Introduction
The Genus Buteo comprises 28 species (Thiollay Reference Thiollay, del Hoyo, Elliott and Sargatal1994) distributed on all continents except Antarctica and Australia. Twenty-one species are found in the New World (Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001) and only three are island endemics: Galapagos Hawk Buteo galapagoensis, Hawaiian Hawk Buteo solitarius and Ridgway’s Hawk Buteo ridgwayi. These three species are considered to be at risk (BirdLife International 2013). Breeding ecology characteristics within the genus Buteo vary from medium-sized, monogamous migrants with high annual productivity to larger, sedentary, polyandrous species with low annual productivity (Ferguson-Lees and Christie Reference Ferguson-Lees and Christie2001).
Ridgway’s Hawk is a forest raptor endemic to Hispaniola in the Caribbean. The species was locally common in areas of Haiti and the Dominican Republic during the beginning of the 20th century (Cory Reference Cory1885, Wetmore and Lincoln Reference Wetmore and Lincoln1934), but is now listed as ‘Critically Endangered’ (BirdLife International 2013). The current global population size is estimated at fewer than 110 pairs, limited to an area of 1,600 km2 of karst rainforest in Los Haitises National Park on the north-east coast of the Dominican Republic (Woolaver Reference Woolaver2011). Forest loss, due to slash–and-burn agriculture, and human persecution of hawks have been major factors in the species’ decline (Woolaver Reference Woolaver2011). Less than 1.5 % of Haiti’s original forest is left, most of which is in the inaccessible uplands of the island and is highly degraded (Rimmer et al. Reference Rimmer, Townsend, Townsend, Fernandez and Almonte2005). The Dominican Republic has not fared much better, with only 10% of its original forest cover remaining, which is under threat of further loss from unregulated logging, slash-and-burn agriculture, and charcoal production (Harcourt and Sayers Reference Harcourt and Sayers1996, Latta et al. Reference Latta, Rimmer, Keith, Wiley, Raffaele, McFarland and Fernandez2006). Previous studies of the species have focused on examining nest success and threats to the species (Thorstrom et al. 2005, Reference Thorstrom, Almonte, Balbuena de la Rosa, Bildstein, Barber and Zimmerman2007). Observations of the reproductive traits of the species have been relatively few. Three nests were monitored by Wiley and Wiley (Reference Wiley and Wiley1981) over one breeding season in 1976. We monitored nesting birds over five breeding seasons from 2005 to 2009 to describe the reproductive traits of the species, determine primary causes of nesting failures, and identify predictors of nest success and fledging rates.
Methods
Study Area
The island of Hispaniola (19°0'N, 71°0'W) is located in the Caribbean, and consists of the nations of Haiti and the Dominican Republic. The cool, wet season is from April to December with eastern regions of the island receiving > 2,000 mm of annual rainfall and the humid wet forests receiving the highest annual amounts at > 3,000 mm. We conducted our study in Los Haitises National Park (19°N, 70°W) which ranges from 0 to 380 m asl in elevation and is on the north-eastern coast of the Dominican Republic (Figure 1). It is a platform karst (eroded limestone) formation, with dense clusters of steep conical hills or mogotes, of nearly uniform height (200–300 m) separated by sinkhole valleys. The Los Haitises region consists of thousands of such mogotes.
Figure 1. Maps showing relative location of Hispaniola in the Caribbean (top) and the island of Hispaniola with the nations of Haiti and Dominican Republic and their respective capital cities: Port-au-Prince (1) and Santo Domingo (2). The boundaries of Los Haitises National Park (3) are delineated in black.
Nest monitoring and breeding
We carried out early season observations for breeding pairs from vantage points on hillsides overlooking valleys to identify nest locations. Due to the topography of the study area, nesting sites were easy to distinguish for each pair and were located in separate valleys. Once found, we visited nests every 1–3 days for easily accessible nests, or every 1–2 weeks for sites that were more difficult to access. During each visit we recorded nest building behaviour, brooding and incubation behaviour and duration, copulations, territorial defence or displays, and pair interactions. Nest observations were carried out with binoculars and a spotting scope from a covered vantage point (10–25 m away) during 4-hr observation sessions, randomised to cover daylight hours.
