Self-management broadly encompasses the tasks required to successfully live with and manage the physical, social and emotional impact of a chronic condition.Reference Taylor, Pinnock, Epiphaniou, Pearce, Parke and Schwappach1 Currently, there is no universally accepted classification of self-management, although it commonly involves the provision of information and education on a condition and its treatment, collaboratively creating an individualised treatment plan, developing skills for self-monitoring symptoms and strategies to support adherence to treatment including medication, psychological techniques, lifestyle and social support. A rapid synthesisReference Taylor, Pinnock, Epiphaniou, Pearce, Parke and Schwappach1 of self-management interventions revealed a robust evidence base for improvement in outcomes of long-term conditions, such as diabetes and asthma, and some evidence for interventions in stroke, hypertension and depression, along with the potential for reducing healthcare resource use. The synthesis concluded that inclusion of self-management should be a requirement for high-quality care for all long-term conditions.
Background
A range of interventions badged as self-management are available for people with long-term conditions falling under the umbrella of severe mental illness (SMI)2 (schizophrenia spectrum disorders, bipolar disorder and major depression), but a recent systematic synthesis regarding their effectiveness is lacking. A 2002 review of interventions for this population identified four key elements that improved the course of illness of those with SMI: (a) providing psychoeducation about mental illness and its treatment, (b) behavioural tailoring to facilitate medication adherence, (c) developing a relapse prevention plan and (d) teaching coping strategies for persistent symptoms.Reference Mueser, Corrigan, Hilton, Tanzman, Schaub and Gingerich3 More recently, an additional focus on patient-defined recovery and personal goals has been incorporated into self-management interventions. Through these elements, self-management interventions are thought to empower individuals by providing the knowledge and skills to enable them to make informed decisions to manage their own care,Reference Mueser and McGurk4 cope with symptoms and reduce susceptibility to relapse and reliance on services.Reference Mueser, Corrigan, Hilton, Tanzman, Schaub and Gingerich3
Rationale for the review
To date, previous reviews of self-management interventions for SMI have focused on broad, nonspecific self-management interventions such as psychoeducationReference Zhao, Sampson, Xia and Jayaram5,Reference Lincoln, Wilhelm and Nestoriuc6 and self-help,Reference Scott, Webb and Rowse7 or they have been confined to specific diagnoses within the SMI populationReference Zou, Li, Nolan, Arthur, Wang and Hu8 – predominantly schizophrenia or psychosis. This limits the generalisabilityReference Mueser, Corrigan, Hilton, Tanzman, Schaub and Gingerich3 of the findings and results in the exclusion of studies focused on broad populations of mental health patients, even though self-management interventions are currently intended for use by a broad group. A comprehensive systematic review and meta-analysis of self-management interventions for people with SMI has not previously been available. Empowering mental health patients and supporting them in making choices about their care are increasingly given weight among the stated goals and values of mental health services and policies: self-management interventions have the potential to help achieve these goals.
The present review
The aim of the present study is to assess the effectiveness of self-management in the typical mixed populations of people with SMI, such as those found in National Health Service (NHS) secondary care settings and in community mental health services in many other systems. We look at the effect of self-management in both the short and longer term in relation to the following prespecified outcomes deemed important from both a commissioning and patient perspective: symptomatic recovery, relapse prevention, reduced need for admission to hospital, self-rated recovery, functioning and quality of life.
Method
A review protocol was developed following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelinesReference Moher, Liberati, Tetzlaff and Altman9 and was registered at PROSPERO (reference: CRD42017043048).
Inclusion criteria
The research question and inclusion criteria were formulated using the PICOS (Participant, Intervention, Comparison, Outcome and Study) design.Reference Moher, Liberati, Tetzlaff and Altman9 This widely used framework supports formulation of focused and rigorous review questions.
Participants
Studies were included if participants were adults aged 18 years and over and diagnosed with a SMI,2 i.e. with a clinical diagnosis10 of schizophrenia spectrum disorders (schizoaffective disorder, delusional disorder and psychosis), bipolar disorder or major depression. Also included were studies with mixed populations of people with these diagnoses (which included those with personality disorder) who were using secondary care mental health services.
