It is known that the risk of hyperkalaemia is increased with the progression of chronic kidney disease (CKD)(Reference Drawz, Babineau and Rahman1). Of 3000–4000 mEq of total body potassium in a 70-kg person, only small amount (∼ 2 %) is found in the extracellular space, and 98 % ∼ of exchangeable potassium is in intracellular space. Serum potassium levels are maintained within a narrow range by the inter-play of insulin, catecholamine, acid-base status and renal handling(Reference Palmer and Clegg2). Hyperkalaemia causes the depolarisation of membrane potential but increases the potassium channel conductance, resulting in tall T waves, P wave loss and QRS wave widening(Reference Weiss, Qu and Shivkumar3). Hence, several clinicians are familiar with reducing the potassium intake in patients with CKD. In the non-CKD population, however, a high dietary potassium intake has been suggested to have beneficial effects. As the dietary potassium increased, systolic and diastolic blood pressure decreased(Reference Binia, Jaeger and Hu4). A decreased potassium intake was associated with an increased all-cause mortality(Reference O’Donnell, Mente and Rangarajan5). Although controversial(Reference Leonberg-Yoo, Tighiouart and Levey6,Reference He, Mills and Appel7) , a decreased potassium intake in the CKD population was also associated with an increased risk of CKD progression(Reference Kim, Park and Yoo8).
CKD patients are at a high risk for developing cardiovascular (CV) events(Reference Kiuchi and Mion9,Reference Ahmed and Al-Attab10,Reference Cha, Kang and Park11) . Therefore, the early detection of CV risk factors is of utmost importance to improve the clinical outcomes. A decreased potassium intake was associated with an increased risk of CV events in the general population(Reference O’Donnell, Mente and Rangarajan5) and increased potassium intake was associated with a decreased risk of CV events and CKD progression in type 2 diabetes patients(Reference Araki, Haneda and Koya12). However, the potential effect of dietary potassium on CV risk has rarely been studied in the CKD population till recently. As a novel CV risk, high-sensitivity troponin T (hs-TnT), the first highly sensitive assay for cardiac troponins, has been associated with current and future CV events(Reference Twerenbold, Jaffe and Reichlin13,Reference Thygesen, Alpert and White14) , even if only slightly elevated(Reference Everett, Brooks and Vlachos15,Reference Ndrepepa, Colleran and Braun16) .
Therefore, the relationship between dietary potassium and serum hs-TnT in the CKD population will expand our knowledge of the potential cardiac effect of dietary potassium in the CKD population. Because approximately 70–80 % of the dietary potassium intake is excreted into the urine(Reference Holbrook, Patterson and Bodner17), urinary potassium excretion has been used as a surrogate for dietary potassium intake(Reference O’Donnell, Mente and Rangarajan5–Reference Kim, Park and Yoo8). In the current study, we first analysed the relationship between urinary potassium excretion and serum hs-TnT levels. Subsequently, we explored whether the relationship between urinary potassium excretion and serum hs-TnT is mediated by serum levels of potassium, using the data from a large number of adults who were enrolled in the KoreaN cohort study for Outcome in patients With Chronic Kidney Disease (KNOW-CKD).
Materials and methods
Participants
The KNOW-CKD is a multicentre, prospective cohort study in Korea with a study population of 2238 patients with non-dialysis CKD stages 1–5 who were enrolled between 2011 and 2016. The detailed design and methods of the KNOW-CKD were previously published (NCT01630486 at http://www.clinicaltrials.gov)(Reference Oh, Park and Park18). Of the 2238 cohort subjects, 605 individuals were excluded, including 103 individuals with missing serum potassium values, 112 individuals missing spot urinary potassium to creatinine ratio, eight individuals with missing serum hs-TnT and 382 individuals with missing other covariates including total CO2 information. Therefore, this study included a total of 1633 patients (online Supplementary Fig. S1).