A nest was defined as reproductively active once an egg had been laid. A nest was classified as successful if at least one young fledged, and failed if it had been active but subsequently did not produce at least one fledgling. Laying, hatching and fledging dates were based on nest observations and nest checks. Fledging rate was defined as the number of fledglings produced per active nest (fledgling nest-1), whereas nest success was the proportion of active nest attempts that fledged at least one young. Hatching success was defined as the percentage of eggs to hatch successfully from a nest and fledgling success as the percentage of nestlings to successfully fledge from a nest. Incubation and brooding refer to when an adult was physically sitting on the eggs or the nestlings. We defined nest attendance as when an adult was physically present at the nest during the nestling stage.
When 15–40 days old, nestlings were placed in cotton bags and lowered to the ground below the nest, where they were measured, ringed (when > 30 days old), and a blood sample collected for DNA analyses. Handling time of nestlings did not exceed 20 minutes per individual. We captured adults using bal-chatri noose traps baited with white domestic mice Mus musculus (Thorstrom Reference Thorstrom1996). Adults were not trapped when the pair was incubating eggs. Ridgway’s Hawks were ringed with coloured and numbered anodised Acraft© aluminium rivet rings in unique combinations to identify individual birds. No more than one ring was placed on a leg and sex was evident by the time nestlings were > 30 days with females being heavier and having thicker tarsi than males (Woolaver Reference Woolaver2011).
We collected the following information for each nest: name of valley, GPS coordinates (WGS84), nest tree species, nest tree circumference at breast height, nest tree height using an optical range finder, nest height from bottom of nest to the ground using a measuring tape if the nest was accessible, or an optical range finder. We also recorded whether it was a first or second nest attempt for that pair in that year. Distances to nearest conspecific nest, and potential competitor’s nests (White-necked Crow Corvus leucognaphalus and Red-tailed Hawk Buteo jamaicensis) were estimated using GPS coordinates and Google Earth©. We also recorded whether the Ridgway’s Hawk nest was constructed on top of a Palmchat Dulus dominicus nest and, if so we noted Palmchat nest status as active or inactive (Table 1).
Table 1. Nest site parameters for Ridgway’s Hawk Buteo ridgwayi nests monitored in Los Haitises, Dominican Republic from 2005 to 2009.
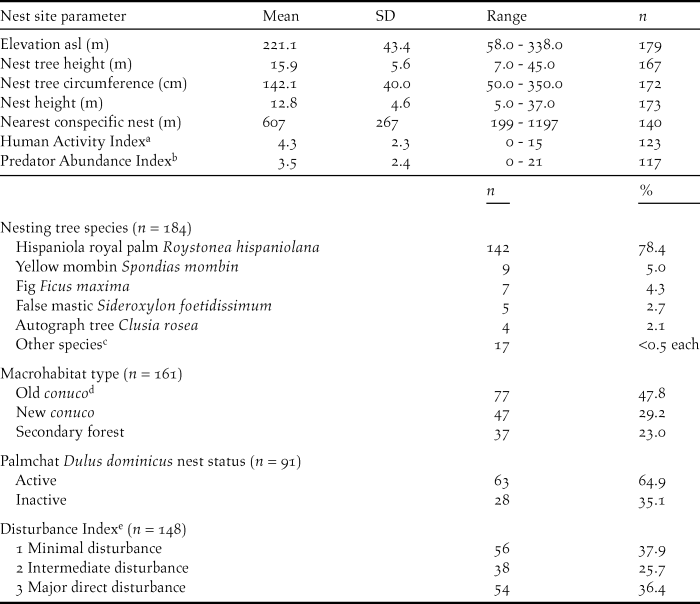
a Number of people observed passing through or working within the nesting valley per 4-hour observation period.
b Number of predators observed within the nesting valley per 4 hour observation period.
c Chicharrón Terminalia domingensis, coconut palm Cocos nucifera, lancewood Nectandra coriacea, masa Tetragastris balsamifera, bullwood Sloanea berteriana, kapok tree Ceiba pentandra, gregorywood Bucida buceras, haya minga Guatteria blainii, seibón Bombacopsis emarginata, bastard hogberry Margaritaria nobilis, pumpwood Cecropia schreberiana, Spanish elm Cordia alliodora, pancho prieto Ziziphus rhodoxylon, river koko Inga vera, and Antilles calophyllum Calophyllum antillanum.
d A conuco is a plot of agricultural land.
e Level of direct disruption to a pair or nest site.