Intervention
Studies were included if patients directly received a self-management intervention that was designed to educate and equip individuals with the skills to manage symptoms, relapses and overall psychosocial functioning.Reference Mueser, Deavers, Penn and Cassisi11 Self-management interventions were delivered in conjunction with treatment as usual (TAU). To investigate the effectiveness of self-management itself, interventions with a broader focus that included self-management as only one of the intervention components were not included in the current review, unless it was possible to ascertain the specific impact of self-management. To be considered a self-management intervention for the purposes of this systematic review, the intervention had to include the following three (of the four) domains identified by Mueser and colleaguesReference Mueser, Corrigan, Hilton, Tanzman, Schaub and Gingerich3 as effective areas of self-management:
(a) psychoeducation about mental illness and its treatment (to make informed decisions about care);
(b) recognition of early warning signs of relapse and development of a relapse prevention plan;
(c) coping skills for dealing with persistent symptoms.
Additionally, the self-management intervention should include a recovery-focused element,Reference Mueser, Deavers, Penn and Cassisi11 such as setting personal goals based on an individual's own hopes for their recovery and learning how to effectively manage their illness in the context of pursuing those goals.
Strategies for medication management, the fourth domain identified by Mueser and colleagues,Reference Mueser, Corrigan, Hilton, Tanzman, Schaub and Gingerich3 was not considered a necessary domain for a self-management intervention to be included in the current review. Making medication management a mandatory domain was considered at odds with a recovery-focused approach; however the majority of studies did include a medication management component.
Comparison
Studies employing either TAU, however defined, or active controls were included in this review.
Outcome
If studies reported on any of the following prespecified outcomes they were included in the meta-analyses:
(a) symptom-focused outcomes;
(b) relapse (or related service use outcomes: number and length of admissions);
(c) recovery-focused outcomes (including measures of overall recovery processes and its components: self-empowerment and efficacy, social connectedness, hope, optimism and the pursuit of a meaningful life);Reference Leamy, Bird, LeBoutillier, Williams and Slade12
(d) functioning (global);
(e) quality of life.
Study design
All randomised controlled trials (RCTs), including cluster RCTs and factorial RCTs, were considered for inclusion. Quasi-randomised studies were excluded.
Exclusion criteria
Studies were excluded for the following reasons.
(a) The intervention had a therapeutic focus beyond that of improving an individual's self-management of their illness (e.g. cognitive remediation, cognitive–behavioural therapy, basic life skills or social skills), which prevented evaluating the specific efficacy of the self-management component.
(b) The intervention was delivered:
(i) to family members (either as the target recipients of the intervention or in addition to the patients);
(ii) as part of or alongside another intervention (e.g. The Life Goals Program, when it was part of the multi-component collaborative care model Life Goals Collaborative Care,Reference Bauer, McBride, Williford, Glick, Kinosian and Altshuler13–Reference Bauer, Biswas and Kilbourne15 was excluded on the basis of the additional nurse care management component).
Search strategy and selection criteria
A systematic search for all relevant literature was conducted using a PRISMAReference Moher, Liberati, Tetzlaff and Altman9 search strategy of the databases Medline, Embase, PsychINFO, DARE and CENTRAL, from their inception until 15 May 2018. The relevant parts of a published search strategy used for the National Institute for Health and Care Excellence (NICE) schizophrenia guidelines16 was used in the current study and details are included in Supplementary Appendix 1 available at https://doi.org/10.1192/bjp.2019.54. Abstracts were screened based on the review protocol (M.L.) and any uncertainties were reviewed to reach a consensus (M.L. and M.F.-A.). Twenty per cent of the full-text articles assessed for eligibility (n = 82) were blindly assessed to meet inclusion and exclusion criteria (M.F.-A. and A.M.). The few cases of disagreement were discussed and consensus was reached. Additionally, a hand search of reference lists was conducted.
