The association of fish and fish oil with cardiac health is linked closely to their contents of n-3 long-chain (LC) PUFA, especially EPA (20 : 5n-3) and DHA (22 : 6n-3)( Reference Harris 1 ). In wild fish, EPA and DHA are obtained from the diet comprising of aquatic plants and algae or other fish( Reference Turchini, Nichols and Barrow 2 ). In farmed fish, EPA and DHA have been supplied by inclusion of fishmeal and fish oil in feeds. Because of the finite supply of fish oil that limits availability and drives up the price, vegetable oils containing n-3 PUFA have been used increasingly to replace fish oil in aquaculture feeds. However, the n-3 PUFA content of vegetable oils is due to the shorter-chain α-linolenic acid (ALA, 18 : 3n-3), and therefore an important commercial issue is the ability of aquaculture species to metabolise ALA to EPA and, in particular, to DHA.
In vertebrates the metabolism of ALA to DHA requires three desaturation and three elongation steps that in many species can be performed by fatty acyl desaturase (FADS) enzymes, Δ6 and Δ5 desaturase, and elongation of very long-chain fatty acid (ELOVL) enzymes, ELOVL5 and ELOVL2. The metabolism of ALA to n-3 LC-PUFA has been studied in several fish species, including rainbow trout (Oncorhynchus mykiss). However, most studies of ALA desaturation and elongation have been conducted on a background of dietary EPA and DHA, which is an inevitable consequence of using fishmeal as a protein source in aquaculture feed( Reference Chen, Nguyen and Semmens 3 – Reference Masiha, Mahboobi Soofiani and Ebrahimi 6 ). There was increased conversion of 14C-labelled ALA and EPA to 14C-DHA in hepatocytes isolated from rainbow trout fed vegetable oil compared with those fed fish oil( Reference Buzzi, Henderson and Sargent 7 ). This suggested that dietary EPA and DHA may be repressing the expression of at least some of the DHA biosynthetic genes, and therefore studies that include dietary EPA and DHA may not allow measurement of the full potential of dietary ALA to act as a source of endogenously produced EPA and DHA.
The recent identification and functional characterisation of ELOVL2( Reference Gregory and James 8 ) and Δ5 desaturase( Reference Abdul Hamid, Carmona-Antoñanzas and Monroig 9 ) in rainbow trout, together with the previously characterised ELOVL5( Reference Meyer, Kirsch and Domergue 10 ) and Δ6 desaturase( Reference Zheng, Seiliez and Hastings 11 ), means that the fatty acid substrate specificities of the four enzymes required for ALA to DHA conversion are well characterised. However, the regulation of these genes in response to dietary n-3 fatty acids has not been fully characterised. We examined the effect of dietary ALA, EPA or DHA, in isolation and in combination, on the regulation of expression levels of the four LC-PUFA biosynthetic genes, FADS2a(Δ6), FADS2b(Δ5), ELOVL5 and ELOVL2, in rainbow trout.
Methods
Animals and housing
A total of 800 mixed-sex diploid rainbow trout fingerlings (approximately 2·5 g) were sourced from the Victorian Department of Primary Industries Hatchery, transferred to Deakin University’s Aquaculture Research Facility, and acclimatised to the new environmental conditions in two 1000 litre tanks for 2 weeks. The fish were fed a commercial diet (Nova SS, 2 mm; Skretting) for 3 d before being fed a low n-3 PUFA reference diet for 2 weeks to reduce baseline tissue EPA and DHA contents. The experiment was conducted in a thermostatically controlled, closed-loop recirculating freshwater aquaculture system, with physical and biological filtration and UV sterilisation. The system was maintained within optimum temperature and water-quality levels, with a 12 h light–12 h dark cycle at 13°C±1°C. All procedures involving fish handling and experimentation conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Deakin University Animal Ethics Committee (project ID G37-2014).
