INTRODUCTION
The first report of the isolation of human Mycoplasma was in 1937, when M. hominis was isolated from a Bartholin's gland abscess [Reference Dienes and Edsall1]. Subsequently, several Mycoplasma species have been detected in the human respiratory and urogenital tracts. Mycoplasmas are usually considered commensals, although some species such as M. pneumoniae, M. hominis, M. genitalium and U. urealyticum have been implicated in pathological conditions. The species that are more frequently isolated from the urogenital tract of sexually active adults are M. hominis and U. urealyticum and, although they have been considered responsible for genital diseases (such as cervicitis, non-gonococcical urethritis, pelvic inflammatory disease), infertility and obstetric complications (such as chorioamniotitis, spontaneous abortions, stillbirth, premature birth, low birthweight in neonates) [Reference Haggerty and Ness2–Reference Kataoka4], not all colonized patients show signs and symptoms of disease. The high prevalence of U. urealyticum colonization observed in asymptomatic women is probably linked to epidemiological factors such as ethnicity (Americans and Africans have higher prevalence), sexual activity with multiple partners, oral contraceptive use, younger age and lower socioeconomic status [Reference Waites, Katz and Schelonka5, Reference Cassell6]. U. urealyticum has also been observed as an opportunistic pathogen during pregnancy but high-density of cervical U. urealyticum colonization may lead to premature rupture of the membranes and preterm delivery [Reference Mitsunari7]. The degree of colonization with U. urealyticum correlates with an adverse effect on pregnancy outcome. This may mean that not all women positive for U. urealyticum should be treated, but only those patients with a high colonization rate. Moreover, M. hominis has been found in about two-thirds of women with bacterial vaginosis and in 10% of women affected by salpingitis and endometritis [Reference Judlin8].
The purpose of this study on women undergoing routine gynaecological examination, is to evaluate the prevalence of cervical U. urealyticum and M. hominis, in order to investigate the relationship between the presence of cervical U. urealyticum or M. hominis and behavioral factors. Moreover, it is interesting to distinguish between detection at high and low density because a high number of microorganisms may be responsible for diseases even in the presence of non-pathogenic microorganisms.
MATERIALS AND METHODS
Study population
Women who attended the Cervico-Vaginal Pathology Unit in the Department of Gynaecology and Obstetrics Sciences and Urology Science, ‘Sapienza’ University of Rome, between 2003 and 2010 for routine gynaecological care were enrolled in the study: informed consent was obtained from all participants. Socio-demographic characteristics, reproductive history, behavioural and sexual information were collected using a questionnaire and a personal interview conducted by trained interviewers. The questionnaire was anonymous and linked to the patient by a code number.
Women were eligible for the study: (1) if they were not currently pregnant nor planning to become pregnant during the following year; (2) if they had an intact uterus and no current referral for hysterectomy; (3) if they had not used vaginal medication in the previous 3 days; (4) if they had not had treatment for cervical disease in the previous 6 months; (5) if they had no clinical signs of herpes virus infection and no diagnosis of other sexually transmitted infections. According to these criteria, 3115 women were enrolled.
Mycoplasma spp. detection
Mycoplasma IST 2 (bioMérieux, France) was used for the isolation of M. hominis and U. urealyticum according to the manufacturer's instructions. The diagnostic kit provided information regarding the presence or absence of M. hominis and U. urealyticum and an estimate of the density of each microorganism with their antimicrobial susceptibilities.
Statistical analysis
The χ 2 test and odds ratio were used to measure the association of M. hominis and U. urealyticum infection with the studied characteristics. Arithmetic means and standard deviations are presented for quantitative variables, age, age at first intercourse and number of lifetime partners. However, because the distributions were not normal (age at first intercourse and number of lifetime partners were extremely positively skewed), neither the t test nor analyses of variance were performed, and the prevalences presented and the distributions were compared directly using the χ 2 test. Statistical tests were considered significant if P ⩽ 0·05. Multivariate logistic regression analysis was used to assess the simultaneous effect of more than one variable on the risk of M. hominis and U. urealyticum infection and to identify possible confounding factors. The variables included in the final logistic regression model were selected on the basis of step-down procedure; i.e. in the first model all the independent variables were included and successively the least significant variables were eliminated. However, the independent variables are highly correlated with each other (e.g. older women are more likely to be parous, those who are young at first intercourse are more likely to have had more partners) and so the final variables included in the model depend on the selection procedure used.