All abstracts were retrieved and added to Mendeley referencing software (version 1.16.3 for MacOS).
Data extraction and quality assessment
Data were extracted and reviewed in Microsoft Excel. Characteristics of the study design, the intervention, participants and outcomes for all available data at all provided time points were extracted. Authors were contacted and asked to provide any missing data. Raw outcome data extracted from papers published before 2012 were kindly provided by the National Collaborating Centre for Mental Health from our group's previous work with them on the development of the NICE schizophrenia guidelines. The relevant studies and outcome data provided from the original search were then extracted according to this current review protocol and checked against the original manuscripts. When a study had three arms, we followed expert guidelinesReference Higgins and Green17 and combined both control groups into a single group to enable pairwise comparison. Mean values were multiplied by −1 to correct for differences in the direction of scales.
Assessment of bias
Assessment of bias was performed by two pairs of researchers (B.H.-S. and A.Y.-U.; M.L. and A.M.) using the Cochrane Collaboration Risk of Bias Tool.Reference Higgins and Green17 Each study was rated for risk of bias due to sequence generation, allocation concealment, blinding of assessors, selective outcome reporting and incomplete data. The blinding of participants in trials of complex interventions is problematic. As such, it is assumed that blinding of participants was at high risk for all studies. Risk of bias was rated as high (weakening confidence in results), low (unlikely to seriously alter results) or unclear. Funnel plots were generated to examine publication bias in analyses with more than ten studies.Reference Sterne, Egger and Smith18
Statistical analysis
Review Manager (RevMan 5.2 for Windows) software was used to conduct the meta-analyses. When outcome data were reported for more than one follow-up point, the time point closest to 1-year post-intervention was used. Where more than one measure was used to report the same outcome in the same study, we prioritised the primary outcome of that study or included the outcome more commonly reported by other studies in the analysis. On the rare event that a study reported both symptomatic relapse and readmission data, we included the readmission data in the analysis. Studies with TAU and active control groups were analysed together.
Effect size calculation
Effect sizes for continuous data were calculated as standardised mean difference (SMD), Hedges' g, and studies were weighted using inverse variance.Reference Higgins and Green17 For dichotomous outcomes we calculated risk ratios and combined studies using the Mantel–Haenszel method.Reference Higgins and Green17 All outcomes are reported with 95% confidence intervals using random-effects modelling. If reported by studies, we used intention to treat data in our analysis.
Heterogeneity
Heterogeneity was assessed through visual inspection of forest plots, the P-value of the χ2 test (Q) and calculating the I 2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than chance.Reference Cuijpers19 A P-value <0.10 and an I 2 >50% suggests substantial heterogeneity. Quantifying inconsistency across studies in this way allowed us to explore the possible reasons for heterogeneity through sensitivity analysis.
Sensitivity analyses were carried out using the one-study-removed method to examine the effect of a specific study on the pooled treatment effect. When a study was identified as substantially contributing to heterogeneity, the potential sources of clinical or methodological heterogeneity were reviewed and compared with the remaining studies to evaluate if their exclusion from the particular meta-analysis was warranted.
Results
Of the 6486 potentially relevant citations, 82 papers were retrieved and assessed for inclusion (Fig. 1). Of these, 20 were excluded because they were not mental health self-management interventions (either they did not meet the three criteria for inclusion, or they covered social skills training only), one study was not completed (protocol paper only), and a further 18 papers were outside of the scope of this review (i.e. self-management was delivered as part of another intervention, or included family members in the intervention). Two papers were included from a reference hand search. A total of 37 RCTs (published across 45 full-text articles) were therefore included in the narrative synthesis. Two were not included in the meta-analysesReference Eckman, Wirshing, Marder, Liberman, Johnston-Cronk and Zimmerman20,Reference Kopelowicz, Wallace and Zarate21 as they did not report usable outcomes.

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow chart.