Macrohabitat for each nesting site valley was divided into three categories: New conuco, Old conuco, and Secondary Forest. Conucos were plots of agricultural land. New conucos were valleys that had been cleared and burned within the last two years for agriculture (primarily beans, maize and root crops such as manioc) and were being actively farmed at the time of the hawk nest attempt. Old conucos were areas that had been cut in the past 3–10 years, but subsequently abandoned and were beginning to regenerate. They contained a mix of native plants such as pumpwood Cecropia schreberiana and agricultural species (mango Mangifera indica, banana Musa acuminata, cacao Theobroma cacao). Secondary Forest sites were areas that had been cut in the past 10–20 years but had been abandoned and allowed to regenerate. They contained a larger proportion of native tree species such as higuillo Piper aduncum, matchwood Schefflera morototoni, West Indian mahogany Swietenia mahagoni, kapok tree Ceiba pentandra, masa Tetragastris balsamifera, American muskwood Guarea guidonia, corcho bobo Pisonia albida, and fourleaf buchenavia Buchenavia tetraphylla. There were no areas of primary forest left on the western side of the National Park where this study was carried out. Patches of primary forest still exist in the centre and east of the park in areas that are relatively inaccessible.
We measured three indices to determine the type and level of disturbances within a breeding pair’s territory: natural predator abundance (Predator Abundance Index), general human activity as number of people in the territory without disturbance to the nest (Human Activity Index), and direct nest or territory disturbance (Disturbance Index). These indices were calculated for each nest by carrying out three 4-hour watches at each nest site and recording: the number of predators observed including active nests of other species within the nesting valley, and number of people observed passing through or working within the nesting valley. Disturbance was measured for each territory by estimating the level of direct disruption to a pair or nest site using information collected during the 4-hour nest watches and from local landowners. This index was divided into three categories. Minimal or no known disturbances to the breeding pair, Intermediate disturbance such as people digging for root crops near the nest tree, and Major direct disturbances to the pair or the nest site such as people using slingshots or throwing rocks at nests or poaching eggs or nestlings.
Statistical analysis
We used an ANOVA to compare laying date among years. A linear regression tested whether fledging rate was correlated to laying date. Mean fledging rate was calculated for each nesting territory over the study period and a linear regression was used to test whether mean fledging rate of a territory was related to the number of years it was active. Because any single year’s nest may fail due to random events (e.g. weather, isolated predation or disturbance), > 3 years of data minimised the influence of rare events and provided a more accurate representation of territory quality.
To determine predictors of nest success, nest site parameters of successful and failed nests were initially compared with univariate tests. We compared continuous variables that met assumptions of normality using t-tests. Variables that violated the assumptions were loge transformed prior to analysis or were analysed using Mann-Whitney U-tests. We used Pearson chi-square tests for categorical parameters. All parameters that had a univariate P-value < 0.25 were then entered into a multivariate model. For multivariate comparison of successful and failed nests, a logistic regression analysis was used to determine which combination of nest site parameters was most useful in predicting nest success.
For determining predictors of fledging rate, nest site parameters were initially analysed separately in a Linear Mixed Model (LMM). Parameters that had a P-value < 0.25 were then entered into a full factorial LMM to determine which combination of nest site parameters was most useful in predicting fledging rate. We carried out further GLIM (Poisson distribution) analyses to verify our LMM results. Values reported in the text are means ± SD. P values < 0.05 indicate a significant result, P values > 0.05 and < 0.10 indicate a trend. Data were analysed using the SPSS statistical package (SPSS 2009).
Results
Nesting biology and behaviour
Observations at nest sites began in January-February, at which time most pairs had already occupied nesting territories and were exhibiting signs of courtship. Construction of nests was observed from 8 January through to 17 July. Nest construction was already underway by several pairs in early January, so the earliest nest building dates were not known. However, with nest construction ranging from 15 to 44 days (see below), and earliest eggs being laid on 15 January, it can be estimated that nest construction may have commenced in some pairs as early as the beginning of December.