Diets and experimental design
Eight iso-proteic and iso-lipidic experimental diets were formulated and manufactured to contain 42 % protein and 20 % lipid. The added dietary oil source was the only variable (Table 1). The oil sources were added at 174 g/kg and included linseed oil (Melrose Laboratories) as a source of ALA, Incromega EPA 500TG oil (Croda International Plc) as a source of EPA and Incromega DHA 500TG oil (Croda International Plc) as a source of DHA. The low n-3 PUFA reference diet contained minimal ALA, EPA and DHA, whereas the other seven diets were formulated to contain high ALA, EPA or DHA in isolation or in combination (ALA+EPA, ALA+DHA, ALA+EPA+DHA or EPA+DHA). Sunflower oil (Nuseed Global) and ARASCO (DSM Nutritional Products) were used to balance the linoleic acid (18 : 2n-6) and arachidonic acid (20 : 4n-6) contents of each of the diets.
Table 1 Formulation and proximate composition (g/kg dry weight) of the experimental diets

ALA, α-linolenic acid (18 : 3n-3); EPA (20 : 5n-3); DHA (22 : 6n-3).
* Ridley Agriproducts.
† Agri Food Ingredients.
‡ The Midfield Group.
§ Bulk Nutrients Grove.
|| DSM Nutritional Products.
¶ Merck KGaA.
** DSM Nutritional Products.
†† Nuseed Global.
‡‡ Cargill Australia.
§§ Melrose Laboratories.
|||| Croda International Plc.
The experimental diets were produced using a meat mincer (Model TJ22-B; Brice Australia) fitted with a 3-mm stainless steel extrusion plate. The feed was dried to 2–3 % moisture in a dark, temperature-controlled fan-forced room at 40°C for 48 h. It was ground and mechanically sieved through a nest of 2, 1 and 0·5-mm screens. The experimental diets used for the duration of the trial were a composite collected from the 2 and 1-mm screens. Diets were stored at approximately 10°C until fed.
The trial consisted of 720 rainbow trout that were weighed and allocated to twenty-four experimental tanks (140 litre tanks). The fish had a mean initial weight of 3·6 (se 0·1) g and were stocked at 30 fish/tank. The fish were assigned one of eight experimental diets (3 tanks/treatment) and were fed to apparent satiety twice daily, at 08.30 hours and 17.00 hours, for a period of 3 weeks. Total feed intake was recorded at the end of the trial and mortalities were recorded throughout the trial.
Sampling and chemical analysis
At the end of the 3-week trial, the fish were euthanised using 30-parts per million AQUI-S (AQUI-S New Zealand Ltd). All fish were weighed and 12 fish per tank were randomly sampled for analysis. The whole liver and a portion of muscle were removed from 6 fish/tank for fatty acid analysis, and samples were stored on ice during the sampling of each tank and then at −20°C. A portion of liver and muscle were removed from the remaining 6 fish/tank for gene expression analysis. These samples were placed immediately into RNAlater, stored on ice during the sampling of each tank, followed by storage at 4°C overnight before being transferred to −80°C.
The proximate compositions of experimental diets were determined according to standard procedures( 12 ). Briefly, protein (N×6·25) was measured using an automated Kjeltec 2300 (Foss Tecator), moisture by drying samples in an oven at 105°C to a constant weight and ash by incinerating samples in a muffle furnace (Wit; C & L Tetlow) at 550°C for 18 h. Lipid content was determined using chloroform–isopropanol (2:1, v/v) extraction basically according to the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 13 ), and 0·005 % (w/v) butylated hydroxyanisole was added to reduce lipid oxidation during processing.