Study characteristics
A detailed breakdown of the characteristics of the studies included in this reviewReference Eckman, Wirshing, Marder, Liberman, Johnston-Cronk and Zimmerman20–Reference Xiang, Weng, Li, Gao, Chen and Xie64 can be found in Supplementary Table 1. Studies included in this review randomised 5790 participants with a median sample size of 107 (range 32–555). The majority of studies were conducted in high-income countries (k = 27), with a smaller but substantial proportion in lower- or middle-income countries (k = 10). The majority of studies (k = 29) included participants who were currently living in the community, with eight studies recruiting from in-patient settings.
The mean age of participants was 40 years and 44% were female. In relation to clinical diagnosis, 18 studies included only participants with schizophrenia spectrum disorder and 7 included only those with a diagnosis of bipolar disorder. The remaining 12 included mixed populations of participants with schizophrenia, psychosis, bipolar, major depressive disorder and personality disorder who were in contact with secondary mental health services.
Across the 37 studies, self-management interventions ranged broadly in duration from 1 to 52 weeks (median duration 12 weeks). Likewise, face-to-face/group contact time also ranged widely from 4 to 96 h (median 23 h). Most interventions were delivered in a group format and facilitated by clinicians (k = 25) or peers (k = 5). The remaining interventions were delivered to participants individually, either as an online, computer-based intervention (k = 2), by a clinician (k = 2) or by a peer (k = 1). Finally, two studies used a combination of group and individual sessions facilitated by a clinician. All interventions were delivered from a manualised protocol, however the depth, detail and fidelity of the intervention to the manual was not always reported in detail. All interventions were delivered in addition to TAU provided in the same respective settings.
Supplementary Table 2 provides a detailed breakdown of the studies reviewed, organised by a preliminary typology of self-management interventions developed as part of this review (further details in Supplementary Table 3).
Controls
Self-management interventions were compared with TAU in 19 studies; waiting list control conditions in 3 studies; and the remaining 12 had active control conditions such as group counselling, occupational therapy or psychoeducation (Supplementary Table 2). A further three were multi-arm studies with active and TAU control groups.
Outcome measures
Supplementary Table 4 outlines the continuous measures used in studies, categorised by outcome type. Dichotomous data were also reported. The outcome measures used across the studies were reported to be well-validated and reliable instruments. Symptom outcomes were reported on measures ranging from self-rated (the Internal State Scale) to those rated by caregivers (Psychosis Evaluation tool for Common use by Caregivers) and those requiring a clinical interview (Positive and Negative Syndrome Scale and Brief Psychiatric Rating Scale). In the majority of studies, relapse was measured as an admission to hospital. A small minority of trials additionally identified relapse in participants when a score reached a cut-off point on a scale, but admission data were given precedence in the present analysis. Measures of quality of life were self-rated, whereas functioning tended to be clinician rated. Measures of recovery which focused on personal recovery as opposed to clinical recovery were exclusively self-rated.
Risk of bias
The risk of bias summary is shown in Fig. 2 and the rating for each individual study can be found in Supplementary Fig. 1. Blinding of participants and personnel is generally considered to be challenging in complex interventions, so risk of bias in this respect was rated as high in all studies except for one.Reference Proudfoot, Parker, Manicavasagar, Hadzi-Pavlovic, Whitton and Nicholas59 Of note, 9 studies were at a high risk of bias for selective reporting of outcomes measured and 18 were unclear. The ‘other bias’ category refers to whether any studies were discontinued due to adverse events, problems with the study design or acceptability of the intervention.