Both sexes participated in nest building. Males tended to do the majority of building during the initial stages prior to shaping of the nest cup (71% of 56 males).The female would then shape the nest cup. Duration of nest construction varied from 15 to 44 days and averaged 21.9 ± 11.8 days (n = 39). Depending on tree species, nests were supported by the bases of palm fronds or in the fork of a large branch. Ridgway’s Hawks used 20 tree species for nest sites, but clearly preferred the Hispaniola royal palm Roystonea hispaniolana (Table 1). Ridgway’s Hawk regularly used the large communal Palmchat nests as platforms upon which to nest, particularly when nesting in a royal palm (Woolaver Reference Woolaver2011).
Average laying date for a first clutch was 20 February ± 15 days (n = 44), and did not vary significantly among years (F 4,41 = 0.59, P = 0.62). Earliest egg dates were in January and the latest in mid-April, spanning 89 days. The majority of eggs were laid two days apart (n = 31), with eggs also being laid three (n = 8) and four (n = 2) days apart. Length of incubation ranged from 33 to 37 days with an average length of 34.9 ± 1.1 days (n = 34). Females performed the majority of the incubation, however males also incubated for significant amounts of time (range 5–250 minutes at a time, n = 21). Hatching was asynchronous and ranged from 1–3 days apart. For the majority of clutches, eggs hatched two days apart (n = 48) with eggs also hatching one (n = 8) and three days apart (n = 3).
Adult attendance in the nest was negatively correlated to nestling age (r 2 = 0.63, P < 0.001). However, even with older nestlings females were rarely seen to travel > 50 m from the nest, unless the male was in attendance at the nest. Similarly, the amount of time adults spent brooding decreased significantly as nestlings aged (r 2 = 0.79, P < 0.001). Males were not observed brooding, but they did attend the nest with nestling(s) for short periods of time ranging from two to 21 minutes at a time (n = 21).
Nestlings fledged as early as mid-April and as late as July. For 4–12 days prior to first flight, young perched on the nest edge and hopped or flapped to nearby palm fronds or branches. Nestlings took their first flights when 41–50 days old. Mean age at fledging was 47.1 ± 2.2 days (n = 42).
None of the pairs monitored raised more than one brood per year. Replacement clutches were not observed after a successful fledging of young, only after nest failures. The proportion of monitored pairs that laid replacement clutches after their first nest failed ranged from 26 to 45% annually, and averaged 35% (n = 84) over the study period. Ridgway’s Hawks laid replacement clutches after failures during both incubation and nestling stages. Although not common, four pairs were observed with replacement clutches even after nests had failed with older feathered nestlings. There were no recorded observations of third nesting attempts. Thirteen of 58 second nesting attempts (22%) were successful. Nesting trees used for second nest attempts averaged 222 ± 157 m from first nest attempts (range 20–562 m, n = 32).
Pairs held clear nesting territories within a valley, which were easily distinguishable due to the topography of the area. Only two adults were observed in a territory during breeding which verified a socially monogamous mating system (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013a). Annual territory re-occupancy rates were high over the study period at 94.5 ± 3.9% (Table 2).
Table 2. Territory re-occupancy rates of Ridgway’s Hawk Buteo ridgwayi in Los Haitises, Dominican Republic from 2005 to 2009.

Reproductive measures and nest outcome
Average clutch size over the five year period was 2.0 ± 0.4 (Table 3) with a modal clutch size of two (n = 63). Brood size varied from 1–3 and averaged 1.4 ± 0.7 (Table 3), with a modal brood size of two (n = 110).
Table 3. Reproductive rates of Ridgway’s Hawk Buteo ridgwayi breeding pairs monitored from 2005 to 2009 in Los Haitises, Dominican Republic.
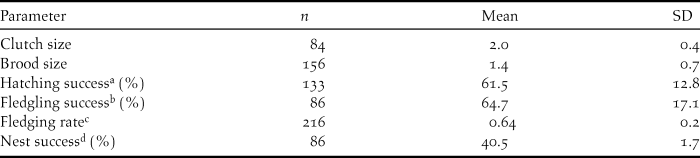
a Percentage of eggs to hatch successfully in a nest.
b Percentage of nestlings to successfully fledge from a nest.
c Number of fledglings produced per active nest.
d Proportion of active nests that fledged at least one young.
