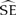Introduction
Many seabird populations are declining, particularly the tubenoses (Procellariiformes; Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). Marine-based threats are often implicated in these declines, as negative interactions (e.g. bycatch in fisheries) contribute to mortality of adults and juveniles at-sea (Lewison and Crowder Reference Lewison and Crowder2003, Lewison et al. Reference Lewison, Crowder, Read and Freeman2004). The movements of seabirds originally inferred from banding studies and shipboard surveys have been refined through data from miniaturized tracking devices (e.g. Brothers et al. Reference Brothers, Reid and Gales1997, Hedd et al. Reference Hedd, Montevecchi, Otley, Phillips and Fifield2012). Understanding the complete annual cycle, including non-breeding distributions, where migration corridors exist, migratory connectivity, and foraging behaviours is key to predicting how populations will respond to changing climate or prey stocks (Grémillet and Boulinier Reference Grémillet and Boulinier2009, Marra et al. Reference Marra, Cohen, Loss, Rutter and Tonra2015), and anthropogenic activities (Lewison and Crowder Reference Lewison and Crowder2003, Anderson et al. Reference Anderson, Small, Croxall, Dunn, Sullivan, Yates and Black2011). Knowledge of the physical and biological processes that influence seabird habitat selection may also assist with identifying significant areas as targets for marine conservation (Lavers et al. Reference Lavers, Miller, Carter, Swann and Clarke2014b).
For some species, such as the Flesh-footed Shearwater, mitigation of some threats, such as cessation of harvesting and reductions in fisheries bycatch off the east coast of Australia, has not reversed population declines on some islands (Gaze Reference Gaze2000, Reid et al. Reference Reid, Hindell, Lavers and Wilcox2013a). Despite its large size and ship-following habits, the Flesh-footed Shearwater has been one of the least studied seabirds in Australia (Powell Reference Powell2009). They breed on islands in southern Australia (Lavers Reference Lavers2015) and northern New Zealand (Waugh et al. Reference Waugh, Tennyson, Taylor and Wilson2013), with many populations known or suspected to be declining (Reid et al. Reference Reid, Hindell, Lavers and Wilcox2013a, Waugh et al. Reference Waugh, Tennyson, Taylor and Wilson2013, Lavers Reference Lavers2015). Increasing awareness of the diversity of pressures faced by Flesh-footed Shearwaters throughout their breeding range, and evidence of a decline at their largest breeding colony on Lord Howe Island (Priddel et al. Reference Priddel, Carlile, Fullagar, Hutton and O’Neill2006), led to the New South Wales population (∼16,500 breeding pairs; Reid et al. Reference Reid, Hindell, Lavers and Wilcox2013a) being listed as Vulnerable in 2000 (Lunney et al. Reference Lunney, Curtin, Ayers, Cogger, Dickman, Maitz, Law and Fisher2000). More recently, Flesh-footed Shearwaters breeding in New Zealand and Western Australia (combined total of ∼45,000 pairs accounting for ∼60% of the world’s population; Waugh et al. Reference Waugh, Tennyson, Taylor and Wilson2013, Lavers Reference Lavers2015) have also been up-listed to Vulnerable (Robertson et al. Reference Robertson, Dowding, Elliott, Hitchmough, Miskelly, O’Donnell, Powlesland, Sagar, Scofield and Taylor2013, DPaW 2015). Owing to recent population declines, the species was up-listed to Near Threatened on the IUCN Red List in 2017 (BirdLife International 2017).
Significant numbers of Flesh-footed Shearwaters are taken as bycatch in the Tasman Sea (Waugh et al. Reference Waugh, Filippi, Kirby, Abraham and Walker2012, Lavers et al. Reference Lavers, Bond, Van Wilgenburg and Hobson2013, Richard and Abraham Reference Richard and Abraham2013) and North Pacific Ocean (DeGange and Day Reference DeGange and Day1991, Tuck et al. Reference Tuck, Polacheck and Bulman2003, Ogi Reference Ogi2008, Artukhin et al. Reference Artukhin, Burkanov and Nikulin2010) where birds from New Zealand and Lord Howe Island forage and over-winter (Rayner et al. Reference Rayner, Taylor, Thompson, Torres, Sagar and Shaffer2011, Lavers et al. Reference Lavers, Bond, Van Wilgenburg and Hobson2013, Reid et al. Reference Reid, Tuck, Hindell, Thalmann, Phillips and Wilcox2013b). Bycatch of Flesh-footed Shearwaters in Western Australia is also a significant issue, with > 500 birds (mostly breeding adults) killed per year (Dunlop Reference Dunlop2007, Lavers Reference Lavers2015). A lack of information on demographics, the timing of migration, routes, and location of wintering grounds for Western Australian Flesh-footed Shearwaters limits our ability to identify additional areas of overlap with marine threats that may influence population trends at the breeding colonies. To inform conservation efforts more effectively, we report preliminary annual survival estimates, and non-breeding movements determined by light-level geolocators, for adult Flesh-footed Shearwaters from Western Australia.