Ethics statement
The protocol of the KNOW-CKD adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) at each participating hospital, including Seoul National University Hospital (IRB number: 1104-089-359), Yonsei University Severance Hospital (IRB number: 4-2011-0163), Kangbuk Samsung Medical Center (IRB number: 2011-01-076), Seoul St. Mary’s Hospital (IRB number: KC11OIMI0441), Gil Hospital (IRB number: GIRBA2553), Nowon Eulji Medical Center (IRB number: 201105-01), Chonnam National University Hospital (IRB number: CNUH-2011-092) and Busan Paik Hospital (IRB number: 11-091). Written informed consent was obtained from all subjects. All data were fully anonymised before we accessed them.
Exposure: estimated dietary potassium intake
Random urine samples were collected after overnight fasting. Urine potassium was measured using the ion-selective electrode method. Additionally, urine creatinine was measured using the Jaffe method in the central laboratory (Lab Genomics).
We assumed that the 24-h urine potassium excretion matched with the dietary potassium intake. However, taking full urine 24 h a day is too burdensome for patients. In this regard, spot urinary potassium to creatinine ratio has been proposed as a good surrogate for 24 h urinary potassium excretion(Reference Tanaka, Okamura and Miura19–Reference Mente, O’Donnell and Dagenais21). Exposure of this study was spot urinary potassium to creatinine ratio (mmol/g Cr), which was calculated from urine potassium (mmol/l)/urine creatinine (mg/dl) × 100.
Outcome: cardiac injury
The serum hs-TnT levels were measured in the central laboratory with a highly sensitive electro-chemiluminescence immunoassay using an Elecsys 2010 analyser (Roche Diagnostics GmbH), which has an analytical measurement range of 3–10 000 ng/l. Cardiac injury was defined as hs-TnT ≥ 14 ng/l, the 99th percentile of a normal reference population(Reference Twerenbold, Jaffe and Reichlin13) and a level proved to be associated with major adverse events(Reference Everett, Brooks and Vlachos15,Reference Ndrepepa, Colleran and Braun16) . We also performed a sensitivity analysis for cardiac injury, defined as a CKD-specific hs-TnT cut-off of ≥ 126 ng/l(Reference Bansal, Zelnick and Ballantyne22).
Other measurements and definitions
Clinical data, including detailed demographic information and baseline laboratory results, were extracted using an electronic data management system (PhactaX). After 5 min of seated rest, the blood pressure was measured using an electronic sphygmomanometer. The BMI was calculated as the weight (kg) per square metre of height (m2). In the central laboratory, serum creatinine was measured using an isotope dilution MS-traceable method, wherein the level of intact parathyroid hormone was measured using a Cobas e411 analyser (Roche Diagnostics GmbH), the level of 25-hydroxyvitamin D was measured using the ADIVIA Centaur XP analyser (Siemens Healthcare Diagnostics) and urine albumin was measured using the immunoturbidimetry. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation(Reference Levey, Stevens and Schmid23). CKD and its stages were defined using the Kidney Disease Improving Global Outcomes 2012 guidelines(Reference Stevens and Levin24). Blood specimens were collected after overnight fasting. The following laboratory variables were measured at the laboratory of each participating centre: values of Hb, leucocytes, blood urea nitrogen, albumin, HDL-cholesterol, TAG, albumin, fasting glucose, serum potassium, Ca, phosphorus and total CO2.
Previous CV disease was defined as a physician diagnosis of coronary artery disease, cerebrovascular disease or peripheral vascular disease at the time of enrolment. The renin-angiotensin system inhibitors are angiotensin-converting-enzyme inhibitors or angiotensin receptor blockers. The Charlson co-morbidity index (CCI) was assessed using the method described previously(Reference Charlson, Szatrowski and Peterson25).
