The systemic inflammatory response syndrome, or SIRS, is the name given to the uncontrolled inflammatory response to an insult (e.g. surgery, trauma, burns) and involving excessive production of inflammatory cytokines, particularly tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-8Reference Bone, Balk, Cerra, Dellinger, Fein, Knaus, Schein and Sibbald1. Sepsis is the presence of SIRS in response to or in combination with an infectionReference Bone, Balk, Cerra, Dellinger, Fein, Knaus, Schein and Sibbald1. The mortality risk of sepsis is about 20 %, and it predisposes to organ failure, which carries an elevated mortality risk. Septic shock is the occurrence of multiple organ failures, metabolic acidosis and hypotension and it carries a mortality risk of 40 to 80 %Reference Bone, Balk, Cerra, Dellinger, Fein, Knaus, Schein and Sibbald1. Together SIRS, sepsis and septic shock are termed “septic syndromes”. Septic syndromes are the leading cause of death in critically ill patients in Western countriesReference Angus, Linde-Zwirble, Lidicker, Clermont, Carcillo and Pinsky2.
Animal studies suggest a central role for inflammatory cytokines in the septic response (see 3 for references) and patients with sepsis show markedly elevated circulating concentrations of TNF-α, TNF-receptor 1, IL-1β and IL-6, with those patients having the highest concentrations being more likely to dieReference Girardin, Grau, Dayer, Roux-Lombard and Lambert4–Reference Arnalich, Garcia-Palomero, Lopez, Jimenez, Madero, Renart, Vazquez and Montiel6. In addition, circulating white cells from septic patients had high levels of activated nuclear factor kappa B (NFκB), a transcription factor that promotes the expression of numerous genes associated with inflammation, and levels of activated NFκB were higher in those patients who went on to dieReference Arnalich, Garcia-Palomero, Lopez, Jimenez, Madero, Renart, Vazquez and Montiel6. Mediators other than inflammatory cytokines are involved in the pathological processes that accompany critical illness. For example, prostaglandin E2 is implicated in sepsis, burns and critical illnessReference Grbic, Mannick, Gough and Rodrick7, Reference Ertel, Morrison, Meldrum, Ayala and Chaudry8, while leukotriene B4 and oxidants released by neutrophils are involved in acute respiratory distress syndromeReference Kollef and Schuster9.
In addition to hyperinflammation, patients with sepsis, burns and trauma can display immunosuppression, characterised by decreased monocyte expression of human leukocyte antigens (HLA), impaired ability of monocytes to stimulate T cells, impaired T cell proliferation, and low production of T helper (Th) 1-type cytokines (e.g. interferon (IFN)-γ) associated with host defence against bacteria and viruses but high levels of the Th2- and Treg-type cytokines (IL-4, IL-10) associated with inhibition of host defence against bacteria and viruses (see 10 for references). It is believed that the immunosuppressed phase of sepsis lags behind the hyperinflammatory phase (Fig. 1) i.e. initially sepsis is characterised by increased generation of inflammatory mediators but as it persists there is a shift towards an anti-inflammatory, immunosuppressed state sometimes called the compensatory anti-inflammatory response syndrome, or CARS although the precise timing of the two phases and the factors that influence their relative magnitudes are not entirely clearReference Heidecke, Hensler, Weighardt, Zantl, Wagner, Siewert and Holzmann11–Reference Tschaikowsky, Hedwig-Geissing, Schiele, Bremer, Schywalsky and Schutter13.

Fig. 1 Hypothetical biphasic immuno-inflammatory response to a traumatic insult. HLA, human leukocyte antigen; NFκB, nuclear factor κ B; ROS, reactive oxygen species. The grey area represents the physiological range.
The concept of immunonutrition
The ability of nutrients to influence the activities of cells of the immune system has been termed “immunonutrition”, although this term has most frequently been associated with the use of specific nutrients or combinations of nutrients in surgical, trauma, burned or critically ill patientsReference Calder14. These patients typically receive artificial nutrition, through the parenteral or enteral routes. The overriding notion of immunonutrition is that nutrients can improve cell-mediated immune responses in a way that is clinically meaningful, but in the context of patients requiring artificial nutrition this concept is extended to include modification of hyper-inflammatory processes (including oxidative stress) and improvement in gut barrier function, so preventing bacterial translocation (Fig. 2).