RESULTS
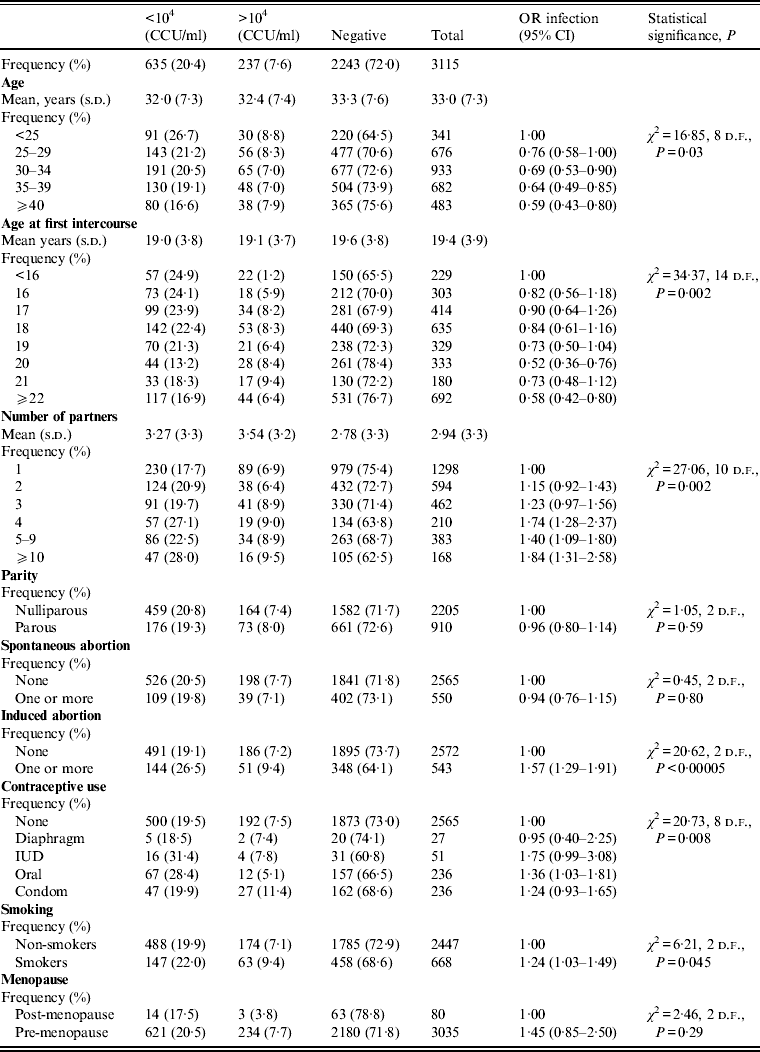
The study compared demographic and behavioural characteristics of 872 women with U. urealyticum infection, 142 women with M. hominis infection and an uninfected group. In the total population U. urealyticum was found in 28% of women; high-density cervical U. urealyticum colonization [>104 colour-changing units (CCU)/ml] was present in 7·6% and <104 CCU/ml in 20·4% (Table 1). The mean age of the women was 33 years (range 14–57 years). The mean age of the U. urealyticum-positive group was 32·2 years (32·0 years for women with high-density cervical U. urealyticum colonization and 32·4 years for women with cervical U. urealyticum colonization <104 CCU/ml), whereas the mean age of U. urealyticum-negative women was 33·3 years.
Table 1. Characteristics of study group, Ureaplasma urealyticum infection (univariate analysis)

CCU, Colour-changing units; OR, odds ratio; CI, confidence interval; s.d., standard deviation; IUD, intrauterine device.
The highest prevalence of U. urealyticum infection was observed in younger women (age <25 years). Comparing age groups shows that the risk of infection decreases with increasing age (P = 0·03). Cervical U. urealyticum colonization <104 CCU/ml was more frequent in women with age at first sexual intercourse <16 years. The mean number of sexual partners was 3·27 in women with low-density cervical U. urealyticum infection, 3·54 in the group with high-density infection but only 2·78 in the U. urealyticum-negative group (P = 0·0001). The risk of U. urealyticum infection increases with the number of sexual partners.