Statistical analysis
In this analysis, there were no missing values. The distributions of the continuous variables were evaluated using histograms and Q–Q plots. It was observed that two variables, CCI and urine albumin-to-creatinine ratio, were not normally distributed. Normally distributed continuous variables have been expressed as mean values and standard deviations, non-normally distributed continuous variables as median and interquartile range and categorical variables as percentage. The differences were analysed using one-way ANOVA for normally distributed continuous variables, Kruskal–Wallis test for non-normally distributed continuous variables and χ 2 test for categorical variables. The P trend was analysed by linear regression for normally distributed continuous variables, Jonckheere–Terpstra test for non-normally distributed continuous variables and the Cochran–Armitage trend test for categorical variables. The OR and 95 % CI for cardiac injury were calculated using logistic regression analyses. In multivariate analyses, covariates were chosen based on clinical relevance (smoking status and renin-angiotensin system inhibitors) and statistical relevance (age, sex, causes of CKD, anti-platelet drugs, anti-lipid drugs, previous CV disease, CCI, BMI, systolic and diastolic blood pressure, fasting glucose, HDL-cholesterol, TAG, serum potassium, Ca, phosphorus, total CO2, 25-hydroxyvitamin D, intact parathyroid hormone, albumin, Hb, leucocyte, blood urea nitrogen, eGFR and urine albumin-to-creatinine ratio) with cardiac injury.
The locally weighted scatterplot smoothing (LOWESS) regression curve on a scatter plot was used to assess the potential non-linear relationship. To identify the potential mediators between exposure and outcome, a causal mediation analysis was performed using 2000 simulations with a ‘mediation’ package(Reference Tingley, Yamamoto and Hirose26). A P value of <0·05 was considered statistically significant. All analyses were performed using R software Version 3.6.2 (R Foundation for Statistical Computing released 2019).
Results
Of 1633 patients, the mean age was 53·6 (sd 12·4) years and 61·7 % were men. The causes of CKD were diabetic nephropathy in 26·6 % of patients, hypertensive nephropathy in 19·8 %, glomerulonephritis in 29·8 % and other causes in 23·8 %. The mean eGFR was 52·9 (sd 31·0) ml/min/1·73 m2 and median urine albumin-to-creatinine ratio was 347·7 (interquartile range 81·4–1049·3) mg/g Cr. The mean serum potassium and spot urinary potassium to creatinine ratio were 4·6 (sd 0·6) mEq/l and 49·5 (sd 22·6) mmol/g Cr, respectively. The distribution of serum hs-TnT was left-skewed with a median 10 (interquartile range 6–17) ng/l (Fig. 1(a)). The overall prevalence of cardiac injury was 33·9%, which increased with the progression of CKD stages (Fig. 1(b)).

Fig. 1. Distribution of hs-TnT and the prevalence of cardiac injury. hs-TnT, high-sensitivity troponin T; SCI, subclinical cardiac injury; CKD, chronic kidney disease.
The clinical characteristics according to the quartile of spot urinary potassium to creatinine ratio are depicted in Table 1. With the increase of quartile of spot urinary potassium to creatinine ratio, age increased, but proportions of men and current smoking decreased. Previous CV disease, usage of renin-angiotensin system inhibitors, anti-platelet drugs, lipid lowering drugs and CCI were not associated with quartiles of spot urinary potassium to creatinine ratio. Proportion of diabetic nephropathy as a cause of CKD linearly decreased with the increase of spot urinary potassium to creatinine ratio quartile. With an increase in the spot urinary potassium to creatinine ratio quartile, the eGFR increased; however, urine albumin-to-creatinine ratio decreased. As the spot urinary potassium to creatinine ratio quartile increased, serum levels of Ca and total CO2 increased, but the values of intact parathyroid hormone, leucocyte and blood urea nitrogen decreased.
Table 1. Baseline characteristics according to the quartile of estimated dietary potassium intake
(Mean values and standard deviations; numbers and percentages; medians and interquartile ranges)

CKD, chronic kidney disease; GN, glomerulonephritis; CV, cardiovascular; RAS, RAS, renin-angiotensin system; CCI, Charlson co-morbidity index; BP, blood pressure; Cr, creatinine; eGFR, estimated glomerular filtration rate; 25-OH Vit.D, 25-hydroxyvitamin D; PTH, parathyroid hormone; BUN, blood urea nitrogen; UACR, urine albumin-to-creatinine ratio.
In post-hoc analysis with Bonferroni correction, *, † and ‡ indicate P < 0·01 when compared with 1Q–3Q, respectively.
Values are expressed as mean values and standard deviations for normally distributed continuous variables, median (interquartile range) in non-normally distributed variables and n (percentage) for categorical variables.