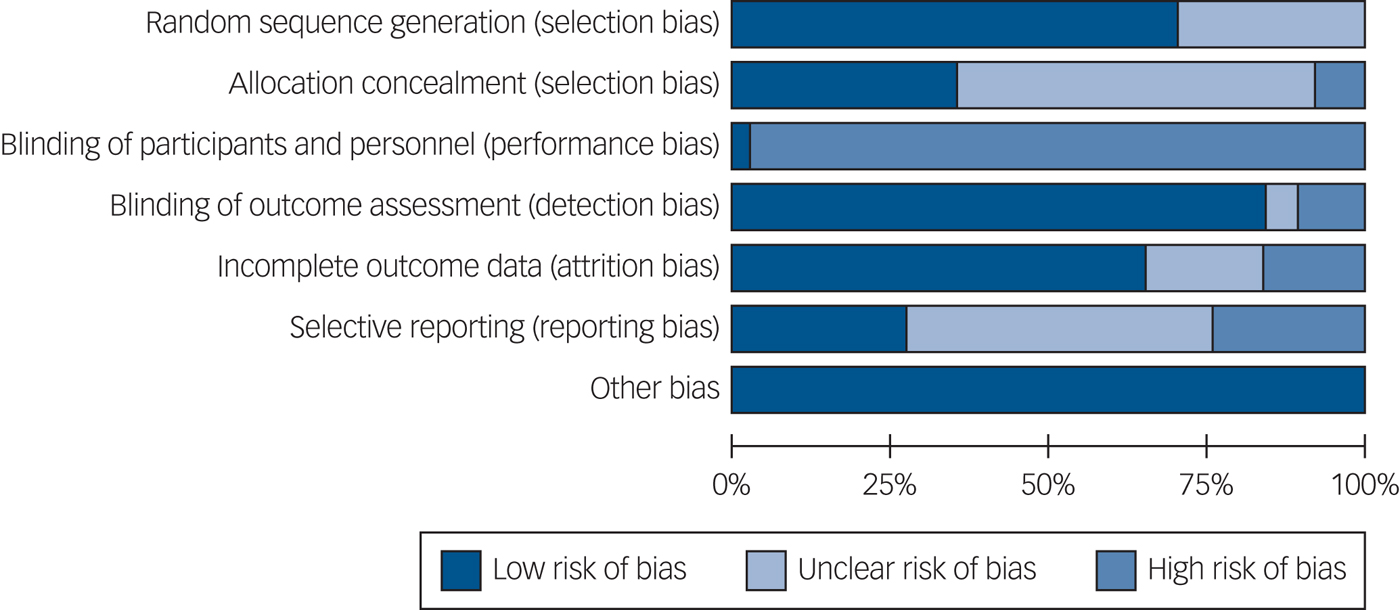
Fig. 2 Cochrane Risk of Bias Summary.
Quantitative synthesis
Data were analysed at two time points: at the end of the treatment intervention (that is, immediately, or within 2 weeks) and at follow-up. The median follow-up length was 41 weeks (range 4–104 weeks) post-treatment; 52 weeks (range 7–130 weeks) post-randomisation. Summary results are outlined in Table 1 (forest plots in Supplementary Fig. 2).
Table 1 Analysis of self-management intervention for people with severe mental illness compared with control (active or treatment as usual) (random-effects model)
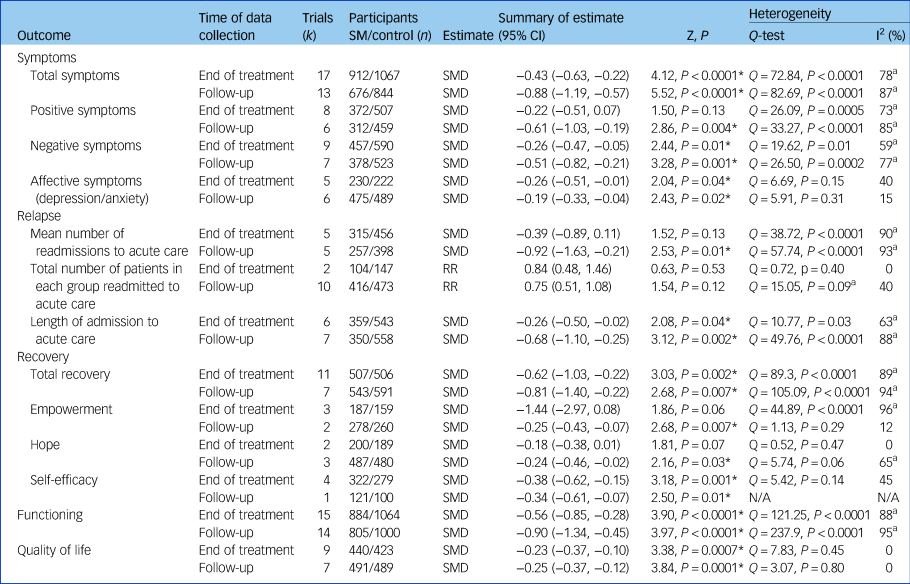
SM, self-management intervention; SMD, standardised mean difference; RR, relative risk.
a. Indicates high heterogeneity: I 2 >50% and/or P-value <0.10.
