a.u., respectively). The lowest expression level was observed in broilers maintained at thermal comfort conditions and fed the MD diet (0·79 a.u.).
A higher expression level of GSS was observed in HS broilers fed the DL1 and DL2 diets. However, no difference was observed among the other treatments.
The highest gene expression level of GPx7 was found in HS broilers fed the higher amounts of methionine (DL1 and DL2). The broilers exposed to HS and fed the MD diet exhibited a higher expression level of GPx7 than those kept at thermal comfort conditions. No difference was observed among the three treatments in broilers kept at thermal comfort conditions.
No interaction effect on the expression level of UCP was observed between diet and temperature. However, the expression level was influenced by both variables. A decreased expression level of UCP was observed in HS broilers in comparison to those kept at thermal comfort conditions (0·27 v. 0·50 a.u.; P= 0·0010). Regarding methionine supplementation, the broilers fed the DL1 and DL2 diets exhibited a lower expression level of UCP than those fed the MD diet (P= 0·0063).
Plasma analyses
Homocysteine content was also influenced by the interaction between environment and methionine supplementation (P= 0·0022). The highest homocysteine level was observed in broilers fed the DL2 diet and kept at thermal comfort conditions, and the lowest level was found in HS broilers fed the MD and DL1 diets (Fig. 1).
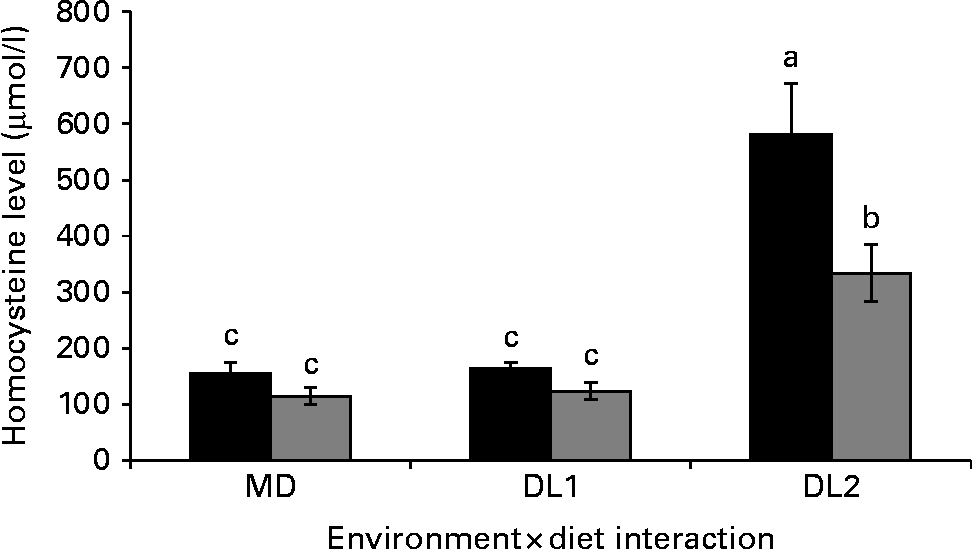
Fig. 1 Effects of the interaction between methionine supplementation and environment on plasma homocysteine level in broilers during the starter period. Values are means, with their standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation. ![]() , Comfort; ■, stress.
, Comfort; ■, stress.
The effects of methionine supplementation and HS on plasma CK, AST and ALT activities in the starter period are presented in Table 6.
Table 6 Plasma analyses of uric acid, creatine kinase (CK), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) activities in broilers during the starter period (Mean values and standard deviations)

MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
An interaction effect between the factors on the activities of uric acid (P< 0·0001) and ALT (P= 0·0024) was observed. The highest level of uric acid was found in broilers maintained at thermal comfort conditions and fed the DL1 diet (652 μmol/l; 10·97 mg/dl), and the lowest level was found in those exposed to HS, regardless of the diet. The highest level of ALT was observed in broilers exposed to HS and fed the MD diet (24·67 units/l).
An environmental effect on CK activity (P= 0·0002) was observed, with lower activity being found in HS broilers. Meanwhile, methionine supplementation had an effect on AST activity (P= 0·0190), with the highest activity being observed in broilers fed the MD diet, and the lowest activity in those fed the DL2 diet.
The effects of methionine supplementation and HS on plasma CK, AST and ALT activities in the grower period are presented in Table 7.