The differences were analysed using one-way ANOVA for normally distributed continuous variables, Kruskal–Wallis test for non-normally distributed continuous variables and χ 2 test for categorical variables.
P trend was analysed by linear regression for normally distributed continuous variables, the Jonckheere–Terpstra test for non-normally distributed continuous variables and the Cochran–Armitage trend test for categorical variables.
Relationship between spot urinary potassium to creatinine ratio and cardiac injury
We explored the relationship between serum potassium, spot urinary potassium to creatinine ratio and serum hs-TnT levels (Fig. 2). With the increase in serum potassium and spot urinary potassium to creatinine ratio, the serum hs-TnT levels steadily increased and decreased, respectively. To identify the potential confounding association of eGFR, we analysed the relationship between eGFR and serum potassium, spot urinary potassium to creatinine ratio and hs-TnT levels (online Supplementary Fig. S2) and found that an increased eGFR was associated with decreased serum levels of potassium and hs-TnT but increased spot urinary potassium to creatinine ratio. To adjust for confounders, we performed multivariate logistic regression analyses (Table 2) and found only spot urinary potassium to creatinine ratio was independently associated with cardiac injury in fully adjusted model: OR 0·917 and 95 % CI (0·841, 0·998) (P = 0·047). After excluding 789 patients for whom 24-h urine potassium excretion results were unavailable, 844 patients remained for the sensitivity analysis. Similarly, serum potassium levels were not associated with cardiac injury, but 24-h urinary potassium excretion showed decreased odds for cardiac injury: OR 0·833 and 95 % CI (0·744, 0·930) (P = 0·001). On the other hand, serum potassium and spot urinary potassium to creatinine ratio were not associated with cardiac injury defined with a CKD-specific hs-TnT cut-off (online Supplementary Table S1).
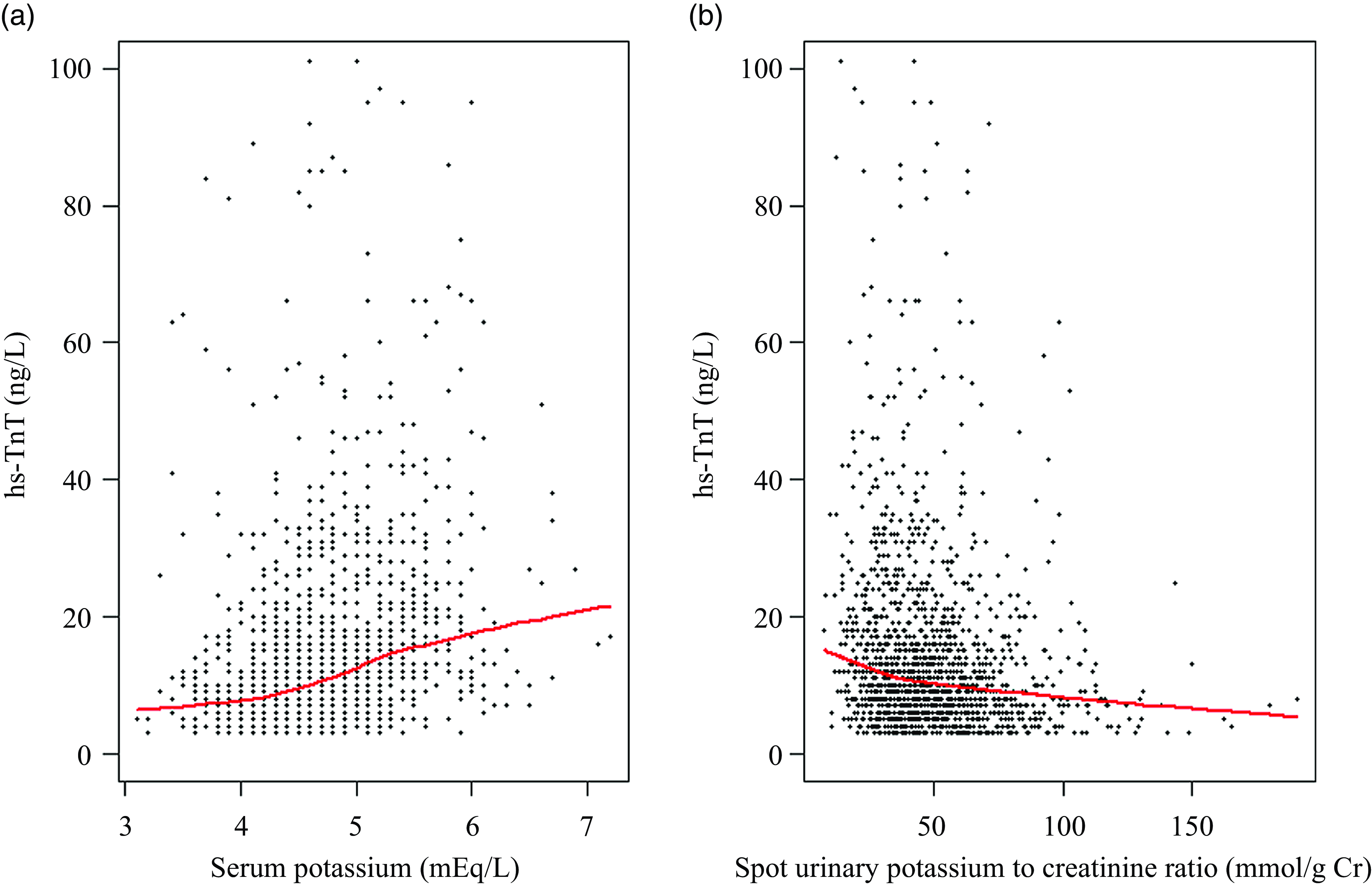
Fig. 2. Relationship between serum potassium, spot urinary potassium to creatinine ratio and hs-TnT. The red line in the scatter plot indicated the LOWESS regression curve. Values of hs-TnT > 100 ng/l were truncated. hs-TnT, high-sensitivity troponin T.