Methods
Study site and capture methods
Shelter (or Muttonbird) Island is 130 m offshore of Torbay, Western Australia (35°03’S, 117°41’E), where ∼200 pairs of Flesh-footed Shearwaters breed (Lavers Reference Lavers2015). Breeding shearwaters were captured from burrows over a two-week period in mid- to late-incubation in 2011–2015. A uniquely numbered band was placed on the right leg. Sex was determined using measurements of head–bill length and minimum bill-depth from both members of breeding pairs (Thalmann et al. Reference Thalmann, Baker, Hindell, Double and Gales2007).
Tag deployment and analysis
Star-Oddi light-level geolocators (mass of tag and attachment: 3 g) were attached to the left leg of 10 adult birds (mean body mass: 599 g) on 22 January 2012. Maximum light measurements were recorded in every 20-minute period as well as water temperature after 20 minutes when the tag was wet, which acted as auxiliary environmental data for estimating location. Prior to deployment, tags were placed in an open area at the deployment location for five days to produce light recordings from which solar elevation estimates could be calculated. After retrieval, raw archived tag data were downloaded, and adjusted for internal clock drift.
At-sea locations were estimated from light intensity recordings using a threshold method: estimation of locations based on sunrise and sunset events and a threshold of 2.5 units (Lisovski et al. Reference Lisovski, Hewson, Klaassen, Korner-Nievergelt, Kristensen and Hahn2012). The corresponding sun elevation angle was estimated using sunrise and sunset times recorded after the deployment of the loggers and when the birds were known to be at the breeding sites. To refine location estimates, we used recorded sea-surface temperature, a spatial mask, and a movement model in a Bayesian framework implemented in the R package ‘SGAT’ (Sumner et al. Reference Sumner, Wotherspoon and Hindell2009, Wotherspoon et al. 2013). The twilight model allows us to estimate locations for a single sunrise/sunset with an expected error distribution. The discrepancy between observed and expected times of twilight was assumed to follow a log-normal distribution. For sunrise, positive values corresponded to an observed sunrise occurring after the expected time of sunrise, whereas positive values for sunset corresponded to an observed sunset before the expected time of sunset. We chose a conservative prior (log-normal distribution: meanlog = 1.5, sdlog = 0.8) since error in twilight detection could vary over the annual cycle. The land mask was based on the premise that birds only utilise marine environments (probability of 0 for positions on land). The probability on sea was further refined for each individual and each day separately, using remotely sensed sea-surface temperatures (weekly means on a 0.25 × 0.25 degree resolution: www.esrl.noaa.gov/psd/data/gridded/data.noaa.oisst.v2.highres.html) and the 60% and 95% percentile of the temperature profiles experienced by the birds. A probability of 1 was assigned to areas with surface temperatures falling within the 60% percentile of the recorded temperature profile. Areas with surface temperatures within the 95% and 100% of the recorded temperature percentile were assigned with probabilities of 0.5 and 0.1 respectively. The potential flight speeds were modelled following a gamma distribution (shape = 1.3, rate = 0.1). For each individual, we used these parameters to run the Markov chain Monte Carlo simulation. The first 10,000 iterations were used for burn-in and tuning. A further 40,000 samples were drawn to evaluate chain convergence before drawing another 5,000 samples to describe the posterior distribution.