Rainbow trout liver fatty acyl desaturase 2a(Δ6), fatty acyl desaturase 2b(Δ5), elongation of very long-chain fatty acid 2 and elongation of very long-chain fatty acid 5 gene expression
A TissueLyser (Qiagen) was used to disrupt and homogenise approximately 15 mg of RNAlater-stabilised liver tissue. Total RNA was extracted with the RNeasy kit (Qiagen) and the quantity and quality of RNA were determined by measuring absorbance at 260 and 280 nm (NanoDrop Technologies Inc.). Quantitative RT-PCR (qRT-PCR) was performed using an Applied Biosystems QuantStudio 7 Flex Real-Time PCR System with the Superscript ІІІ Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen Australia Pty Ltd). Each 10 µl reaction contained 10 ng of complementary DNA (cDNA), 5 µl 2× SYBR Green reaction mix, 0·2 µl SuperScript III RT/Platinum Taq Mix and 200 nm of each primer (Table 2). The cycling conditions were as follows: at 50°C for 3 min, at 95°C for 5 min, 40 cycles of 95°C for 15 s and 60°C for 30 s, followed by 40°C for 1 min and a melt curve analysis of 1°C increments from 60 to 95°C. Agarose gel electrophoresis of the products and melt curve analysis were used to ensure that a single specific product of expected size was obtained. Amplifications were carried out with negative controls (no cDNA template control). Gene expression data are expressed as mean values with their standard errors (n 3) normalised for the geometric mean of the expression levels of two housekeeping genes, β-ACTIN and elongation factor 1α, in relative units.
Table 2 Primers used for quantitative RT-PCR amplification of fatty acyl desaturase (FADS) 2a(Δ6), FADS2b(Δ5), elongation of very long-chain fatty acid (ELOVL) 5, ELOVL2, β-ACTIN and elongation factor 1α (EF1α), including the GenBank accession number of the sequence used for primer design
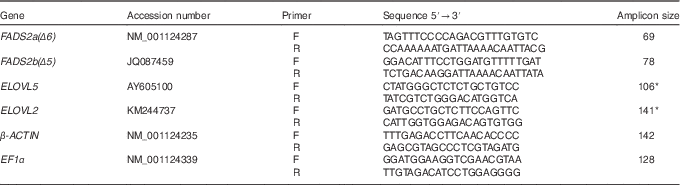
* Primers previously published( Reference Gregory and James 8 ).
Fatty acid analysis
Total lipid was extracted from feeds and trout tissues using chloroform–isopropanol (2:1, v/v) basically according to the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 13 ). Lipid weight was determined after drying the extracted lipid under N2. Phospholipid fatty acids of liver and muscle tissues were separated by TLC using a mobile phase of petroleum ether–acetone (3:1, v/v). Total lipid from feeds or phospholipid fatty acids from liver and muscle tissue were methylated in 1 % (v/v) sulphuric acid in methanol for 3 h at 70°C to prepare fatty acid methyl esters that were extracted in heptane and analysed by GC as described previously( Reference Gregory, See and Gibson 14 ). The identity of each fatty acid peak in the chromatogram was ascertained by comparing its retention time to authentic lipid standards (Nu-Chek Prep). Fatty acid methyl esters were quantified using GC Chemstation software (Agilent Technologies). All solvents contained 0·005 % (w/v) butylated hydroxyanisole as an antioxidant. The amount of each fatty acid was expressed as a percentage of the total amount of all fatty acids.
Statistical analysis
The expression of LC-PUFA biosynthetic genes in fish consuming the experimental diets compared with the low n-3 PUFA reference diet was analysed by one-way ANOVA with Dunnett’s post hoc test. The liver and muscle fatty acid data were analysed by one-way ANOVA with Tukey’s post hoc test. All analyses were carried out using Graphpad Prism version 5.03 for Windows (Graphpad Software). Statistical significance was set at P<0·05.
Results
Experimental diets
The low n-3 PUFA diet contained minimal ALA, EPA and DHA with the total n-3 PUFA being 1·0 % of total fatty acids (Table 3). The high-ALA diet contained 35·7 % ALA, the high-EPA diet contained 29·0 % EPA, and the high-DHA diet contained 33·0 % DHA (Table 3). The experimental diets with a combination of two n-3 PUFA (ALA+EPA, ALA+DHA or EPA+DHA) contained equal amounts of both n-3 PUFA, which were approximately half of the amount in the high-ALA-, -EPA- or -DHA-only diets (Table 3). Likewise, the ALA+EPA+DHA diet contained equal amounts of ALA, EPA and DHA, which were each one third of the amount in the high-ALA-, -EPA- or -DHA-only diets (Table 3). The total n-6 PUFA concentrations were 17·0–21·5 % of total fatty acids across all diets (Table 3). The total lipid content was similar across all diets, with an average of 20·8 % (Table 1).