Table 2. Relationship between serum potassium level, urinary potassium excretion and cardiac injury
(Odds ratios and 95 % confidence intervals)

* In model 1, the covariates were age, sex, smoking status, causes of chronic kidney disease, administration of renin-angiotensin system inhibitors, anti-platelet drugs, anti-lipid drugs, previous CVD, age-adjusted Charlson co-morbidity index, BMI, systolic and diastolic blood pressure, fasting glucose, HDL-cholesterol and TAG levels.
† In model 2, the covariates were variables of model 1 with Ca, phosphorus, 25-hydroxy vitamin D, intact parathyroid hormone, albumin, Hb and leucocytes.
‡ In model 3, the covariates were variables of model 2 with blood urea nitrogen, estimated glomerular filtration rate, total CO2 and urine albumin-to-creatinine ratio.
OR and 95 % CI for cardiac injury were calculated using logistic regression.
Mediation and subgroup analysis
To identify the potential mediating association of serum potassium levels with the relationship between spot urinary potassium to creatinine ratio and cardiac injury, we performed a mediation analysis (Fig. 3). It was observed that approximately 6·4 % of the association of the spot urinary potassium to creatinine ratio with cardiac injury was mediated by serum potassium levels, which was not statistically significant (P = 0·368). We found significant modification between spot urinary potassium to creatinine ratio by age (P interaction = 0·015), eGFR (P interaction = 0·021) and CCI (P interaction = 0·002) (Table 3). Increased spot urinary potassium to creatinine ratio was associated with cardiac injury only in age < 55 years, eGFR ≥ 46 ml/min/1·73 m2 and CCI < 4.

Fig. 3. Mediation analysis for the relationship between spot urinary potassium to creatinine ratio and cardiac injury. The exposure was spot urinary potassium to creatinine ratio, the mediator was serum potassium and the outcome was cardiac injury. The relationship between exposure and outcome was calculated with multivariate logistic regression analysis, and that between exposure and mediator was calculated with multivariate linear regression analysis. Prop. mediated, proportion mediated.