Adult survival analysis
We captured and banded 114 adult birds from their burrows in November 2012, December 2013, January 2015, and December 2015, and coded breeding seasons by the year in which they began (2011–2012 is denoted as “2011”), resulting in four periods over which to estimate survival (2011–2012 through 2015–2016 breeding seasons). We estimated apparent adult survival (ϕ) and encounter probabilities (p) following Lebreton et al. (Reference Lebreton, Burnham, Clobert and Anderson1992) in Program MARK (White and Burnham Reference White and Burnham1999). Given the relatively small number of marked individuals, we did not formally consider emigration (Pradel et al. Reference Pradel, Rioux, Tamisier and Lebreton1997; Prévot-Julliard et al. Reference Prévot-Julliard, Lebreton and Pradel1998), but searched for marked birds in a 10 m buffer around three small, well-defined sub-colonies (see Discussion and Figure S1 in the online supplementary material). We also did not consider models including breeding propensity, but enumerated birds with encounter histories that suggested skipped breeding (i.e. birds not present for 1–2 years between encounters). Birds that had not been seen in the last two encounter periods could not be assigned confidently.
We constructed four models with time-varying and time-invariant estimates of ϕ and p (Table S1). Models were ranked using Akaike’s Information Criteria adjusted for small sample size (AICc; Akaike Reference Akaike1974, Burnham and Anderson Reference Burnham and Anderson2002). We tested for goodness-of-fit using 100 parametric bootstraps to generate the mean deviance and overdispersion (Jones et al. Reference Jones, Hunter and Robertson2002, Bond et al. Reference Bond, Jones, Williams and Byrd2013). The observed deviance and overdispersion were then divided by their respective bootstrap mean, and the higher of these two values was used to adjust for overdispersion and extrabinomial error (ĉ). This value of ĉ was used to adjust model likelihoods using quasi-AICc (QAICc). We generated parameter estimates and unconditional standard errors (![]() $\widehat {SE}$) weighted across all four models by Akaike weight (wi; Burnham and Anderson Reference Burnham and Anderson2002), and examined mark-recapture model assumptions using TEST 2 and TEST 3 in U-CARE (Choquet et al. Reference Choquet, Lebreton, Gimenez, Reboulet and Pradel2009).
$\widehat {SE}$) weighted across all four models by Akaike weight (wi; Burnham and Anderson Reference Burnham and Anderson2002), and examined mark-recapture model assumptions using TEST 2 and TEST 3 in U-CARE (Choquet et al. Reference Choquet, Lebreton, Gimenez, Reboulet and Pradel2009).
Results
Tracking data
Three geolocators were recovered from shearwaters on 27 November 2012 (two additional birds were recaptured, but the tags were missing). The three recovered tags ceased operating after 110–130 days, providing data for a total of 373 days at sea (Table 1). Birds A (♂) and B (♀) reared chicks to fledging successfully while the chick of bird C (♀) died of an unknown cause. The post-breeding migration of successful breeders commenced between 2–16 May with bird A requiring 6.5 days to travel northwest ∼ 5,900 km to the south coast of Sri Lanka (Figure 1a). Bird C remained in the southern Indian Ocean until ∼ 8 April 2011 before heading north along the coast of Western Australia for approximately 23 days and then west towards Sri Lanka (Figure 1c; Table 1).
Table 1. Sex and breeding status of adult Flesh-footed Shearwaters tracked using geolocation tags on Shelter Island, Western Australia.
A,B,CIndicates relevant panel for this bird presented in Figure 1.
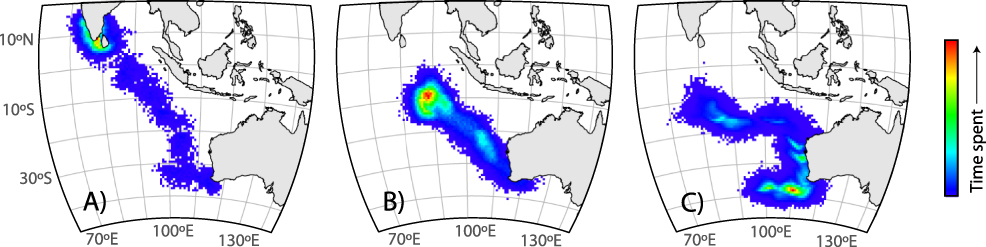
Figure 1. Map of relative time spent by migrating Flesh-footed Shearwaters carrying light-level geolocator tracking devices: a) successful breeding male, b) successful breeding female, and c) female whose chick died in early February. The density represents measures of relative time spent per area across the individual tracking period, incorporating the spatial uncertainty inherent in the model. Since individuals were tracked for different periods (36, 11.5, and 101 days), the scale of time spent is different for each individual. Bin size is 57 x 59 km.
Adult survival
Of the 114 breeding adults banded during December and January 2012–2015, 38 were recaptured during 2012–2015. An additional 22 adults were banded in 2015–2016 (i.e. no opportunity for recapture).