Table 7 Plasma analyses of uric acid, creatine kinase (CK), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in broilers during the grower period (Mean values and standard deviations)

MD, without methionine supplementation; DL1, recommended level of methionine supplementation; DL2, excess methionine supplementation.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Plasma CK (P< 0·0001) and ALT (P= 0·0004) activities were influenced by the interaction between temperature and diet. CK activity was found to be higher in broilers maintained at thermal comfort conditions and fed the DL1 diet (1908·00 units/l). The highest ALT activity was observed in HS broilers fed the MD diet (10·00 units/l), and the lowest activity in those maintained at thermal comfort conditions and fed the DL2 diet (5·17 units/l).
Uric acid content was influenced by both temperature and diet. An increased level of uric acid was observed in broilers kept at thermal comfort conditions (259 v. 190 μmol/l 4·36 v. 3·20 mg/dl; P= 0·0002). Regarding the diet, the level of uric acid increased in those fed the DL1 diet (P= 0·0017).
The treatments did not influence the activity of AST in broilers from 22 to 42 d of age.
Discussion
Broiler, layer and breeder production are quite affected by high temperatures found in some tropical countries during the summer season. These high temperatures cause damage to the performance and the yield of parts of chickens, which can be explained by the physiological changes that occur in the bird's body( Reference Geraert, Padilha and Guillaumin 9 , Reference Yunianto, Hayashi and Kaneda 10 ). When birds are exposed to HS, environmental and postural mechanisms such as reduction in FI and increase in water intake are used primarily in an attempt to reduce metabolic heat production and increase heat dissipation( Reference Mujahid, Yoshiki and Akiba 23 ). In broilers exposed to HS environment at 42 d of age, a huge decrease in weight was observed, even though the period of evaluation was only 24 h. Although the observed reduction cannot be explained completely, research in the literature has shown that broilers exposed to high temperatures increase their plasma corticosterone levels, which stimulates a huge increase in the breakdown of proteins in the bird's body( Reference Yunianto, Hayashi and Kaneda 10 ).
In the present study, it was observed that acute HS (38°C for 24 h) increased the body temperature of even the birds in the starter period (1–21 d old). The higher body temperature observed in HS broilers can induce metabolic changes such as increased ROS production and increased lipid peroxidation; thus, increased body temperature can contribute to HS-induced oxidative stress( Reference Lin, Decuypere and Buyse 24 ). ROS are produced mainly as a function of proton leakage during phosphorylative oxidation; however, the mechanism of ROS production in HS birds is not yet fully known( Reference Tan, Yang and Fu 25 ). The effects of HS are possibly due to an accelerated rate of ROS formation or an increase in ROS reactivity( Reference Bai, Harvey and McNeil 26 ).
The production of ROS in broilers exposed to high temperatures has also been correlated with the potential of the mitochondrial membrane and the expression of the UCP gene( Reference Fink, Rezka and Herlein 27 ). A greater mitochondrial membrane potential is associated with higher ROS production, and higher UCP mRNA production is associated with a lower production of free radicals, resulting in less cellular damage due to decreased ROS content. A greater mitochondrial membrane potential and decreased UCP mRNA expression has been observed in broilers exposed to HS( Reference Mujahid, Akiba and Toyomizu 7 ).
UCP can reduce ROS production by affecting decoupling during the production of ATP( Reference Abe, Mujahid and Sato 16 ); therefore, maintaining the appropriate levels of UCP mRNA transcripts could help to combat the overproduction of ROS and oxidative stress that is caused by acute HS. The expression of UCP is influenced by environmental factors such as HS( Reference Mujahid, Sato and Akiba 6 ) and nutritional status( Reference Evock-Clover, Poch and Richards 28 ). As found in the literature( Reference Mujahid, Sato and Akiba 6 , Reference Gasparino, Voltolini and Del Vesco 14 ), we also observed lower expression levels of UCP in broilers exposed to HS in the present study. Regarding methionine supplementation, we observed that broilers fed the MD diet exhibited higher expression levels of UCP. This result is consistent with that reported previously that broilers fed a MD diet exhibit a worse feed conversion ratio or feed efficiency( Reference Del Vesco, Gasparino and Oliveira Neto 29 ). Despite the beneficial effect of UCP in reducing the damage to DNA and cell proteins, as it reduces the production of free radicals, a higher mRNA expression of UCP may worsen the feed conversion ratio, as it reduces the production of ATP( Reference Ojano-Dirain, Toyomizu and Wing 30 , Reference Raimbault, Dridi and Denjean 31 ).