*P < 0.05.
Symptoms
A total of 17 studies (n = 1979) found a small but significant effect of self-management on total symptoms at post-treatment (SMD −0.43, 95% CI −0.63 to −0.22). At follow-up, 13 studies (n = 1520) demonstrated a marked effect of self-management on total symptoms (SMD −0.88, 95% CI −1.19 to −0.57). There was no significant effect on positive symptoms at post-treatment, however at follow-up (k = 6, n = 771) there was a moderate effect (SMD −0.61, 95% CI −1.03 to −0.19). Self-management had a small effect on negative symptoms at post-treatment (SMD −0.26, 95% CI −0.47 to −0.05) and a moderate effect at follow-up (SMD −0.51, 95% CI −0.82 to −0.21). When looking at symptoms of depression and anxiety, five studies (n = 452) favoured self-management both at end of treatment (SMD −0.26, 95% CI −0.51 to −0.01) and follow-up (SMD −0.19, 95% CI −0.33 to −0.04, k = 6, n = 964).
Relapse/readmission
Self-management did not have an effect on the total number of patients readmitted at either time point (SMD 0.84, 95% CI 0.48–1.46, and SMD 0.75, 95% CI 0.51–1.08, respectively), however there was an effect at follow-up on the mean number of readmissions (SMD −0.92, 95% CI −1.63 to −0.21). A small effect (SMD −0.26, 95% CI −0.50 to −0.02) was demonstrated on length of hospital admissions immediately following treatment (k = 6, n = 902), whereas a moderate effect (SMD −0.68, 95% CI −1.10 to −0.25) was found at follow-up (k = 7, n = 908).
Self-rated recovery
In relation to overall self-rated recovery, self-management was favoured over control at both time points with a moderate effect size (SMD −0.62, 95% CI −1.03 to −0.22) immediately following treatment (k = 11; n = 1013) and a large effect at follow-up (k = 7, n = 1134, SMD −0.81, 95% CI −1.40 to −0.22).
Empowerment
At the end of treatment (k = 3, n = 346) self-management interventions did not increase sense of empowerment (SMD −1.44, 95% CI −2.97 to 0.08), however at follow-up (k = 2, n = 538) there was a small but significant effect (SMD −0.25, 95% CI −0.43 to −0.07).
Hope
Self-management did not influence hope at end of treatment (k = 2, n = 389, SMD −0.18, 95% CI −0.38 to 0.01). At follow-up three studies with 967 participants showed a small but significant effect favouring self-management over control (SMD −0.24, 95% CI −0.46 to −0.02).
Self-efficacy
Four studies (n = 601) reporting on self-efficacy at end of treatment favoured self-management (SMD −0.38, 95% CI −0.62 to −0.15). One study provided data for self-efficacy at follow-up (n = 221), which also favoured self-management (SMD −0.34, 95% CI −0.61 to −0.07).
Functioning
At the end of treatment (k = 15, n = 1948), there was evidence of a moderate effect of self-management on functioning (SMD −0.56, 95% CI −0.85 to −0.28). At follow-up (k = 14, n = 1805) this increased to a large sized effect of self-management on social and functional disability (SMD −0.90, 95% CI −1.34 to −0.45).
Quality of life
Immediately following the end of the intervention, evidence from nine studies (n = 863) showed a small but significant effect of self-management on participants' self-rated quality of life (SMD −0.23, 95% CI −0.37 to −0.10) which was maintained at follow-up (k = 7, n = 980, SMD −0.25, 95% CI −0.37 to −0.12).