Table 3 Fatty acid composition (percentage of total fatty acids) of the experimental diets

ALA, α-linolenic acid (18 : 3n-3); EPA, 20 : 5n-3; DHA, 22 : 6n-3; SDA, stearidonic acid (18 : 4n-3); DPA, docosapentaenoic acid (22 : 5n-3); LC-PUFA, long-chain PUFA.
* Total n-3 LC-PUFA is the sum of 20 : 3n-3, 20 : 4n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
Growth performance
The final body weights of rainbow trout were not statistically different between any of the dietary treatments (Table 4). Similarly, no significant differences were observed for weight gain, dry feed intake, feed conversion ratio, specific growth rate and survival between any of the dietary treatments (Table 4).
Table 4 Growth performance and feed efficiency of rainbow trout fed the experimental diets for 3 weeksFootnote * (Mean values with their standard errors; n 3)

ALA, α-linolenic acid (18 : 3n-3); EPA, 20 : 5n-3; DHA, 22 : 6n-3; IBW, initial body weight; FBW, final body weight; WG, wet weight gain.
* There were no significant differences between dietary treatments in any parameter.
† WG (%), percentage weight gain=100×((FBW−IBW)/IBW).
‡ FCR, feed conversion ratio=dry feed intake (g)/WG (g).
§ SGR (% gain/d), specific growth rate=100×(ln FBW−ln IBW)/21 d.
|| Survival (%)=100×(number of fish remaining on day 21/initial number of fish).
The effect of dietary n-3 PUFA on liver long-chain PUFA biosynthetic gene expression
The effect of dietary ALA, EPA or DHA on the regulation of expression levels of the four LC-PUFA biosynthetic genes was compared with the low n-3 PUFA diet. Dietary ALA reduced the expression of FADS2a(Δ6) and FADS2b(Δ5), although the reduced level of FADS2a(Δ6) was not statistically significant (Fig. 1(a) and (b)). Dietary EPA significantly reduced the expression of ELOVL2 only (Fig. 1(d)). Dietary DHA reduced the expression of all four genes, with FADS2a(Δ6) and FADS2b(Δ5) down-regulated by 4·2-fold each, but the reduced ELOVL5 expression did not reach statistical significance (Fig. 1).

Fig. 1 Rainbow trout liver fatty acyl desaturase (FADS)2a(Δ6) (a), FADS2b(Δ5) (b), elongation of very long-chain fatty acid (ELOVL)5 (c) and ELOVL2 (d) gene expression after dietary intervention. Values are means (n 3), with standard errors represented by vertical bars normalised for the geometric mean of the expression levels of two housekeeping genes, β-ACTIN and elongation factor 1α (EF1α), in relative units (RU). The mean value was significantly different from that of the low n-3 PUFA diet: ** P<0·01, *** P<0·001. ALA, α-linolenic acid (18 : 3n-3); EPA (20 : 5n-3); DHA (22 : 6n-3).
The effect of combinations of dietary ALA, EPA or DHA on FADS2a(Δ6), FADS2b(Δ5), ELOVL5 and ELOVL2 expression was compared with the effect of the low n-3 PUFA diet. Dietary EPA+DHA significantly reduced the expression of FADS2a(Δ6), FADS2b(Δ5) and ELOVL2, although the 13·7-fold down-regulation of FADS2b(Δ5) was greater than the 4·8- and 4·5-fold down-regulation of FADS2a(Δ6) and ELOVL2, respectively (Fig. 1). Dietary ALA+EPA+DHA or ALA+DHA also significantly reduced the expression of FADS2a(Δ6), FADS2b(Δ5) and ELOVL2 (Fig. 1). Dietary ALA, EPA or DHA, in isolation or in combination, did not significantly change the expression of ELOVL5 compared with the low n-3 PUFA diet (Fig. 1(c)).