The organism's defence against ROS may be mediated by non-enzymatic and enzymatic antioxidants that are mainly represented by superoxide dismutase and catalase enzymes and by the GSH defence system( Reference Kuss 32 ). GSH is involved in a variety of biological actions, including protection against toxic compounds and mainly defence against free radicals( Reference Morand, Rios and Moundras 33 ). In the organism, GSH can be biosynthesised by three amino acids: glutamic acid; glycine; cysteine. Cysteine, in turn, can be synthesised in the body via the methionine metabolic pathway, which is composed of methylation, remethylation and trans-sulfuration. In the remethylation pathway, homocysteine is converted to methionine by the action of two enzymes: methionine synthase and BHMT. Trans-sulfuration occurs in two stages: in the first stage, homocysteine reacts with serine by the action of CBS, resulting in the synthesis of cystathionine; in the second stage, cystathionine is metabolised by cystathionine β-lyase, resulting in the synthesis of cysteine( Reference Stipanuk 34 ).
The synthesis of GSH also occurs in two steps. In the first step, a link between the amino acids cysteine and glutamic acid occurs by the action of the γ-glutamylcysteine synthetase enzyme. This reaction results in the synthesis of γ-l-glutamyl-l-cysteine. In the second phase, the dipeptide is linked to glycine by GSS( Reference Huber, Almeida and Fátima 18 ).
It has been estimated that approximately 50 % of GSH is generated from homocysteine, and that under oxidative stress conditions, the production rate increases through the stimulation of the trans-sulfuration pathway via the increased expression and activity of CBS and the inhibition of methionine synthase, which are the enzymes responsible for the synthesis of cysteine and methionine, respectively( Reference Mosharov, Cranford and Banerjee 20 , Reference Persa, Pierce and Ma 21 ).
In the present study, we evaluated the gene expression levels of CBS, BHMT, GSS and GPx7, and found that acute HS and higher methionine content increased the expression levels of CBS, GSS and GPx7. These results suggest that birds exposed to HS attempt to avoid increased ROS production by increasing the expression levels of genes that are part of or that contribute to the antioxidant system of GSH. Because adequate levels of methionine are required for greater efficiency of the antioxidant system, better results were observed in broilers fed the DL1 and DL2 diets.
We found that the gene expression of BHMT was lower in HS broilers. Since remethylation was inhibited, the amount of BHMT mRNA was found to be lower even when the HS birds were fed the MD diet, thereby indicating that the organism under stress can stimulate the production of GSH even when fed diets that are poor in methionine. In contrast, we observed a higher gene expression level of BHMT in broilers kept at thermal comfort conditions and fed the MD diet. This result was expected because during normal metabolism, remethylation is favoured when there is a low methionine or S-adenosylmethionine concentration( Reference Stipanuk 34 , Reference Finkelstein 35 ).
Homocysteine is an endogenous amino acid formed as an intermediate product of methionine metabolism. In the body, most of the homocysteine is linked to proteins, and most of the portion that is in the free form is either oxidised and forms dimers (homocysteine) or combines with cysteine( Reference Dietrich-Muszalska, Malinowska and Olas 36 ). A high concentration of homocysteine in the blood is known as hyperhomocysteinaemia and is associated with several diseases. When the normal metabolism of trans-sulfuration and remethylation is disturbed, usually by CBS deficiency, cysteine levels are decreased and there is lower antioxidant capacity; meanwhile, methionine levels may increase dramatically, which may cause a disease known as homocystinuria type I( Reference Ramakrishnan, Sulochana and Lakshmi 37 ).
Because of the importance of homocysteine in methionine metabolism and its involvement in the synthesis and action of enzymes that were used in the experiments of the present study, we evaluated plasma homocysteine concentration in broilers between 1 and 21 d of age. We observed that, in general, HS broilers showed a lower homocysteine concentration. The highest concentration of homocysteine was observed in broilers that were maintained at thermal comfort conditions and fed the DL2 diet. The broilers fed the DL2 diet and exposed to HS exhibited higher homocysteine concentrations than those fed the MD and DL1 diets, regardless of the environment. These results are consistent with the other results of the present study. A lower homocysteine concentration in HS broilers was expected because higher amounts of cysteine are produced from homocysteine through the increased action of the CBS enzyme. In addition, higher homocysteine concentrations observed in broilers fed the DL2 diet correspond to our hypothesis because with higher methionine levels in the diet, requirements of this amino acid are more easily met; therefore, a lower gene expression level of BHMT was observed, resulting in increased plasma homocysteine concentration.
