Introduction
Acquired immunodeficiency syndrome (AIDS) is a deadly infectious disease caused by the human immunodeficiency virus (HIV). As of December 2016, 36.7 million people were living with HIV/AIDS worldwide, with more than 35 million deaths resulting from the disease [1]. In Taiwan, a total of 33 428 HIV-infected cases were reported to the Taiwan Centers for Disease Control by the end of 2016, with 5523 (16.5%) deaths due to the disease [2]. With the success of highly active antiretroviral therapy (HAART), people living with HIV/AIDS (PLWHA) are ageing, and more chronic diseases (e.g. cardiovascular diseases (CVDs)) are being diagnosed in this population.
When HIV infects the host, it can cause platelet activation and endothelial dysfunction [Reference Kline and Sutliff3], which could lead to the development of atherosclerosis. In rat models, HIV can enter myocytes directly and cause myocardial damage [Reference Fiala4], which may result in myocardial dysfunction. Despite evidence suggesting that HIV might play an important role in the pathogenesis of CVDs, the association of HIV infection with the subsequent development of CVDs has not been extensively studied. A previous study showed that HIV infection was accompanied by more extensive atherosclerosis after adjustment for traditional risk factors for CVDs [Reference Boccara5]. Moreover, a Veterans Aging Cohort Study followed up 2391 male HIV-infected patients and found that HIV infection was a risk factor for the development of heart failure [Reference Butt6]. Since CVDs have become the major driver of HIV-associated morbidity and mortality [Reference Farahani7], it is imperative to determine the incidences of CVDs in PLWHA.
HAART is effective on reducing mortality among PLWHA. Since the introduction of HAART, PLWHA are living longer and more comorbidities (e.g. CVDs) are reported in this population. Although HAART plays an important role on improving outcome among PLWHA, the effect of HAART on the development of CVDs is still unclear. A previous report demonstrated that HAART could significantly reduce the levels of CVD markers (e.g. endothelial dysfunction, platelet and monocyte activations) at 12 weeks after HAART initiation [Reference O'Halloran8]. However, some reports showed that HAART could cause immune reconstitution inflammatory syndrome and may initiate the development of CVDs [Reference Capeau9, Reference Boccara10].
Knowledge of the current incidence of CVDs among PLWHA is important for determining the best allocation of medical resources. Therefore, we conducted a nationwide population-based cohort study to determine the incidence of CVDs among PLWHA in Taiwan.
Methods
Data source
This cohort study used data from the Taiwan Centers for Disease Control HIV Surveillance Database from 2000 through 2014. In Taiwan, by law, medical professionals must report all new HIV cases to the Taiwan Centers for Disease Control within 24 h of diagnosis. All reported HIV-infection cases in Taiwan were diagnosed by positive HIV-1 western blot or polymerase chain reaction analysis. All HIV-infected individuals in Taiwan have been offered free-of-charge HAART since 1997 [Reference Chen and Kuo11]. This study was approved by the institutional review board of Taipei City Hospital.
Study subjects
In this cohort study, adult PLWHA (aged ⩾15 years) from 2000 through 2014 were selected from the Taiwan Centers for Disease Control HIV Surveillance Database. All PLWHA were followed until the diagnosis of CVDs, death or 31 December 2014. Death events were determined by the death certificate database of Taiwan.
Control group
To calculate the expected rates of CVDs, control subjects were obtained from a database linked by the Office of Statistics of the Department of Health using the National Health Insurance Research Database (NHIRD) and death certificate database. The control group consisted of 2 million individuals randomly sampled from the Registry for Beneficiaries of the NHIRD [12], which maintains the registration data and all the original claims data of every beneficiary of the National Health Insurance programme from 2000 to 2014. There were approximately 23.72 million individuals in the NHIRD registry. Random selection was performed using the SAS software. There was no significant difference in age and gender distribution between the patients in the randomly selected subset and the original set of individuals in the NHIRD [12].