Table 3. Subgroup analysis for the relationship between spot urinary potassium to creatinine ratio and cardiac injury, according to the status of confounders
(Odds ratios and 95 % confidence intervals)

eGFR, estimated glomerular filtration rate; CCI, Charlson co-morbidity index; UACR, urine albumin-to-creatinine ratio; P int, P for interaction.
Subgroup was categorised based on median value.
Adjusted OR and 95 % CI per 10 mmol/g Cr increase of spot urinary potassium to creatinine ratio were calculated using multivariate logistic regression analysis, entering into age, sex, smoking status, causes of chronic kidney disease, administration of renin-angiotensin system inhibitors, anti-platelet drugs and anti-lipid drugs, previous CVD, CCI, BMI, systolic and diastolic blood pressure, fasting glucose, HDL-cholesterol, TAG, Ca, phosphorus, total CO2, 25-hydroxy vitamin D, intact parathyroid hormone, albumin, Hb, leucocyte, blood urea nitrogen, eGFR, total CO2 and UACR as covariates.
When covariates were chosen as subgroup, they were excluded from the model.
Discussion
Among clinicians, it is a regular practice to reduce the potassium intake in CKD patients. Additionally, several CKD patients are educated to avoid high-potassium foods. In this study, we can also identify the habitual patterns that patients with good kidney function consumed more potassium, while patients with poor kidney function avoided potassium intake (online Supplementary Fig. S2-B). However, until recently, there have been no official recommendations to limit the potassium intake in patients with CKD. Specific codes of conduct have not been suggested even in the KDIGO 2012 guideline with respect to the consumption of potassium in CKD patients(27). In the meantime, several studies have consistently suggested the harm of a low potassium diet and potential CV benefits of a high-potassium diet(Reference Binia, Jaeger and Hu4,Reference O’Donnell, Mente and Rangarajan5,Reference Kim, Park and Yoo8,Reference Araki, Haneda and Koya12) . Nonetheless, there have been no studies to evaluate the relationship between urinary potassium excretion and cardiac injury in CKD patients. In addition, whether the relationship between urinary potassium excretion and cardiac injury is mediated by serum potassium levels has never been studied.
Cardiac troponins are structural proteins of the heart. Traditionally, the elevation of cardiac troponins means myocardial cell death(Reference Thygesen, Alpert and White14). However, as the assays of troponin became more sensitive, much lower levels of troponin can be determined, and the elevation of troponins in those once-undetectable ranges now reflects not only myocardial death but also the injury of structures and functions of the heart(Reference Twerenbold, Jaffe and Reichlin13). Among others, hs-TnT was the first highly sensitive assay for cardiac troponin, and the most validated, and 14 ng/l for hs-TnT has been suggested to be a good cut-off for cardiac injury(Reference Twerenbold, Jaffe and Reichlin13), not only as the 99th percentile of a normal reference population but also the value that can predict future CV events and mortality(Reference Everett, Brooks and Vlachos15,Reference Ndrepepa, Colleran and Braun16) . In this study, the overall prevalence of cardiac injury in stable non-dialysis CKD patients was 33·9 %, which was concordant with the known findings that CKD patients are a high-risk group for CV events(Reference Kiuchi and Mion9–Reference Cha, Kang and Park11). The prevalence of cardiac injury increased with the increase of CKD stages, and approximately 82·0 % of CKD stage 5 patients had cardiac injury. Therefore, the factors associated with cardiac injury need to be studied further.
In this study, we found that the serum potassium levels were not associated with cardiac injury, after adjusting for the markers of kidney function and damage. We propose two possible explanations for these results. First, the risk of arrhythmia is profoundly increased only when serum potassium is in extreme range, such as < 3·0 mEq/l or ≥ 7·0 mEq/l(Reference Weiss, Qu and Shivkumar3,Reference Pezhouman, Singh and Song28) . However, the serum potassium levels in this population were too stable. The range of serum potassium was between 3·1 mEq/l and 7·2 mEq/l, and only two patients showed serum potassium ≥ 7·0 mEq/l in this population. Second, the pathophysiology of dyskalaemia-related arrhythmia was mostly changing the excitation and relaxation of membrane potential, not the structural proteins(Reference Weiss, Qu and Shivkumar3). Since troponins are structural proteins, the serum levels of potassium may not be associated with the serum levels of hs-TnT.