Of the 216 nests monitored, nest success was 61.5% for the incubation period, and 64.7 % for the nestling period. Overall nest success was relatively constant from year to year, averaging 40.5 % (Table 3).
Over the study period, number of fledglings per reproductively active nest (fledgling nest-1) was 0.64 ± 0.20 per pair (Table 3) but was unequally distributed among pairs. The 10 most productive nesting territories, representing 25% of the breeding population, produced 40% of the fledglings. Fledging rate was not correlated to laying date (r 2 = -0.03, P = 0.18) and was not correlated with number of years a nesting territory was active (r 2 = -0.004, P = 0.94). This was verified by field observations of birds returning to monitored nesting valleys, and individual nest trees, regardless of nest success the previous year.
Human disturbance, both confirmed and suspected, accounted for 43% and 18% of all known nest failures, respectively. Confirmed human disturbances included: nest trees burned or cut down (n = 3), poaching or intentional killing of eggs/nestlings (n = 9), and abandonment due to excessive human activity such as rocks and sticks thrown at the nest (n = 5). Suspected human disturbances included the disappearance from nests of older healthy nestlings during successive years at sites where landowners were openly known to persecute the hawks, or where people were suspected of poaching nestlings for food (n = 8).
Other causes of nesting failure included: infestation by botfly Philornis pici (n = 6), infertile eggs (n = 5), death of young nestlings from exposure during heavy rains and winds (n = 4), and nests falling to the ground due to a broken supporting palm frond (n = 4). Further causes of nest failure suspected by the authors but not confirmed were: predation by Red-tailed Hawk, Stygian Owl Asio stygius, and Hispaniolan boa Epicrates striatus.
Predictors of nest success and fledging rate
Two of the measured nest parameters, nest height and Disturbance Index, exhibited univariate P-values < 0.25 when comparing successful and failed nests (Table 4) and were therefore included in a final model. The final logistic regression model, controlling for year and area, found the Disturbance Index to be a significant predictor of nest success, with nest height as a potential predictor (disturbance: χ 2 2 = 5.57, P = 0.02, nest height: χ 2 1 = 3.41, P = 0.09). We found significantly less direct disturbance in territories with successful nests. Nests which were higher in nesting trees showed a trend to be more successful than nests lower in trees, with successful nest height averaging 14.0 ± 3.6 m (n = 66) and failed nest height averaging 12.1 ± 4.0 m (n = 107).
Table 4. Results of univariate tests for the effect of nest site parameter on nest success and fledging rate of Ridgway’s Hawk Buteo ridgwayi nests from 2005 to 2009 in Los Haitises, Dominican Republic. P-values in bold indicate variables included in multivariate models.

a Number of people observed within the nesting valley per 4 hour observation period.
b Number of predators observed within the nesting valley per 4 hour observation period.
c Level of direct disruption to a pair or nest site divided into three categories. Minimal disturbance, Intermediate disturbance (e.g. people digging for root crops near nest tree), and Major direct disturbance (e.g. people throwing rocks at nests or poaching nestlings).
Similarly, Disturbance Index (F = 2.62, P < 0.01) and nest height (F = 1.71, P = 0.03) were significant predictors of fledging rate (Table 4) within the final LMM model. GLIM (Poisson distribution) analyses supported these findings (data not presented). Nests with the least amount of direct nest site disturbance produced significantly more fledglings, as did nests higher up in the nesting tress. Of 44 nests with heights ≤ 10 m: 79% failed, 21% fledged one young, and 9% fledged two young. By comparison, of 15 nests with heights >16 m: 11% failed, 24% fledged one young, and 65% fledged two young (nest success: χ 2 1 = 6.72, P < 0.01, fledging rate: F 1,172= 9.09, P < 0.001).
Predator and Human Activity Indices were not predictors of nest success or fledging rate (Table 4) and did not affect nest outcome. Similarly, the presence or absence of a Palmchat nest and whether the Palmchat nest was active or inactive did not predict Ridgway’s Hawk nest success or fledging rate. This was also true for macrohabitat type and distance to nearest conspecific nest (Table 4).