Variables and measures
The outcome of new cases of CVDs was identified by selecting those with insurance claims whose recorded data contained the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of CVDs [Reference Chao13, Reference Liang14]. CVDs were categorised as heart diseases, cerebrovascular accidents, peripheral arterial disease and chronic kidney disease. Heart diseases included coronary artery disease (ICD-9-CM code 410-414), myocardial infarction (ICD-9-CM code 410), percutaneous coronary intervention (ICD-9-CM code 36.01, 36.02, 36.05, 36.06 and 36.09), coronary artery bypass surgery (ICD-9-CM code 36.1), sudden cardiac death (ICD-9-CM code 427.5, 798.1 or 798.2), heart failure (ICD-9-CM code 4280) and atrial fibrillation (ICD-9-CM code 427.31). Cerebrovascular accidents included all-cause stroke (ICD-9-CM code 430–437), ischaemic strokes (ICD-9-CM code 433-437) and haemorrhagic strokes (ICD-9-CM code 430–432). Peripheral arterial disease and chronic kidney disease were defined as ICD-9-CM codes 443.9 and 580–587, respectively. A person was considered to have a new onset of CVD only if the condition occurred in an inpatient setting or after three or more outpatient visits.
Statistical analysis
The incidence density (ID) and standardised incidence rate (SIR) for each CVD were calculated in this cohort of PLWHA. For the calculation of ID in each CVD, PLWHA were excluded if they had received a diagnostic code for this CVD before being included in the study. Person-years analysis was performed, stratifying for age, sex, calendar period and type of CVDs in order to estimate ID and SIR in the PLWHA cohort. The start date for the calculation of person-years was the date of HIV diagnosis and the end date was 31 December 2014 or the date of incident CVD or death during the follow-up period. ID of each CVD was calculated by dividing the number of observed cases by the total person-years at risk for that CVD. SIR for each CVD was calculated by dividing the observed number of cases by the number that would be expected if age-, sex- and calendar period-specific rates of the comparison population applied. In this study, the 2 million controls were used for calculating SIRs. The 95% confidence interval (CI) was calculated using the Poisson distribution.
To examine the effect of HAART on the incidence of CVDs, this study analysed the data after stratifying PLWHA by HAART. For calculating ID of each CVD, a person was considered to receive HAART if the person received HAART before the new onset of this CVD. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA). A two-tailed P-value <0.05 was considered statistically significant.
Results
Participant selection
We identified 26 355 individuals who had received a new diagnosis of HIV from 1 January 2000 through 31 December 2014. After excluding those younger than 15 years (n = 35) and those with incomplete data (n = 48), the remaining 26 272 patients were included in the analysis. The overall mean (s.d.) age was 32.3 (10.2) years; 93.9% of the subjects were male and 73.4% of the subjects received HAART.
Incidence and SIR of different types of CVDs among PLWHA
Table 1 shows the new onset, incidence and SIR of different types of CVDs. The most common new onset of CVD was coronary artery disease (n = 811; ID = 557.13/100 000 person-years), followed by chronic kidney disease (n = 729; ID = 495.99/100 000 person-years) and all-cause stroke (n = 432; ID = 292.60/100 000 person-years). SIR of different types of CVDs was calculated using IDs of CVDs among PLWHA and the general population. Compared with the general population, increased SIRs were seen in coronary artery disease (SIR = 1.11, 95% CI 1.04–1.19), percutaneous coronary intervention (SIR = 1.32, 95% CI 1.18–1.47), coronary artery bypass surgery (SIR = 1.47, 95% CI 1.29–1.66), sudden cardiac death (SIR = 3.01, 95% CI 2.39–3.73), heart failure (SIR = 1.50, 95% CI 1.31–1.70) and chronic kidney disease (SIR = 1.95, 95% CI 1.81–2.10). Furthermore, compared with the general population, a decreased SIR was seen in atrial fibrillation (SIR = 0.53, 95% CI 0.37–0.73).