Heterogeneity and sensitivity analyses
Of the 22 meta-analyses, 17 had high levels of heterogeneity as assessed by an I 2 >50% and/or a significant χ2 test. The one-study-removed methodReference Higgins and Green17 was used to explore sources of statistical heterogeneity. Although high heterogeneity was identified in a range of meta-analyses, it did not appear to be driven by just one study. An evaluation of clinical and methodological characteristics resulted in the decision to not remove any studies. A full account of the sensitivity analysis is in Supplementary Appendix 2.
Publication bias
Funnel plots were created for the six meta-analyses that had more than ten studies (see Supplementary Fig. 3). The small number of studies and participants across these studies meant that it was difficult to discern any evident publication bias.
Post hoc analysis
A post hoc subgroup analysis of TAU-only and active control-only studies was conducted (see results in Supplementary Table 4). No differential pattern of outcomes between the different comparators was found.
Discussion
This is the first comprehensive systematic review and meta-analysis evaluating self-management interventions for people with SMI. The reviewed evidence suggests that self-management does confer benefits across a broad range of outcomes. Specifically, self-management has a positive impact on total symptom severity, negative symptoms and the symptoms of depression and anxiety, both at end of treatment and at 1-year follow-up. Self-management was found to influence positive symptoms at follow-up only. The effect size for self-management on total symptom severity was comparable to or better than those found in recent meta-analyses of cognitive–behavioural therapy for psychosis (CBTp): pooled effect size −0.33 (95% CI −0.47 to −0.19)Reference Jauhar, McKenna, Radua, Fung, Salvador and Laws65 and 0.40 (95% CI 0.252–0.58).Reference Wykes, Steel, Everitt and Tarrier66 At longer-term follow-up (approximately 1 year post-intervention) self-management had a large effect (SMD −0.88, 95% CI −1.19 to −0.57), although the high heterogeneity should be noted.
Despite the positive effect on symptoms, the findings were inconsistent for variables related to relapse and readmission. This was in contrast to a previous meta-analysis of self-management interventions for those with schizophrenia only,Reference Zou, Li, Nolan, Arthur, Wang and Hu8 which found a significant impact on relapse and readmission. In the present review, few studies reported relapse as an outcome and, of those that did, only a small number of participants experienced relapse events which may account for the lack of effect. The paucity of data impedes making any comment on the effect of self-management on relapse. However, self-management did demonstrate a small to moderate effect in terms of reducing the average length of hospital admissions, both at the end of treatment and 1 year follow-up.
Self-management did demonstrate a significant, medium-sized effect on global functioning and a small but significant effect on quality life at both end of treatment and 1 year follow-up. Furthermore, self-management seems to confer a benefit on outcomes valued especially highly by consumers,Reference Slade and Longden67 i.e. outcomes related to personal recovery and an individual's sense of empowerment, hope and self-efficacy. A moderate to large effect on overall recovery and self-efficacy was seen at both end of treatment and follow-up; the effect on the recovery-related concepts of empowerment and hope were significant at follow-up only.
Methodological limitations of primary studies
Although all studies included in this review were RCTs, there was variation in the reporting of sequence generation, allocation concealment and – as is common in complex interventions – blinding of participants and personnel was not always consistent. The greatest cause for concern was the selective reporting of outcomes which was noted or not clearly reported in two-thirds of the studies reviewed. Furthermore, the relatively small number of studies and participants in some studies meant that it was difficult to discern any evident publication bias. These limitations must be considered alongside the findings presented in this review to avoid an overestimate of the benefit of self-management.
Strengths and limitations of the review
This review gives a broad indication of the effectiveness and potential value of self-management interventions for people with SMI. A strength of this review is the generalisability of the findings to current practice. For instance, we included a diagnostically heterogeneous sample of people with SMI, representative of those on caseloads in secondary care mental health services and included samples from a wider range of countries and cultures.