Uric acid, similar to so many other metabolites, has been reported in the literature as one of many elements that exhibit antioxidant activity. At the physiological pH range, it is commonly found in the form of urate, a powerful ROS scavenger released into the bloodstream by deleterious reactions such as Hb auto-oxidation or peroxide production by macrophages. Urate can inactivate an oxidant before they can react with biological molecules such as DNA, proteins and lipid membranes( Reference Sautin and Johnson 38 ).
Birds possess specific mechanisms that contribute to increased urate concentrations in the blood, such as the absence of the enzyme uricase and the ability to encapsulate uric acid with proteins. Studies have indicated a relationship between higher uric acid concentrations and the decreased presence of oxidative stress markers( Reference Klandorf, Rathore and Iqbal 39 , Reference Simoyi, Van Dyke and Klandorf 40 ).
In the present study, we observed lower uric acid content in broilers exposed to HS than in those kept at thermal comfort conditions. This result indicates that under stress conditions, a higher concentration of uric acid was used to combat ROS production, by decreasing their concentration in these broilers. The broilers fed the methionine-supplemented diets had higher uric acid concentration. This suggests that stress demands an increased concentration of this antioxidant in the plasma, and that this increased level results from methionine supplementation because the presence of this amino acid can increase FI and concomitantly glycine intake, which is a necessary element for the synthesis of uric acid. Methionine supplementation( Reference Bunchasak, Sooksridang and Chaiyapit 41 ) and increased FI have been linked to increased plasma uric acid concentration levels in broiler chickens( Reference Machin, Simoyi and Blemings 42 ).
The activity of the enzyme CK can be considered as a certain kind of oxidative stress marker, since previous studies in the literature link oxidative stress to decreased CK activity( Reference Glaser, Leipnitz and Straliotto 43 , Reference Aksenova, Butterfield and Zhang 44 ), possibly via the oxidation of the thiol group. The activity of the enzyme can be preserved by endogenous GSH, which serves as a protective agent during the half-life of the enzyme in the circulation; the loss of activity under certain conditions cannot be recovered when the extracellular GSH concentration is decreased, even in the presence of thiol-reducing agents( Reference Gunst, Langlois and Delanghe 45 ). In the present study, we observed that broilers exposed to HS demonstrated lower activity of this enzyme; and we also observed that in stress conditions, broilers fed the DL1 diet demonstrated greater activity than those fed the MD diet. This result may be due to a protective role of GSH in CK activity.
Similar to CK activity, the activities of AST and ALT enzymes in the plasma have been consistently associated with stress. This is due to the fact that these enzymes are released into the blood when the body suffers some kind of injury( Reference Khan, Alhomida and Sobki 46 ). However, unlike CK, increased activity of AST and ALT has been observed in animals under stress( Reference Kumar Das, Hiran and Mukherjee 47 – Reference Ismail, Al-Busadah and El-Bahr 49 ). In the present study, increased activity of these enzymes was observed in HS broilers fed the MD diet. A deficiency in methionine, as we have observed, may have contributed to the decreased action of GSH system components, and thus resulting in greater damage to the birds.
These results allow us to suggest that under HS conditions in which the body temperature was greatest, methionine supplementation could mitigate the effects of stress, since the supplementation contributed to the increased expression of genes related to cysteine and GSH production as well as to the increased expression of the GPx7 gene. The broilers exposed to stress and fed the methionine-supplemented diets showed better results in the activities of enzymes used as stress markers, which could be due to higher antioxidant capacity.
Acknowledgements
The present study was supported by the Brazilian National Council for Research – CNPq (E. G., grant no. 483751/2012-0). CNPq had no role in the design or analysis of the study or in the writing of this article.
The authors' contributions are as follows: A. P. D. V., E. G. and A. R. d. O. N. were responsible for the conception and design of the study; A. P. D. V., E. G. and M. A. M. S carried out the gene expression analysis; A. P. D. V., V. Z. and D. d. O. G. conducted the experiment, contributed to the data collection, and carried out the plasma analysis; E. G. performed the statistical analysis; A. P. D. V. and E. G. were responsible for the data interpretation. All authors contributed and approved the final version of the manuscript.
None of the authors has any conflict of interest to declare.