There was no trap-dependence (TEST 2.CT: χ22 = 1.782, p = 0.41; TEST 2.CL: χ21 = 0.936, P = 0.33), and survival did not depend on when animals were marked or recaptured (TEST 3.SR: χ23 = 1.467, P = 0.69; TEST 3.SM: χ22 = 0.000; P = 0.99). The top-ranked model included variable survival and a constant encounter probability (wi = 0.46), followed by a model in which survival was constant over time, but encounter probability varied (wi = 0.26, ΔQAICc = 1.16), and one where both survival and encounter probabilities were constant (wi = 0.23, ΔQAICc = 1.40). The model where survival and encounter probabilities varied over time received the least support (Table S1). Model-averaged parameter estimates gave annual apparent survival rates (± ![]() $\widehat {SE}$) between 0.634 ± 0.130 and 0.835 ± 0.164, and encounter probabilities between 0.391 ± 0.086 and 0.486 ± 0.147 (Table 2). Overall, the annual survival and encounter probabilities over the 2011–2015 study period were 0.731 ± 0.073 SE (95% CI: 0.567–0.849) and 0.428 ± 0.078 SE (95% CI: 0.286–0.584), respectively.
$\widehat {SE}$) between 0.634 ± 0.130 and 0.835 ± 0.164, and encounter probabilities between 0.391 ± 0.086 and 0.486 ± 0.147 (Table 2). Overall, the annual survival and encounter probabilities over the 2011–2015 study period were 0.731 ± 0.073 SE (95% CI: 0.567–0.849) and 0.428 ± 0.078 SE (95% CI: 0.286–0.584), respectively.
Table 2. Model-averaged parameter estimates unconditional standard errors (![]() $\widehat {SE}$), and 95% confidence intervals of apparent annual survival (ϕ) and encounter probability (p) of Flesh-footed Shearwaters. Survival intervals are presented based on the beginning of the breeding season such that “2011-2012” indicates survival from the 2011–2012 to 2012–2013 breeding season.
$\widehat {SE}$), and 95% confidence intervals of apparent annual survival (ϕ) and encounter probability (p) of Flesh-footed Shearwaters. Survival intervals are presented based on the beginning of the breeding season such that “2011-2012” indicates survival from the 2011–2012 to 2012–2013 breeding season.

Of the 114 marked individuals, we determined breeding propensity for 48: 12 (25%) were absent in one (n = 7) or two (n = 5) years of the study. Among marked individuals, 30% of birds bred in the next breeding season given that they bred in the current season, though this included birds which were never encountered subsequently. Using only birds known to be alive, 50% of birds were breeding the following year.
Discussion
We provide much needed insight into the movement and survival of a declining population of Flesh-footed Shearwaters from Western Australia. Based on at-sea observations, Gibson-Hill (Reference Gibson-Hill1953) and De Silva and Perera (Reference De Silva and Perera1995) predicted that Western Australian Flesh-footed Shearwaters would overwinter in the northern Indian Ocean. The limited geolocator data from 2012 confirm at least a portion of the Western Australian Flesh-footed Shearwater population spends the non-breeding period in the Arabian Sea and Bay of Bengal (Figure 1). Incomplete tracking data (GPS devices ceased transmission in the central Indian Ocean) for Flesh-footed Shearwaters from King George Sound, Western Australia, also indicated this migration route and over-wintering area (Powell Reference Powell2009). This region is highly productive and a major upwelling region that attracts a diversity of marine predators (Prasad and Nair Reference Prasad and Nair1960, Ballance et al. 1996, Vinayachandran et al. Reference Vinayachandran, Chauhan, Mohan and Nayak2004, Catry et al. Reference Catry, Ramos, Le Corre and Phillips2009). Wintering Flesh-footed Shearwaters also appeared to exploit this area, being one of the most commonly recorded seabirds in the region, and in the highly productive waters of the Gulf of Oman (Nezlin et al. Reference Nezlin, Polikarpov and Al-Yamani2007) during mid-April to August (Ballance et al. 1996, Campbell et al. Reference Campbell, Smiles, Roberts, Judas and Pedersen2017), suggesting the area is frequented by the Western Australian breeding population.