Discussion
Ridgway’s Hawks are socially monogamous and territorial, and raise only one brood per year (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013a). Although females were the primary incubator, males were also observed incubating 5–250 minutes at a time (Woolaver Reference Woolaver2011). Similar behaviour has been noted in the White-throated Hawk Buteo albigula and tropical populations of Broad-winged Hawk Buteo platypterus (Trejo et al. Reference Trejo, Ojeda, Sympson and Gelain2004, Henstengberg and Vilella Reference Hengstenberg and Vilella2005), however incubation behaviour of males is not well documented in Neotropical raptors (Newton Reference Newton1979, Thorstrom and Quixchan Reference Thorstrom and Quixchan2000, Schulze et al. Reference Schulze, Cordova, Seavy and Whitacre2000). In the Red-shouldered Hawk Buteo lineatus, Ridgway’s Hawk’s closest taxonomic relative (Amaral et al. Reference Amaral, Sheldon, Gamauf, Haring, Riesing, Silveira and Wajntal2009), the female carries out the majority of the incubation (Dykstra et al. Reference Dykstra, Hays, Crocoll and Poole2008).
The mean clutch size in our study was slightly larger than described by Wiley and Wiley (Reference Wiley and Wiley1981), but similar to other Buteo species in the tropics (see Table 5; Newton Reference Newton2013). Avian clutch sizes are thought to be limited by food availability and/or predation pressures (Lack Reference Lack1947, Reference Lack1948, Skutch Reference Skutch1967). Food limitation was found to be a main factor limiting clutch size in Savanna Hawk Buteogallus meridonalis (Mader Reference Mader1982). Relatively low non-human predation pressures (this study), and an adequate food supply (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013b) may have potentially selected for 3-egg clutches in Ridgway’s Hawk. Brood size and length of the nestling period for Ridgway’s Hawk varied somewhat from the larger island Buteo species (Table 5), but was consistent with other similar-size tropical Buteo species.
Table 5. Reproductive biology of island Buteo species and southern population of Red-shouldered Hawk.

a Modal.
b Mean.
c Pairs generally attempted to breed 2 out of 3 years.
d Puerto Rican subspecies B. p. brunnescens.
e Puerto Rican subspecies B. j. jamaicensis.
f Subspecies B. l. extimus.
Nearly all nesting territories we monitored over consecutive years were re-occupied. This high re-occupancy rate suggests that these are traditionally used territories. The widespread habitat loss likely limits options for territory switching, and it is not unexpected that Ridgway’s Hawk would have a high re-occupancy rate. High re-occupancy rates were also documented in the endangered Puerto Rican Sharp-shinned Hawk Accipiter striatus venator (Delannoy and Cruz Reference Delannoy and Cruz1988) and the Puerto Rican Broad-winged Hawk Buteo platypterus brunnescens (Hengstenberg and Vilella Reference Hengstenberg and Vilella2005).
Nesting trees of Ridgway’s Hawk were predominantly Hispaniola royal palms, with much less frequent use of emergent native hardwoods. Neotropical raptors have been most commonly reported to nest in emergent native hardwoods (Santana et al. Reference Santana, Laboy, Mosher and Temple1986, Thorstrom and Quixchan Reference Thorstrom and Quixchan2000, Delannoy and Tossas Reference Delannoy and Tossas2002, Hengstenberg and Vilella Reference Hengstenberg and Vilella2005) and we are not aware of other published accounts of Neotropical raptors using palm trees for the majority of their nesting sites. On the neighbouring island of Puerto Rico, the Broad-winged Hawk inhabits forests with similar structure, including a mix of emergent hardwoods and a similar species of palm tree (Puerto Rico royal palm Roystonea borinquena), yet there are no published records of these hawks nesting in palms. The availability of native nesting trees for Broad-winged Hawks in Puerto Rico was reported to be high, with an average of 85 suitable trees per 4 ha (Delannoy and Tossas Reference Delannoy and Tossas2002). This is not the case for Ridgway’s Hawk where each nesting valley of 2–5 ha contained c.10–25 potentially suitable nesting trees and since annual cutting and burning of the forest has continued unabated this number has likely been reduced even further (L. Woolaver pers. obs.). We observed some pairs with nest attempts in both hardwoods and palm trees within a breeding season and the few historical records of Ridgway’s Hawk nests have been from both native hardwood (five records) and royal palm (one record), suggesting that the predominance of palm-tree nesting in the current study may not be a fixed trait but could be a recent phenomenon resulting from the reduction of native hardwoods in nesting valleys (Wetmore and Lincoln Reference Wetmore and Lincoln1934, Wiley and Wiley Reference Wiley and Wiley1981, Dod Reference Dod1992).