Regarding limitations, heterogeneity was found to be high across many of the meta-analyses and, although a certain amount of heterogeneity is inevitable, we have tried to mitigate this through the use of random-effects modelling.Reference Higgins and Green17 A further potential limitation is from the risk of bias quality assessment of the studies included in this review. Interestingly, readmission rates and service use outcomes were infrequently measured by studies. We recommend the inclusion of this outcome in future studies of self-management. We also encourage collection and reporting of important sample characteristics such as participants' length of illness. Fewer than half of the included studies reported on length of illness – a potential mediator of the effectiveness of self-management interventions.
The choice to pool together comparisons of self-management against TAU or against active controls in the same analyses could be criticised. A post hoc subgroup analysis of TAU-only and active control-only studies showed no differential pattern of outcomes between the different comparators. Arguably, TAU varies hugely among the included studies and all of the active controls are treatments which might be available from a multidisciplinary community mental health team. Thus, irrespective of whether TAU and active controls are combined or not, the analysis is evaluating the addition of self-management to highly varied care.
The absence of patient and public involvement in this review is a limitation. Its inclusion would have been particularly useful in developing the operationalisation of self-management, as well as contributing to the interpretation and implications of findings from a patient's perspective. A final limitation in conducting this review was the lack of consensus of how to define the concept known as self-management. Our review is based on a clear operationalisation of self-management, however there is still substantial variation in such interventions.
Implications for practice
Although self-management for this population has been previously recommended at a guideline level,16,68 it remains to be routinely implemented at a service level. On the basis of this review, there is a strong case for including self-management as a high priority for psychosis services and generic community mental health services, alongside interventions such as CBTp or employment support. The diagnostically mixed populations in many studies may have been an impediment to identification of self-management as a high priority in guidance focused on specific groups, but our study supports recommendations from policy bodies and patient groups which state that self-management should be at the core of care for all long-term health conditions, physical and mental.69,Reference Foot, Gilburt, Dunn, Jabbal, Seale and Goodrich70 Self-management interventions are relatively straightforward compared with other psychotherapeutic interventions and can be delivered across settings and in a variety of ways (including group, individual or digital therapies, bibliotherapy or a combination of these), increasing potential for wide implementation. In this population they are often supported: support may be from clinicians, but also from peers. One may hypothesise that peer support could be especially effective in empowering patients and increasing self-efficacy to manage their illness. Effective implementation of these interventions has the potential to alter the long-term course of both the mental and physical health of people with SMI.
Research implications
In terms of future research, demonstrating whether there are clear effects on relapse and readmission is likely to require large, methodologically robust trials that include these outcomes along with cost-effectiveness analysis. The high heterogeneity in this review suggests there are important differences in the content and implementation or context of self-management interventions which influence how effective they may be. There are likely a number of potential contributors: length of intervention, contact time, facilitator (clinician or peer) and type of self-management intervention (from proposed subtypes). Future intervention studies would also benefit from the inclusion of measures of potential mediators and moderators: for instance, the addition of cognitive outcomes will be important for assessing the role of cognitive factors in mediating improvements in functioning. Additionally, structured development of future self-management programmes in conjunction with patients is recommended.Reference Milton, Lloyd-Evans, Fullarton, Morant, Paterson and Hindle71
Accordingly, there is a need to explore what forms of self-management are most effective, feasible and acceptable, and for whom. Possible study paradigms include realist evaluation of what works for whom, mechanistic studies or a broader systematic review that would have in its scope naturalistic studies using a variety of methods to look at experiences and outcomes of delivering self-management in various ways. Nevertheless, the evidence that self-management already has positive effects on a range of important outcomes is already substantial: thus research is now needed on how to overcome implementation barriers and embed self-management in a sustained and widespread way to routine care for people with long-term mental health conditions, and how to evaluate the effect of this. Implementation–evaluation designs have potential to address these questions.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.54.
Funding
This paper reports work undertaken as part of the CORE Study, which is funded by the National Institute for Health Research under its Programme Grants for Applied Research programme (reference RP-PG-0109-10078). The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.





eLetters
No eLetters have been published for this article.