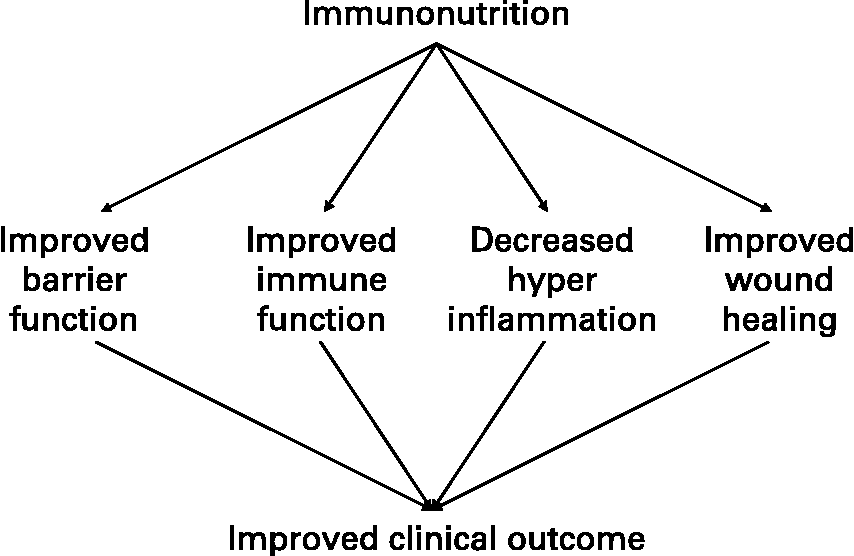
Fig. 2 The concept of immunonutrition in the context of surgical or critically ill patients.
Scientific rationale for the nutrients included in immune-modulating artificial nutrition
Nutrients considered for inclusion in immune-modulating artificial nutrition are generally those that have been shown to act in relevant animal models to improve immune function, regulate inflammation, maintain or improve gut barrier function, or improve antioxidant defences, and which have been shown to be safe and efficacious (assessed according to the defined study outcome which has not always been a clinical endpoint) in clinical trials in the relevant patient groups. In addition, theoretical considerations, experimental data from in vitro studies and healthy volunteer studies, and clinical findings in other patient groups have played a role in influencing the makeup of immune-modulating artificial nutrition. It is important to appreciate that artificial nutrition contains macro and micronutrients including carbohydrate, lipid, protein and/or peptides, and the full range of vitamins and minerals; immune-modulating artificial nutrition contains additional nutrients or increased amounts of nutrients normally present. Nutrients that have been identified as potentially important as components of immune-modulating artificial nutrition are:
Glutamine
Arginine
N-acetyl cysteine (as a cysteine precursor)
Branched chain amino acids
Nucleotides
Long-chain n-3 fatty acids
Antioxidant vitamins
Trace elements
Taurine
The scientific rationale for the inclusion of these nutrients is summarized in Table 1.
Table 1 Scientific rationale for inclusion of nutrients in immune-modulating artificial nutrition

Trials of immunonutrition in surgical and critically ill patients
Glutamine
Enteral glutamine increased the blood ratio of CD4+ to CD8+ cells in intensive care patientsReference Jensen, Miller, Talabiska, Fish and Gianferante26, whilst parenteral administration in post-colorectal surgery patients increased mitogen-stimulated proliferation of blood lymphocytesReference O'Riordain, Fearon, Ross, Rogers, Falconer, Bartolo, Garden and Carter27. Another study in post-operative patients who received glutamine parenterally also showed increased blood lymphocyte numbersReference Morlion, Stehle, Wachtler, Siedhoff, Köller, König, Fürst and Puchstein28 and more recently, parenteral glutamine for 48 hours after major abdominal surgery was shown to result in better maintenance of the HLA-DR expression on circulating monocytesReference Spittler, Sautner, Gornikiewicz, Manhart, Oehler, Bergmann, Függer and Roth29. Patients with oesophageal cancer being treated with radio-chemotherapy also had higher blood lymphocyte counts and better lymphocyte proliferative responses if they consumed glutamine for 28 daysReference Yoshida, Matsui, Shirouzu, Fujita, Yamana and Shirouzu30. Furthermore, in addition to a direct immunological effect, glutamine, even given parenterally, appears to improve gut barrier function in patients at risk of infectionReference van der Hulst, van Kreel, von Meyenfeldt, Brummer, Arends, Deutz and Soeters31, an effect that is likely to decrease translocation of bacteria from the gut and hence eliminate a key source of infection.