In this study, contraceptive use [condom, intrauterine device (IUD), oral contraceptives] was associated with infection, but the number of women using an IUD and diaphragm was very small. IUD and oral contraceptive users had a higher prevalence of U. urealyticum (39·2% and 33·5%, respectively).
The risk of U. urealyticum infection is statistically associated with previous induced abortion and smoking, while a previous spontaneous abortion, parity and menopausal status are not significantly associated with this infection. The characteristics of women with low- and high-density cervical U. urealyticum infection are reported in Table 1. In univariate analysis the characteristics significantly associated with U. urealyticum presence are also age at first sexual intercourse (P = 0·002), the number of sexual partners (P = 0·002), previous induced abortion (P < 0·00005), contraceptive use (P = 0·008) and smoking habit (P = 0·045) (Table 1). Multivariate logistic regression analysis shows that IUD, number of sexual partners and age were independently significantly associated with the presence of U. urealyticum. The infection decreases with age ⩾35 years (OR 0·76, 95% CI 0·64–0·90) and tends to increase in women with a higher number of sexual partners [partners (n = 4): OR 1·71, 95% CI 1·25–2·33; partners (n = 5–9): OR 1·39, 95% CI 1·08–1·78; partners (n⩾10): OR 1·80, 95% CI 1·28–2·54) and IUD users (OR 1·41, CI 95% 1·11–1·80).
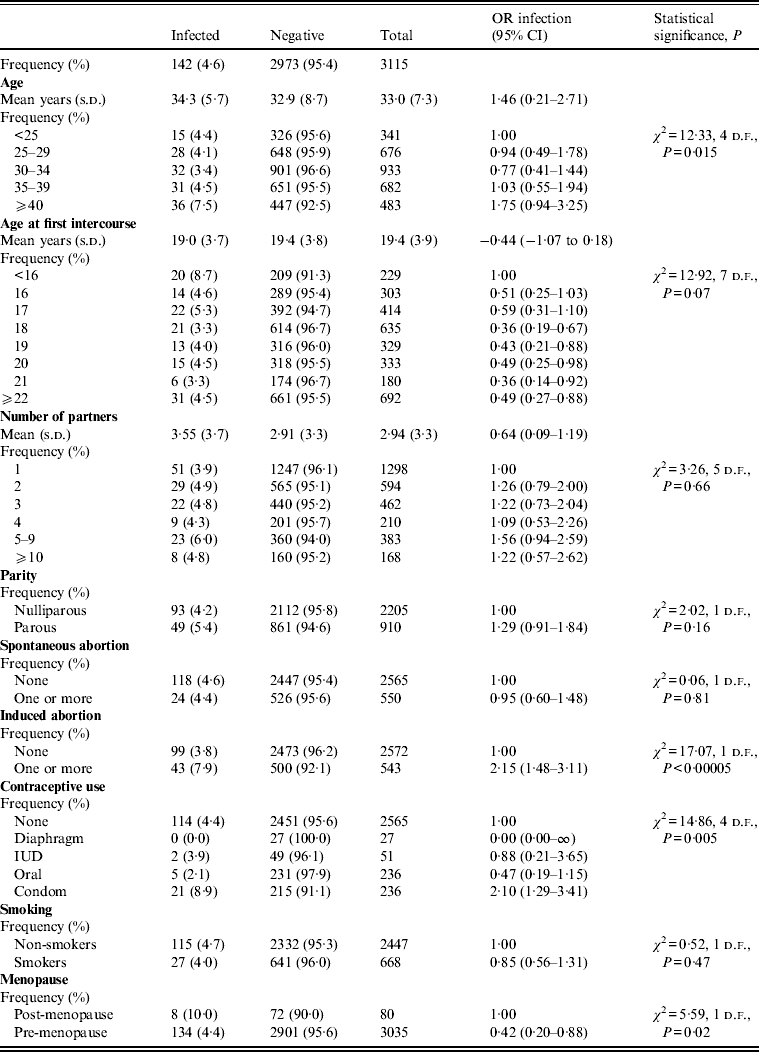
Table 2 shows the demographic and behavioural characteristics of M. hominis-positive and -negative women. In the total population, M. hominis was found in 142 (4·6%) women. The mean age of these women was 34·3 years while it was 32·9 for negative women. The difference is statistically significant (P = 0·02). The M. hominis infection is significantly associated with older age (⩾40 years, P = 0·015), in contrast to U. urealyticum, and more frequent in women with younger age at first intercourse (<16 years). No strong evidence of a difference between positive and negative women regarding age at first intercourse was observed. The mean of number of sexual partners was 3·55 for infected women and 2·91 for negative women (P = 0·02). Moreover, for M. hominis, the risk of infection increased in women with a history of previous induced abortion, but not in those with a history of previous spontaneous abortion and parity. Of the contraceptive methods reported, only 27 women used a diaphragm and none of these were positive. M. hominis infection was significantly more frequent in condom users (OR 2·10, 95% CI 1·29–3·41), but may tend to decrease in women who used oral contraceptives (OR 0·47, 95% CI 0·19–1·15) or IUD (OR 0·88, 95% CI 0·21–3·65) even though the number of IUD and oral contraceptive users was very small.