The effect of dietary n-3 PUFA on the liver phospholipid n-3 PUFA composition
The proportion of stearidonic acid (SDA, 18 : 4n-3) in the liver increased with dietary ALA (Table 5). The proportion of EPA in the liver increased with dietary ALA and EPA, with the latter resulting in larger increases (Table 5). EPA levels in the liver were significantly higher with dietary ALA alone compared with those with dietary ALA+DHA (Table 5). Liver docosapentaenoic acid (DPA, 22 : 5n-3) increased with dietary ALA or EPA, in isolation or in combination with each other or with DHA, with the exception of dietary ALA+DHA in which DPA levels remained as low as with dietary DHA alone (Table 5). Liver DHA in fish consuming the low n-3 PUFA diet was significantly lower than in fish on any other diet. The proportion of DHA in the liver was increased by 3- or 3·7-fold when fish were consuming dietary ALA or EPA, respectively, compared with fish consuming the low n-3 PUFA diet (Table 5). Fish consuming dietary DHA had significantly higher liver DHA levels compared with that in fish on any other diet, with the exception of fish consuming dietary EPA+DHA.
Table 5 Lipid content (percentage of wet weight) and fatty acid composition (percentage of total fatty acids) of liver phospholipids after dietary intervention (Mean values with their standard errors; n 3)

ALA, α-linolenic acid (18 : 3n-3); EPA, 20 : 5n-3; DHA, 22 : 6n-3; SDA, stearidonic acid (18 : 4n-3); DPA, docosapentaenoic acid (22 : 5n-3); LC-PUFA, long-chain PUFA.
a,b,c,d,e,f,g Mean values within a row with unlike superscript letters were significantly different (P<0·05) as determined by ANOVA.
* Total n-3 LC-PUFA is the sum of 20 : 3n-3, 20 : 4n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
The effect of dietary n-3 PUFA on the muscle phospholipid n-3 PUFA composition
The proportion of SDA in muscle increased with dietary ALA (Table 6). The proportion of EPA in muscle increased with dietary ALA and EPA, with the latter resulting in larger increases, similar to the pattern observed in the liver (Table 6). The percentage of DPA in the muscle increased to a greater extent with dietary EPA alone compared with dietary EPA and DHA (ALA+EPA+DHA and EPA+DHA diets) (Table 6). Muscle DHA in fish consuming the low n-3 PUFA diet was significantly lower than that in fish on any other diet (Table 6). In these fish, the proportion of DHA in muscle was 21·7 % of total fatty acids (Table 6), which was higher than the proportion of DHA in the liver, which was 10·8 % of total fatty acids (Table 5). The proportion of DHA in muscle was increased by 1·2- or 1·6-fold when fish were consuming dietary ALA or EPA, respectively, compared with the low n-3 PUFA diet (Table 6).
Table 6 Lipid content (percentage of wet weight) and fatty acid composition (percentage of total fatty acids) of muscle phospholipids after dietary intervention (Mean values with their standard errors; n 3)

ALA, α-linolenic acid (18 : 3n-3); EPA, 20 : 5n-3; DHA, 22 : 6n-3; SDA, stearidonic acid (18 : 4n-3); DPA, docosapentaenoic acid (22 : 5n-3); LC-PUFA, long-chain PUFA.
a,b,c,d,e,f Mean values within a row with unlike superscript letters were significantly different (P<0·05) as determined by ANOVA.
* Total n-3 LC-PUFA is the sum of 20 : 3n-3, 20 : 4n-3, 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
Discussion
This study is unique in that all of the four LC-PUFA biosynthetic genes were examined and the effects of individual n-3 PUFA concentrations in the diet were assessed. There have been studies that have directly or indirectly examined the effect of fish oil on some of the elongase and/or desaturase enzymes, and in general these were fish oil–vegetable oil comparisons( Reference Cleveland, Francis and Turchini 4 – Reference Masiha, Mahboobi Soofiani and Ebrahimi 6 , Reference Panserat, Hortopan and Plagnes-Juan 15 – Reference Turchini and Francis 17 ). In addition, the vegetable oil diets containing ALA had a background of EPA and DHA from the inclusion of 7–58 % fishmeal in the dietary formulations, which made examining the effect of ALA on the LC-PUFA biosynthetic genes challenging( Reference Cleveland, Francis and Turchini 4 – Reference Masiha, Mahboobi Soofiani and Ebrahimi 6 , Reference Thanuthong, Francis and Manickam 16 , Reference Turchini and Francis 17 ). Dietary fishmeal was reduced to 5·8 % in the present study, resulting in experimental diets with minimal EPA and DHA, both 0·3 % of total fatty acids.