The timing of departure from the breeding colony varies by location and year, with most tracked Flesh-footed Shearwaters from Lord Howe Island (2005–2008; Priddel et al. Reference Priddel, Carlile, Fullagar, Hutton and O’Neill2006, Reid et al. Reference Reid, Hindell and Wilcox2012) and New Zealand departing between 20 and 30 April. In Western Australia, our data indicate departures between 2–16 May 2012 (Table 1) while five adult shearwaters carrying GPS satellite tags in 2008 departed King George Sound as early as 23 March (Powell Reference Powell2009). The overall timing of the breeding season does not differ among sites (Warham Reference Warham1958, Powell et al. Reference Powell, Wooller and Bradley2007). The eastern population of Flesh-footed Shearwaters requires around 20 days to migrate 9,000 km to their wintering grounds in the Sea of Japan (Rayner et al. Reference Rayner, Taylor, Thompson, Torres, Sagar and Shaffer2011, Reid et al. Reference Reid, Tuck, Hindell, Thalmann, Phillips and Wilcox2013b), while those from Western Australia reach their wintering grounds off Sri Lanka (∼ 5,900 km; Table 1) in around six days. Seabird flight is strongly influenced by wind (Spear and Ainley Reference Spear and Ainley1997, Weimerskirch et al. Reference Weimerskirch, Louzao, de Grissac and Delord2012, Yonehara et al. Reference Yonehara, Goto, Yoda, Watanuki, Young, Weimerskirch, Bost and Sato2016), with migration following low-cost “wind-highways” linking breeding and wintering areas (Felicísimo et al. Reference Felicísimo, Muñoz and González-Solis2008). During May, Western Australian shearwaters completing their migration likely exploit the South-west Monsoon Current which flows northward, then curves east over the Arabian Sea and south-east towards India (Pocklington Reference Pocklington1979, de Vos et al. Reference de Vos, Pattiaratchi and Wijeratne2013).
Our estimates of adult Flesh-footed Shearwater survival, while based on a small sample, are low compared to other shearwaters (Table S2). Adult survival could be influenced negatively by several factors, including disturbance from humans and feral species (Lavers Reference Lavers2015), climate change and trophic decline (Bond and Lavers Reference Bond and Lavers2014), ingested plastic, and contaminants (Lavers et al. Reference Lavers, Bond and Hutton2014a). Western Australian Flesh-footed Shearwaters tracked using GPS in 2015 (J. Lavers unpubl. data) suggest adult birds forage in coastal waters during the breeding season, only occasionally venturing beyond the continental shelf (Powell Reference Powell2009). The cooler inshore waters around the mouth of King George Sound are spawning grounds for Australian pilchards Sardinops sagax neopilchardus (Fletcher and Tregonning Reference Fletcher and Tregonning1992), the shearwaters’ key prey species (Lavers Reference Lavers2015). This reliance on pilchards, combined with the species’ gregarious and aggressive nature around fishing vessels (Wahl and Heinemann Reference Wahl and Heinemann1969), brings Flesh-footed Shearwaters into regular contact with purse seiners targeting pilchards in King George Sound (Dunlop Reference Dunlop2007, Lavers Reference Lavers2015).
Flesh-footed Shearwaters breeding in New Zealand are exposed to many of these same pressures (Waugh et al. Reference Waugh, MacKenzie and Fletcher2008, Bond and Lavers Reference Bond and Lavers2011, Buxton et al. Reference Buxton, Currey, Lyver and Jones2013), yet adult survival is higher (75.6 to 94.0%, Barbraud et al. Reference Barbraud, Booth, Taylor and Waugh2014) than in Western Australia (Table 2). The low encounter rate at Shelter Island may explain this discrepancy, reflecting emigration to nearby colonies or low breeding propensity. Breeding site fidelity is high in Procellariiformes (Warham Reference Warham1990, Brooke Reference Brooke2004), including some Flesh-footed Shearwater populations (Barbraud et al. Reference Barbraud, Booth, Taylor and Waugh2014). Low nest-site fidelity could result from human disturbance (Carey Reference Carey2011), and Shelter Island is visited often by the public. The low survival estimates from Shelter Island could also indicate a lower rate of mate retention. Birds whose mate did not survive (e.g. killed as bycatch) would need to find a new mate, which could manifest in lower nest-site fidelity and therefore reduced probability of recapture (Mills and Ryan Reference Mills and Ryan2005).