Table 1. Standardised incidence rates of cardiovascular diseases in Taiwanese HIV-1/AIDS patients aged ⩾15 years enrolled in the National Health Insurance system between 2000 and 2014 (n = 26 272)

CI, confidence interval.
a Events per 100 000 person-years.
b The SIR is significant.
SIRs of CVDs in male and female PLWHA
Table 2 shows SIR of different types of CVDs was calculated in male and female PLWHA. Compared with the general population, male PLWHA had a higher risk of coronary artery disease (SIR = 1.10, 95% CI 1.02–1.18), percutaneous coronary intervention (SIR = 1.37, 95% CI 1.23–1.53), coronary artery bypass surgery (SIR = 1.50, 95% CI 1.31–1.70), sudden cardiac death (SIR = 2.84, 95% CI 2.23–3.56), heart failure (SIR = 1.41, 95% CI 1.22–1.62) and chronic kidney disease (SIR = 1.91, 95% CI 1.77–2.06), but had a lower risk of atrial fibrillation (SIR = 0.48, 95% CI 0.33–0.69). Also, female PLWHA had a higher risk of sudden cardiac death (SIR = 6.78, 95% CI 2.92–13.36), heart failure (SIR = 2.51, 95% CI 1.71–3.56), all-cause stroke (SIR = 1.52, 95% CI 1.11–2.04), haemorrhagic stroke (SIR = 3.09, 95% CI 1.73–5.10) and chronic kidney disease (SIR = 2.59, 95% CI 1.97–3.33).
Table 2. Standardised incidence rates of cardiovascular diseases in Taiwanese HIV-1/AIDS patients aged ⩾15 years enrolled in the National Health Insurance system between 2000 and 2014 (n = 26 272)
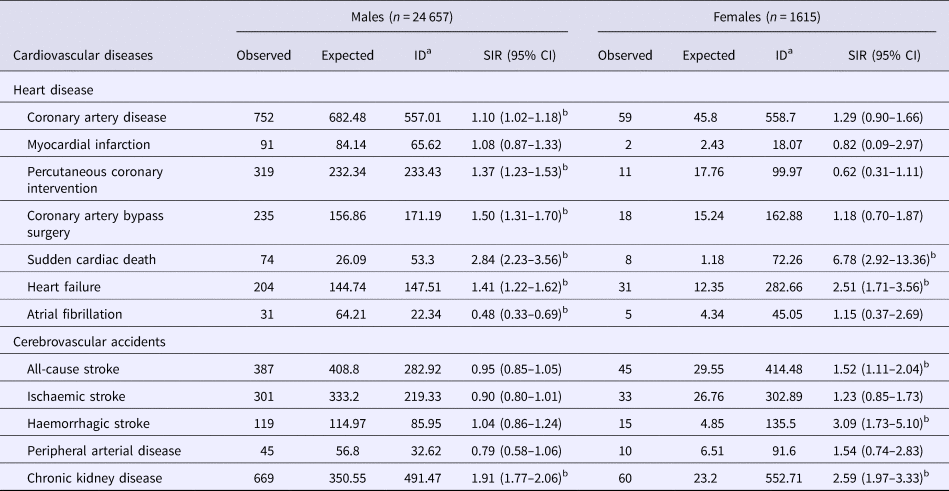
CI, confidence interval; ID, incident density; SIR, standardised incidence rate.
a Events per 100 000 person-years.
b The SIR is significant.
Incidence and SIRs of CVDs among PLWHA with and without HAART
Table 3 shows the incidence and SIR of different types of CVDs among PLWHA by HAART status. The SIRs for all-cause, ischaemic and haemorrhagic stroke were higher in PLWHA who did not receive HAART, but were lower in PLWHA who received HAART. Moreover, PLWHA who received HAART and those who did not receive HAART have higher risks of coronary artery disease, percutaneous coronary intervention, coronary artery bypass surgery, sudden cardiac death, heart failure and chronic kidney disease.