Table 2. Characteristics of study group, Mycoplasma hominis infection (univariate analysis)

OR, Odds ratio; CI, confidence interval; s.d., standard deviation; IUD, intrauterine device.
In contrast to U. urealyticum infection, age at first intercourse, number of sexual partners, and smoking were not associated with M. hominis infection. In univariate analysis, the characteristics significantly associated with M. hominis were previous induced abortion (P < 0·00005), contraceptive method (P = 0·005) and post-menopause status (P = 0·02). In multivariate logistic regression analysis, previous induced abortion (OR 2·00, 95% CI 1·37–2·91), condom use (OR 2·11, 95% CI 1·29–3·43) and age at first intercourse >16 years (OR 0·51, 95% CI 0·31–0·84) were significantly associated with M. hominis infection. Previous induced abortion and condom use double the risk of M. hominis infection whereas age at first intercourse >16 years halves the risk.
DISCUSSION
The study provides information on the prevalence of U. urealyticum and M. hominis infection in gynaecological outpatients referred for occasional or routine gynaecological examinations. In Italy, few studies have been published regarding the prevalence of Mycoplasma species and their risk factors. In this study U. urealyticum was detected in 28% of the 3115 enrolled women while M. hominis was isolated in 4·6%. In previous studies, U. urealyticum was found in 40–80% of sexually mature asymptomatic women, in 5% of children, in 40% of sexually inactive women, in 67% of sexually active women, in 25% of post-menopausal women, and in 72% of women with multiple sexual partners [Reference Volgmann, Ohlinger and Panzig9, Reference Wang10]. Colonization increases with the number of different sexual partners, and the proportion of women colonized is greater than that of men, suggesting greater female susceptibility. In addition, the prevalence of U. urealyticum, in both men and women, is higher than that of M. hominis [Reference Taylor-Robinson3]. The prevalence of M. hominis has been found to be 21–53% in other studies [Reference Waites, Katz and Schelonka11]. A low incidence of Mycoplasma from pre-pubertal growth was also reported. After puberty, colonization of genital mycoplasmas occurs primarily through sexual contact [Reference Gupta12]. M. hominis and U. urealyticum can be part of the normal vaginal flora of women but both may play a role in obstetric complications and genital diseases.
Information from previous studies has accumulated slowly, mainly due to the technically challenging culture methods needed to link the organism to clinical conditions [Reference Larsen and Hwang13]. The high prevalence of mycoplasmas observed in healthy patients might suggest that not all cases of infection should be treated. Genital diseases such as cervicitis in women, urethritis in men and adverse pregnancy outcomes (infertility, postpartum endometritis, chorioamnionitis, spontaneous abortion, stillbirth, premature birth, perinatal morbidity and mortality, pneumonia, bacteraemia, meningitis, bronchopulmonary dysplasia) [Reference Czikk, McCarthy and Murphy14–Reference Al17] occur in only a sub-population of individuals who are colonized in the lower tract by genital Mycoplasma. The risk factors for upper tract involvement are unknown.