Unlike serum potassium levels, higher urinary potassium excretion was associated with lower odds of cardiac injury, even after adjusting for markers of kidney function and damage. The relationship between urinary potassium excretion and cardiac injury was independent of serum potassium levels. We confirmed that the relationship between the urinary potassium excretion and cardiac injury was not mediated by serum potassium levels in mediation analysis. We postulated that the beneficial association of a urinary potassium excretion with cardiac injury may be derived from the intimate relationship between the high-potassium diet and healthy food patterns(Reference Clase, Carrero and Ellison29). Or, high fruit and vegetables diets that are high in potassium are also high in dietary bicarbonate equivalents. In this study, patients with higher spot urinary potassium to creatinine ratio quartile showed higher levels of total CO2, suggesting certain high-potassium diets might reduce serum potassium levels to a certain degree by treating metabolic acidosis(Reference Goraya, Simoni and Jo30). Therefore, whether the potential beneficial renal and cardiac effects of a high-potassium diet(Reference Binia, Jaeger and Hu4,Reference O’Donnell, Mente and Rangarajan5,Reference Kim, Park and Yoo8,Reference Araki, Haneda and Koya12) were from potassium per se need to be tested in future interventional studies.
In a sensitivity analysis, both serum potassium levels and urinary potassium excretion were not associated with cardiac injury defined as a CKD-specific hs-TnT cut-off of ≥ 126 ng/l, even in univariate analysis (online Supplementary Table S1). This may be because patients with hs-TnT ≥ 126 ng/l are highly related to myocardial infarction, myocarditis and congestive heart failure(Reference Twerenbold, Jaffe and Reichlin13), which are much more serious conditions than cardiac injury and are overwhelmingly influenced by other factors rather than potassium intake.
This study has several strengths. First, this was the first study to simultaneously evaluate the potential inter-relationship between serum potassium, urinary potassium excretion and cardiac injury in patients with large-scale non-dialysis CKD. Second, there have been no studies on whether the potential beneficial association of high-potassium intake was from potassium per se or not. Although inconclusive, our study results may provoke research interests and lead to subsequent studies in this field. Finally, the study was based on a globally recognised CKD cohort (KNOW-CKD)(Reference Orlandi, Huang and Fukagawa31,Reference Alencar de Pinho, Levin and Fukagawa32) . The multicentre setting minimised selection and centre-specific bias. In addition, the wide range of baseline kidney function and inclusion of various causes of CKD may augment the reliability of our study results.
However, this study also has some limitations. First, this was a cross-sectional study. Therefore, a causal relationship cannot be ascertained using the study results. Second, only half of the cohort participants had 24-h urine potassium excretion. Therefore, the primary exposure of the study was a surrogate, not measured values. However, since we used the most validated surrogate for urinary potassium excretion(Reference Tanaka, Okamura and Miura19–Reference Mente, O’Donnell and Dagenais21), and the study purpose was hypothesis generation, not confirming a causal relationship, this limitation might be acceptable. Third, the single ethnicity of the patients in this study limits our ability to generalise our results.
In conclusion, higher urinary potassium excretion was associated with lower odds of cardiac injury. However, the potential cardiac association of higher urinary potassium excretion was not mediated by serum potassium levels. Future studies need to be conducted to confirm the results of this study.
Acknowledgements
This study was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102 and 2022-11-007).
Conceptualization: S. W. L. Data Curation: S. A. S., J. Y. J., Y. K. O., K. B. L. Formal Analysis: H. K. M., S. K. P., S. W. L. Funding Acquisition: K.-H. O., C. A. Investigation: H. K. M., S. W. L. Methodology: S. K. P., C. A. Project Administration: H. K. M., K.-H. O., S. W. L. Resources: K.-H. O., C. A., S. A. S., J. Y. J., Y. K. O., K. B. L. Software: S. K. P. Supervision: K.-H. O. Validation: S. K. P. Visualization: H. K. M. Writing – Original Draft: H. K. M. Writing – Review & Editing: S. W. L.
There are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114523002064