Table 3. Standardised incidence rates for cardiovascular diseases in Taiwanese HIV-1/AIDS patients aged ⩾15 years, by HAART status

PLWHA, people living with HIV/AIDS; HAART, highly active antiretroviral therapy; ID, incident density; SIR, standardised incidence rate; CI, confidence interval.
a Events per 100 000 person-years.
b The SIR is significant.
Discussion
This nationwide cohort study found that PLWHA had a higher risk of incident coronary artery disease, percutaneous coronary intervention, coronary artery bypass surgery, sudden cardiac death, heart failure and chronic kidney disease, but had a lower risk of atrial fibrillation. When the effect of HAART on incident CVD was considered, the risks of incident all-cause, ischaemic and haemorrhagic stroke were higher in PLWHA who did not receive HAART, but were lower in PLWHA who received HAART.
This study showed that PLWHA had higher risks of incident coronary artery disease, percutaneous coronary intervention and coronary artery bypass surgery. A previous report showed that PLWHA had more extensive atherosclerosis compared with HIV-uninfected individuals [Reference Grunfeld15]. HIV-associated vasculopathy and immune dysfunction may account for the development of atherosclerosis and higher risks of coronary artery disease in PLWHA. When HIV infects the host, HIV virion or its particles (e.g. GP120 or TAT) can stimulate the endothelium directly and increase endothelial permeability, which assists the invasion of leukocytes into vessel walls and results in vascular inflammation [Reference Kline and Sutliff3, Reference Kuller16]. The HIV virion can also cause endothelial dysfunction, an early marker of atherosclerosis, which leads to platelet adhesion and aggregation, blood-clotting activation and fibrinolysis derangement, favouring a prothrombotic state [Reference Kline and Sutliff3, Reference Neuhaus17]. While the HIV-associated vasculopathy (e.g. atherosclerosis) is exacerbated, it may cause vascular constriction and thrombotic occlusion, which could lead to the development of coronary artery disease.
HIV-associated immune dysfunction may also account for the higher risks of coronary artery disease in PLWHA. A previous study showed that HIV infection could cause immune dysfunction, including the depletion of CD4+ T cells and the activation of CD8+ T cells [Reference Sodora and Silvestri18]. CD8+ T-cell activation and a lower CD4:CD8 ratio have been shown to be associated with higher carotid intima–media thickness by computed tomography angiography [Reference Lang19, Reference Lo20]. A study also showed that CD8+ T cells activation remained in PLWHA with undetectable plasma HIV RNA levels after receiving HAART [Reference Hunt21], which could cause chronic vascular inflammation and increase the risk of coronary artery disease in HIV-infected individuals.
This study found that PLWHA had a higher risk of heart failure than HIV-uninfected individuals. A previous study revealed that PLWHA had a higher prevalence of heart failure than HIV-uninfected individuals (7.2% vs. 4.4%) [Reference Al-Kindi22]. However, longitudinal studies to determine the association of HIV infection with the subsequent development of heart failure are scarce. A Veterans Aging Cohort Study showed that HIV-infected patients had a 1.2- to 1.8-fold higher risk of developing heart failure compared with non-HIV patients [Reference Butt6, Reference Freiberg23]. The increased risk of heart failure in PLWHA persisted among those without coronary artery disease [Reference Butt6]. The present study followed up 26 272 PLWHA and found that PLWHA had a 1.5-fold higher risk of incident heart failure compared with non-HIV patients.