Some shearwaters, including Flesh-footed Shearwaters in New Zealand, may not breed annually (Mougin et al. Reference Mougin, Jouanin and Roux1997, Waugh et al. Reference Waugh, Jamieson, Stahl, Filippi, Taylor and Booth2014), similar to our findings. Many factors influence whether individuals breed in a given year, including prior breeding success, disturbance, and survival/return of a partner (Chastel Reference Chastel1995, Le Bohec et al. Reference Le Bohec, Gauthier-Clerc, Gremillet, Pradel, Bechet, Gendner and Le Maho2007, Finkelstein et al. Reference Finkelstein, Wolf, Goldman, Doak, Sievert, Balogh and Hasegawa2010). Of Flesh-footed Shearwaters encountered at least once after marking, half were not encountered the year following a breeding attempt, suggesting a relatively low breeding frequency relative to similar species (typically > 75%; Chastel Reference Chastel1995). The small number of marked birds relative to the number of parameters in a multi-state model was too small to examine breeding propensity (Burnham and Anderson Reference Burnham and Anderson2002).
Finally, heavy rainfall events can result in nest abandonment by burrowing seabirds (Thompson and Furness Reference Thompson and Furness1991, Tiller et al. Reference Tiller, Klomp, Fullagar and Heyligers2013) and are implicated in the low site fidelity observed in December 2012 and 2015 when storms flooded burrows on Shelter Island (J. Lavers pers. obs.). To account for this movement, recapture effort was expanded to include unmarked burrows within a 10 m radius of each sub-colony. However, during subsequent breeding seasons, some banded birds were found breeding in new burrows up to 50 m away from their original capture location indicating a high degree of movement within the colony. The survival estimate for Shelter Island is, therefore, conservative.
Factors influencing the survival of Flesh-footed Shearwaters on their overwintering grounds are poorly understood. Large-scale fishing fleets operate in the northern Indian Ocean, and data on seabird bycatch are extremely limited due to lack of reporting, however Flesh-footed Shearwaters have been observed following, and negatively interacting with, vessels in this area (Huang and Liu Reference Huang and Liu2010, IOTC 2015). Small numbers are harvested and sold by local fishermen in southern India after the birds become hooked (Abdulali and Grubh Reference Abdulali and Grubh1982).
As sentinels of ocean health, seabirds, including those from Western Australia, tell a worrying story of increasing levels of marine pollution, changing oceanography and climate, and reductions in fish stocks (Bond and Lavers Reference Bond and Lavers2011, Chambers et al. Reference Chambers, Devney, Congdon, Dunlop, Woehler and Dann2011, Dunlop et al. Reference Dunlop, McNeill and Cannell2013). Seabirds face pressures both on land and at sea, yet factors contributing to mortality and morbidity are often examined in isolation (Lavers Reference Lavers2007, Costello et al. Reference Costello, Coll, Danovaro, Halpin, Ojaveer and Miloslavich2010, Coll et al. Reference Coll, Piroddi, Albouy, Ben Rais Lasram, Cheung, Christensen, Karpouzi, Guilhaumon, Mouillot, Paleczny, Palomares, Steenbeek, Trujillo, Watson and Pauly2012). Effective conservation requires targeted on-island management with concurrent protection of areas used by seabirds during breeding, migration, and over-wintering (Louzao et al. Reference Louzao, Hyrenbach, Arcos, Abelló, de Sola and Oro2006, Lavers et al. Reference Lavers, Miller, Carter, Swann and Clarke2014b). Enhanced protection of productive upwelling zones that form key staging grounds for Flesh-footed Shearwaters would provide much-needed protection for this declining species, as well as a suite of marine wildlife that also use these habitats.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000084
Acknowledgements
BirdLife Tasmania, Trading Consultants Ltd, W.V. Scott Charitable Trust, and the Integrated Marine Observing System (IMOS) provided funding or equipment for this research. Special thanks to C. & G. Biddulph, P. Collins, A. Fidler, M. Hindell, C. McMahon, J. Pridham, P. Sharp, M. Stadler, S. Stuckenbrock, S. Toole, the Two Hands Project, and V. Wellington for providing generous support. Discussions with A. Breton improved the survival analysis. Research was undertaken with approval from the University of Tasmania animal ethics committee (permit no. A12279 and A13598) and Western Australian Department of Parks and Wildlife (permit no. CE004240 and SF010159). The manuscript benefited from discussions with A. Kumar, R. Jeyabaskaran, M. Hindell, J. Praveen, and two anonymous reviewers improved previous drafts.