The present study found that the highest expressions of all four LC-PUFA biosynthetic genes were in fish fed low dietary n-3 PUFA. The expressions of FADS2a(Δ6), FADS2b(Δ5) and ELOVL2 were variously repressed by the individual n-3 PUFA, none of which repressed the expression of ELOVL5. Of the fatty acids tested, dietary DHA had the largest and most consistent effect in down-regulating all four genes, including reduced expression of ELOVL5, although it was not statistically significant. This is a possible explanation for some of the effects of dietary ALA on the liver content of some of the downstream products of its metabolism. For example, the proportion of SDA resulting from dietary ALA was decreased by the addition of DHA to the diet (1·1 v. 0·3 % of total fatty acids). This could be due to the observed DHA repression of FADS2a(Δ6), which codes for Δ6 desaturase, the enzyme responsible for SDA synthesis. Likewise, the proportion of DPA in fish fed dietary ALA was decreased by the addition of DHA to the diet (1·8 v. 0·5 % of total fatty acids). This could be due to the observed DHA repression of ELOVL2, and subsequently the ELOVL2 enzyme responsible for DPA synthesis from EPA.
Irrespective of diet, fish fed diets supplemented with n-3 PUFA showed higher levels of DHA in the liver compared with fish fed the low n-3 PUFA diet. For example, fish fed dietary ALA showed a 3-fold increase in liver DHA compared with fish fed the low n-3 PUFA diet, despite dietary DHA being identical (0·3 % of total fatty acids). Similar changes were seen in muscle DHA. Clearly, these juvenile fish exhibited an active LC-PUFA biosynthetic pathway that had an impact on the phospholipid fatty acid composition of the liver and muscle tissue. It is possible that adult fish may be less responsive but the use of juveniles allowed greater experimental manipulation.
Endogenously synthesised DHA appeared to have a different effect from dietary DHA on FADS2a(Δ6) and FADS2b(Δ5) gene expression. For example, dietary EPA resulted in 40·4 % DHA in the liver with no change in expression of FADS2a(Δ6) or FADS2b(Δ5) compared with the low n-3 PUFA diet of 10·8 % DHA. However, dietary EPA+DHA down-regulated the expression of FADS2a(Δ6) and FADS2b(Δ5) by 4·8- and 13·7-fold, respectively, but resulted in the same amount of liver DHA (42·1 %) as that in the dietary EPA group. This is a very important and novel observation that warrants further tailored investigations. Our findings on reduced FADS2a(Δ6) expression in fish fed dietary EPA+DHA are in agreement with a previous report in rainbow trout that found that FADS2a(Δ6) expression was 4·1-fold lower in fish fed a fish oil diet compared with a vegetable oil diet devoid of n-3 LC-PUFA( Reference Panserat, Hortopan and Plagnes-Juan 15 ). Similarly, in Atlantic salmon, dietary EPA+DHA significantly down-regulated the expression of Δ5fad, Δ6fad and elovl2 compared with a vegetable oil diet low in n-3 LC-PUFA; yet dietary EPA alone had no effect( Reference Thomassen, Rein and Berge 18 ).
Previously, liver ELOVL5 expression was shown to be unchanged between rainbow trout fed a fish oil diet and those fed increasing dietary ALA (8·2–32·4 % of total fatty acids)( Reference Thanuthong, Francis and Manickam 16 ). We have now found that neither dietary ALA, EPA nor DHA in isolation or in combination has a significant effect on the expression of ELOVL5 compared with a diet low in n-3 PUFA. Of particular interest is the differing effect of dietary EPA on significantly down-regulating the expression of ELOVL2, but not ELOVL5. Previous functional characterisation of the ELOVL2 and ELOVL5 enzymes demonstrated that their substrate specificities towards EPA were similar at the concentrations examined, but ELOVL2 could further elongate EPA to DPA and then to 24 : 5n-3( Reference Gregory and James 8 ). Therefore, the down-regulation of ELOVL2 by dietary EPA may have a downstream effect on the synthesis of DPA and subsequently DHA, which ELOVL5 cannot compensate for because of the lack of activity towards DPA( Reference Gregory and James 8 ).