Nest success and fledging rate
Nest success found in this study (40%) was similar to the 34% reported by Thorstrom et al. (Reference Thorstrom, Almonte, Balbuena de la Rosa, Bildstein, Barber and Zimmerman2007) for Ridgway’s Hawk from 2002 to 2005. The Broad-winged Hawk and Sharp-shinned Hawk of Puerto Rico were found to have 50% and 29% nest success, respectively. Puerto Rican Sharp-shinned Hawks suffered not only from high nestling mortality, but also desertion of clutches (Delannoy and Cruz Reference Delannoy and Cruz1988), which was not seen to be a factor in Ridgway’s Hawk nest failures.
Ridgway’s Hawks produced only one brood per season, similar to other tropical Buteo species, with an annual fledging rate of 0.64 fledglings (Table 3). This is nearly identical to that reported by Wiley and Wiley (Reference Wiley and Wiley1981) (0.66) and Thorstrom et al. (Reference Thorstrom, Almonte, Balbuena de la Rosa, Bildstein, Barber and Zimmerman2007) (0.60) based on smaller sample sizes. Productivity in raptors has been attributed to prey availability (Newton Reference Newton1979) and predator abundance (Sergio et al. Reference Sergio, Marchesi and Pedrini2003). An index of prey density collected during a concurrent study (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013b) indicated that there was no shortage of food within the study area during the 5-year period, and no association between prey delivery rates and either nest success or fledging rate.
A recent molecular study has revealed that Ridgway’s Hawk has experienced a genetic bottleneck, and that inbreeding is occurring in the population (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013c), but that infertility of eggs (high rate of infertile eggs or embryonic death) appeared to be low at 9%. This is encouraging since small, island populations can be seriously compromised by conditions associated with bottlenecks and inbreeding, including low fertility (Frankham Reference Frankham1998, Swinnerton Reference Swinnerton2001) or embryo mortality (Glenn et al. Reference Glenn, Stephan and Braun1999). The small remaining population of Takahe Porphyrio hochstetteri, exhibits hatch success rates of 30%, due to inbreeding depression (Jamieson et al. Reference Jamieson, Roy and Lettink2001) whereas infertility rates in the Endangered Pink Pigeon Columba mayeri are greater than 50% (Swinnerton Reference Swinnerton2001).
Direct persecution to breeding pairs was the main cause of nest failure in this study. Thorstrom et al. (Reference Thorstrom, Almonte, Balbuena de la Rosa, Bildstein, Barber and Zimmerman2007) also concluded that most Ridgway’s Hawk nest failures were due to human activity, including slash-and-burn agriculture, deliberate cutting of nesting trees, poaching nestlings for food, and direct persecution of hawks at nest sites. The misconception that Ridgway’s Hawks are poultry predators has led to their unfortunate persecution and is most likely a substantial factor (along with habitat loss) for the species’ rapid population decline throughout Hispaniola. This misconception is fuelled by confusion with the larger, sympatric Red-tailed Hawk, which does prey on chickens. Human disturbance at nest sites has been documented to negatively impact breeding behaviour resulting in breeding failures in a number of raptor species (Arroyo and Razin Reference Arroyo and Razin2006, González et al. Reference González, Arroyo, Margalida, Sánchez and Oria2006, Zuberogoitia et al. Reference Zuberogoitia, Zabala, Martínez, Martínez and Azkona2008, Margalida et al. Reference Margalida, Moreno-Opo, Arroyo and Arredondo2011).