The question of who should be assessed for Mycoplasma presence, and whether or not to treat the infection to prevent obstetric and gynaecological diseases, remains open [Reference Volgmann, Ohlinger and Panzig9]. The pathogenic potential of U. urealyticum seems to be related to high bacterial density (>10000 CCU/ml). In a recent study, we found a significant association between human papillomavirus (HPV) infection and Chlamydia trachomatis. Interestingly, there was also an association between HPV and U. urealyticum, but only in patients with a high colonization rate [Reference Verteramo18]. Further, high-density cervical U. urealyticum colonization may be a risk factor for premature rupture of membranes, chorioamnionitis and preterm delivery [Reference Abele-Horn19, Reference Randelović20].
In the present study, high-density cervical U. urealyticum was found in 7·6% (237/3115) of women and low-density infection in 20·4% (635/3115) of women. In the future, we believe that it would be useful to study the characteristics of women with high-density cervical U. urealyticum colonization in order to identify possible risk indicators of genital diseases or obstetric complications.
One of the strengths of this study is that the results start to fill the gap in the knowledge of the epidemiology of these infections, but caution should be exercised when trying to generalize the results. Almost all health indicators show considerable variability in the geographical regions of Italy, and the patients in this study are from the capital, Rome, which can hardly be considered typical of all Italy. Between countries, there is likely to be even more variability. These data may be considered an addition to current knowledge and a first indication of the local prevalence of the infections. However, the associations between the identified risk factors and the prevalence of the infections may be more generalizable, but this must be confirmed by other studies in other environments. Furthermore, the responses to sensitive questions on sexual history and behaviour may not be accurate. The recall may be distorted by shyness or simply bad memory. For example the response to the question on the number of lifetime sexual partners may be reasonably precise for one, two or three partners, but at higher numbers some women may feel reluctant to state, or indeed forget, the true value. There is evidence of ‘heaping’ at 5, 10, 15, 20 and even 30 partners with associated reduced frequencies at 9, 11, 19 and 21 partners. Grouping the frequencies at these higher values may reduce the effect of numerical preference.
It is also true that some of the significant results may be due merely to chance. We adopted the 5% level of significance to indicate ‘strong evidence’ of an association, but we performed many tests and one in 20 results should be ‘significant’ even when the null hypotheses are true. Our sample size of >3000 women is not particularly small, but when divided between infection status and the categories of the risk factors the numbers quickly become small. Some of our results on the effect of each of the various contraceptive methods on the risks of infection are based on very small numbers; the statistical power is low, which may cause real associations to be missed.
It is interesting to observe that a history of previous induced abortion, as opposed to previous spontaneous abortion, increases the risk of infection for both M. hominis and U. urealyticum. Another surprising finding is that M. hominis is more common in older women and post-menopausal women while U. ureayticum is more common in younger women. U. urealyticum seems to be sexually transmitted and to present the same demographic and behavioural characteristics of other sexually transmitted diseases such age at first sexual intercourse, number of sexual partners, IUD use and smoking, whereas the infection decreases with increasing age.
Morever, Tibaldi et al. [Reference Tibaldi21] found the highest prevalence of U. urealyticum infection in younger women (age <25 years) and the risk of infection decreased with increasing age. The prevalence was higher for women with 0–8 and 9–13 years of education, and those not having a steady partner. It was also increased for women who had more than one previous abortion, were currently using an IUD, had inconsistently used condoms or spermicide, had more than one partner in the previous 6 months, and more than two lifetime partners. The prevalence in women was greater than for men, suggesting greater female susceptibility, probably due to biological differences. The characteristics associated with M. hominis infection are also condom use and young age at first intercourse (<16 years). Age at first intercourse >16 years halved the risk, whereas condom use doubled the risk. M. hominis can occur as an endogenous organism in the vaginal flora from the intestinal tract. It is possible that the use of condoms, causing vulvar and vaginal erythema, favours M. hominis growth. The increased frequency of M. hominis in post-menopausal and older women, and the unprotective role of condoms suggest a non-sexual mode of transmission and strengthens the hypothesis of an opportunistic microorganism.
The data presented in this study correlate the presence of M. hominis and U. urealyticum with some risk factors associated with these infections and may help to identify individuals predisposed to complications, restricting the number of patients being treated with both clinical and economic advantages.
ACKNOWLEDGEMENTS
We are grateful to all study participants for their contributions and to the MIUR-Italy (Ministero dell'Università, Istruzione, Ricerca scientifica) for financial support.
DECLARATION OF INTEREST
None.