Atlantic salmon is closely related to rainbow trout and has Δ6 desaturase( Reference Zheng, Tocher and Dickson 19 , Reference Monroig, Zheng and Morais 20 ), Δ5 desaturase( Reference Hastings, Agaba and Tocher 21 ), Elovl5a( Reference Hastings, Agaba and Tocher 21 , Reference Morais, Monroig and Zheng 22 ), Elovl5b( Reference Morais, Monroig and Zheng 22 ) and Elovl2( Reference Morais, Monroig and Zheng 22 ) enzymes that have been functionally characterised and shown to be responsible for the metabolism of ALA to DHA. The expression of the Atlantic salmon Δ6fad_a, Δ6fad_b, Δ6fad_c, Δ5fad, elovl5a, elovl5b and elovl2 liver transcripts has been examined in response to dietary fish oil replacement with vegetable oil diets low in n-3 LC-PUFA and high in C18 PUFA. Collectively these studies showed that Δ6fad_a, Δ5fad and elovl2 expression was up-regulated in vegetable oil-fed Atlantic salmon compared with fish oil-fed salmon( Reference Zheng, Tocher and Dickson 19 , Reference Monroig, Zheng and Morais 20 , Reference Morais, Monroig and Zheng 22 – Reference Zheng, Torstensen and Tocher 27 ), as we have seen in rainbow trout. However, in many of these studies the expression of the Atlantic salmon elovl5a and elovl5b genes was inconsistent with reports of down-regulation of elovl5a ( Reference Xue, Hixson and Hori 25 ), up-regulation of elovl5b ( Reference Morais, Monroig and Zheng 22 ) or no effect on elovl5a or elovl5b ( Reference Morais, Monroig and Zheng 22 , Reference Morais, Pratoomyot and Taggart 24 , Reference Xue, Hixson and Hori 25 ) in response to dietary vegetable oil compared with fish fed fish oil. The Atlantic salmon elovl5a and elovl5b may be regulated differently by dietary n-3 PUFA or by conditions such as water temperature and fish size, although this is not fully understood. In comparison, juvenile rainbow trout ELOVL5 expression does not seem to be changed significantly in response to different dietary n-3 PUFA when reared at water temperatures between 13 and 15°C( Reference Thanuthong, Francis and Manickam 16 ).
The present gene regulatory study has shown that FADS2a(Δ6), ELOVL5 and ELOVL2 are most highly expressed when diets are high in ALA with no added EPA or DHA. Conversely, the expression of all four genes was down-regulated by dietary EPA and DHA, with DHA having the larger and more consistent effect, although ELOVL5 expression was the least responsive to dietary n-3 PUFA changes. These findings should be considered when optimising diets containing vegetable oils and/or fish oil or fishmeal to achieve maximum DHA synthesis. A study using larger fish and a longer feeding period is warranted to determine the impact on the percentage of DHA in the edible muscle portion of rainbow trout.
Acknowledgements
The authors are grateful to Cindy Hall (Rheumatology Unit, Royal Adelaide Hospital, Australia), Karen Hermon, Fernando Norambuena, James Emery, Michael Lewis and Amber Chen (School of Life and Environmental Sciences, Deakin University, Australia) for their assistance during the tissue collection. The authors also thank Karen Hermon for her assistance with the proximate analysis.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. K. G., D. R. T, M. J. J. and G. M. T. designed the research; M. K. G., R. O. C. and G. M. T. conducted the research; G. M. T. and M. J. J. provided the essential materials; M. K. G. and M. J. J. analysed the data; and M. K. G., D. R. T. and M. J. J. wrote the paper. All authors read and approved the final version of the manuscript.
The authors have no conflicts of interest to declare.