Fortunately, nest success and fledging rate were not affected by lower levels of general human activity within the territory (e.g. people walking through a territory or working in the valley). Most nests were in close proximity to human activity due to an extensive trail system throughout Los Haitises National Park and most valleys within Los Haitises were used to some extent for root crop cultivation. Ridgway’s Hawks tolerated fairly extensive human activity within their territory and successfully fledged young while utilising small fragments of forest within human-altered habitat, as long as they were not directly persecuted. Wiley (Reference Wiley1986) noted that Ridgway’s Hawk had been historically recorded in varying degrees of human-altered habitat types. Thorstrom et al. (Reference Thorstrom, Almonte, Balbuena de la Rosa, Bildstein, Barber and Zimmerman2007) also recorded pairs occupying pasturelands, coconut plantations, and forest edge habitats. This adaptability to human-modified habitats has likely been a contributing factor to their continued survival in the Dominican Republic. Ridgway’s Hawk is closely related to Red-shouldered and Broad-winged Hawks (Amaral et al. Reference Amaral, Sheldon, Gamauf, Haring, Riesing, Silveira and Wajntal2009) both of which are woodland Buteo species adaptable to a range of human-altered habitats (del Hoyo et al. Reference del Hoyo, Eliott and Sargatal1994, Dykstra et al. Reference Dykstra, Hays, Daniel and Simon2000, Reference Dykstra, Hays, Daniel and Simon2001). Fortunately, Ridgway’s Hawks have persisted within the final sanctuary of Los Haitises, despite increased human activity and habitat modification.
Conservation implications and recommendations
Nest success and fledging rate of the remaining population of Ridgway’s Hawk were comparable to those of other similar-sized Neotropical raptors. The lack of evidence for compromised productivity (such as low clutch size and number fledged, low hatching success) suggests that reproductive parameters were likely not a significant factor in the species’ decline. Natural nest failure from predation, parasites, and weather was also not a major limiting factor for the species, although caution is required to ensure that such factors do not become a problem if the population continues to decline to lower levels. Most nest failures were a result of direct human disturbance to the nest or persecution of nesting adults or nestlings and, along with habitat loss, represent the most serious threats to the species survival.
The survival of Ridgway’s Hawk in its natural environment depends on the effective management and protection of Los Haitises National Park. Los Haitises National Park is a “paper park” with no clear boundaries or effective protection (Geisler et al. Reference Geisler, Warne and Barton1997). It has experienced massive, uncontrolled immigration since the 1980s. The current situation has been complicated by the repeated expulsion and re-colonisation of the Park, with the addition of commercialisation of root-crop cultivation (Marizán Reference Marizán1994). Two initial actions are recommended: firstly, an awareness campaign should target the National Parks and government of the Dominican Republic regarding the significance of Ridgway’s Hawk as a Hispaniolan endemic on the brink of extinction. Secondly, a national workshop should be held that includes all stakeholders in the use and conservation of Los Haitises National Park, including local communities, government, conservation and development NGOs, and tourism operators. Conservation planning should also focus on modifying the traditional practice of burning after clearing an area for agriculture. Ridgway’s Hawks and local farmers can co-exist. As long as native rainforest remains on the mogotes, there will be sufficient prey to support hawks (Woolaver et al. Reference Woolaver, Nichols, Morton and Stutchbury2013b) and farmers can continue to produce crops in the fertile valleys below. However, the traditional practice of burning after cutting has a devastating effect on the remaining patches of forest. Even if it is too difficult on a political scale to reduce the level of agricultural use within the Park, eliminating burning as a traditional practice at the individual farm level (within the park) would allow remaining forest patches on the mogotes to continue providing habitat for Ridgway’s Hawk and other endemics.
Acknowledgements
We thank N. Collar, J. Wiley, R. Thorstrom, S. Latta, E. Fernández, and D. Wege for their advice during our study. Many people contributed significantly to this research by searching for hawks and collecting field data, including J. Almonte, J. Cespedes, N. Corona, S. Balbuena de la Rosa, T. Bueno Hernandez, P. de Leon Franco, T. Nichols, J. Sinclair, H. Jorge Polanco, and J. Vetter. J. Brocca, E. Fernández, and the Sociedad Ornitólogica Hispaniola provided much appreciated logistical support. All research was carried out with the permission of the Subsecretaría de Estado de Áreas Protegidas y Biodiversidad, and the Secretaría de Estado de Medio Ambiente y Recursos Naturales, Republica Dominicana. Our research was funded by Wildlife Preservation Canada, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the James Bond Fund of the Smithsonian Institution. Previous versions of this manuscript have been improved based on comments by J. Wiley, A. Margalida, and an anonymous reviewer. We particularly thank E. Williams and K. Wallace for their support throughout the study.