Direct HIV-induced myocardial damage may account for the higher risk of incident heart failure in PLWHA. In vitro studies of human and rat cardiomyocytes have shown that HIV can enter myocytes directly through pathways independent of C-C chemokine receptor type 5 [Reference Fiala4, Reference Twu24]. HIV invasion of cardiac myocytes could cause myocardial inflammation and cytokine release [Reference Magnani and Dec25]. HIV-related proinflammatory cytokines (e.g. tumour necrosis factor-α and interleukin-1β) can promote the expression of inducible nitric oxide synthase in cardiomyocytes [Reference Monsuez26]. High concentrations of nitric oxide and tumour necrosis factor-α could induce cardiomyocyte apoptosis and lead to depressed heart function [Reference Monsuez26].
This study showed that the risks of all-cause, ischaemic and haemorrhagic strokes were lower in PLWHA receiving HAART, but were higher in PLWHA not receiving HAART. Previous cohort studies showed that HIV infection was associated with a higher risk of stroke, particularly in those with a severely immunocompromised status [Reference Durand27, Reference Rasmussen28]. HIV-related atherosclerosis and aneurysmal arteriopathy may explain the higher risks of incident ischaemic and haemorrhagic stroke in PLWHA [Reference Neuhaus17, Reference Goldstein, Timpone and Cupps29]. HIV infection can cause endothelial dysfunction and induce the development of atherosclerosis [Reference Neuhaus17], which could increase the risk of ischaemic stroke in PLWHA. The HIV virion can also cause vasculitis of the vasa vasorum and result in intramural arterial ischaemia, which could lead to aneurysmal dilation and a potential increase in the risk of intracranial haemorrhage [Reference Goldstein, Timpone and Cupps29, Reference Chetty, Batitang and Nair30]. When PLWHA receive HAART, the antiretroviral therapy regimens could significantly suppress viral replication and prevent the subsequent development of ischaemic and haemorrhagic strokes in this population.
This study also found that PLWHA had a higher risk of chronic kidney disease than HIV-uninfected individuals. HIV infection has been associated with a higher risk of chronic kidney disease [Reference Campos, Ortiz and Soto31]. Our study showed that the incidence of chronic kidney disease was 437.1 and 639.1 per 100 000 person-years in PLWHA receiving and not receiving HAART, respectively. Theories regarding the excess risk of chronic kidney disease in this population include a direct effect of HIV on kidney injury, HIV-related immune complex kidney disease, consequences of opportunistic infections and complications of HAART [Reference Cohen, Kopp and Kimmel32]. Because chronic kidney disease could shorten the lifespan of PLWHA, the screening and treatment of chronic kidney disease is imperative in this population [Reference Estrella and Fine33].
This nationwide population-based cohort study has several strengths. First, our research design, which included an unbiased subject selection and strict HIV diagnosis, supported the validity of these findings. Moreover, this cohort study traced all PLWHA and control subjects with referral bias being minimised because all medical care was covered by the Taiwan National Health Insurance. Furthermore, the study's large sample size was powered to detect even very small differences between patients with and without HIV infection.
Limitations
Two limitations should be considered when interpreting these findings in this study. First, the diagnoses of CVDs that rely on administrative claims data recorded by physicians or hospitals may be less accurate than diagnoses made in a prospective clinical setting; however, there is no reason to suspect that the validity of claims data would differ from a patient's HIV status. Second, the external validity of our findings may be a concern because almost all our enrolees were Taiwanese. The generalisability of our results to other non-Asian ethnic groups requires further verification. However, our findings suggest new avenues for future research.
Conclusions
In conclusion, this nationwide long-term cohort study found an association between HIV infection and incident CVD. Compared with the general population, PLWHA had a higher risk of incident coronary artery disease, percutaneous coronary intervention, coronary artery bypass surgery, sudden cardiac death, heart failure and chronic kidney disease, but had a lower risk of atrial fibrillation. As PLWHA are ageing, clinicians need to be alert regarding the higher risk of CVDs in this population.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors are grateful for statistical consultation at the Biostatistical Consultation Centre, National Yang-Ming University, Taipei, Taiwan.
Conflict of interest
None.