The improvements in immune function with glutamine administration appear to result in clinical advantage. Parenteral glutamine following bone marrow transplantation reduced infections and length of hospital stayReference Ziegler, Young, Benfell, Scheltinga, Hortos, Bye, Morrow, Jacobs, Smith and Antin32, with a later report showing that glutamine resulted in higher total blood lymphocyte, T lymphocyte and CD4+ lymphocyte numbers after patients' dischargeReference Ziegler, Bye, Persinger, Young, Antin and Wilmore33. Very low birthweight babies who received a glutamine-enriched premature feeding formula had a much lower rate of sepsis than babies receiving a standard formulaReference Neu, Roig, Meetze, Veerman, Carter, Millsaps, Bowling, Dallas, Sleasman, Knight and Auestad34, and in a study of patients in intensive care, glutamine decreased mortality compared with standard PN and changed the pattern of mortalityReference Griffiths, Jones and Palmer35. In a more recent study, in which patients received enteral glutamine vs. standard enteral feed from within 48 hours of the initiation of trauma, there was a significant reduction in the 15-day incidence of pneumonia, bacteremia and severe sepsis in the glutamine group, although this was not associated with reduced mortalityReference Houdijk, Rijnsburger, Jansen, Wesdorp, Weiss, McCamish, Teerlink, Meuwissen, Haarman, Thijs and van Leeuwen36. This improved clinical outcome in patients receiving glutamine was associated with increased monocyte expression of HLA-DRReference Boelens, Houdijk, Fonk, Nijveldt, Ferwerda, Von Blomberg-Van Der Flier, Thijs, Haarman, Puyana and Leeuwen37 and increased ex vivo IFN-γ production by T cellsReference Boelens, Houdijk, Fonk, Puyana, Haarman, von Blomberg-van der Flier and van Leeuwen38. Enteral glutamine was found to decrease infection rate, Pseudomonas aeruginosa bacteremia, and mortality in adult burned patientsReference Garrel, Patenaude, Nedelec, Samson, Dorais, Champoux, D'Elia and Bernier39.
Novak et al. Reference Novak, Heyland, Avenell, Drover and Su40 conducted a meta-analysis of 14 studies of glutamine (parenteral or enteral) in surgical or critically ill patients excluding bone marrow transplantation and premature infant studies. Glutamine use was associated with decreased infectious complications (RR = 0·81) and length of hospital stay (2·6 d shorter) and a trend towards lower mortality (RR = 0·78). In surgical patients, glutamine decreased infections and length of hospital stay, but did not affect mortality. Mortality benefits were seen in the critically ill, especially when glutamine was used parenterally at high dose. Murray and PindoraReference Murray and Pindoria41 conducted a meta-analysis of parenteral glutamine in bone marrow transplantation and showed decreased length of hospital stay (6·2 d shorter) and reduced development of positive blood cultures (RR = 0·23). They concluded that bone marrow transplant patients with gastrointestinal failure should receive parenteral glutamine.
Arginine
Oral arginine supplementation for 7 days post-surgery was associated with an increased number of circulating CD4+ cells and an enhanced response of peripheral blood lymphocytes to mitogens by day 7Reference Daly, Reynolds, Thom, Kinsley, Dietrick-Gallagher, Shou and Ruggieri42. Although the arginine-supplemented group achieved a positive nitrogen balance (by day 6), there was no difference in clinical outcome compared with the placebo group. Intravenous arginine has been given in various conditions including in surgical and intensive care unit patients, although these did not evaluate immune outcomes (see 43 for references).
N-acetyl cysteine
Infusion of N-acetyl cysteine infusion into patients with sepsis increased blood glutathione concentration, decreased plasma concentrations of IL-8 and soluble TNF receptors, and improved respiratory function with a decreased number of days in intensive careReference Spapen, Zhang, Demanet, Vleminckx, Vincent and Huyghens44, Reference Bernard, Wheeler, Arons, Morris, Paz, Russell and Wright45. While not affecting mortality rates, N-acetyl cysteine also shortened hospital length of stay.
Fatty acids
The lipid typically used in parenteral nutrition is soybean oil, in which n-6 linoleic acid comprises about 50 % of fatty acids present. A meta-analysis of total parenteral nutrition suggested that inclusion of lipids might be detrimental as far as complications are concernedReference Heyland, MacDonald, Keefe and Drover46, at least in very ill patients. A recent study in gastrointestinal surgery patients showed that the amount of n-6 fatty acids infused was one of two predictors of the length of hospital stay, the other being the delay in starting nutrition supportReference Koch and Heller47. Despite this, clinical trials with soybean oil based lipid emulsions provide conflicting evidence with some showing immunosuppressive effects, perhaps linked to poorer patient outcomes, while others show no effect on immune outcomes (see 23 for references). Nevertheless, there is an increasing view that using lipid emulsions entirely based upon soybean oil is not optimal. One approach to decreasing the linoleic acid content in lipid emulsions is partial replacement of soybean oil with long-chain n-3 fatty acid rich fish oil. This not only decreases n-6 fatty acid content but increases n-3 fatty acid provision. Several studies with fish oil containing lipid emulsions have been conducted in post-surgery patients, demonstrating decreased production of some inflammatory mediators (leukotriene B4, TNF-α, IL-6) and better preservation of some immune functions (e.g. monocyte expression of HLA-DR, IFN-γ production) (see 10,23 for references). Some studies have also reported shorter postoperative stays in intensive care and in hospital with parenteral fish oil but most report no differences in infection rates or mortality (see 10,23). One study reported that perioperative fish oil decreased need for mechanical ventilation, readmission to intensive care, mortality and length of hospital stayReference Tsekos, Reuter, Stehle and Boeden48 and similar findings were seen in the 230 post surgical patients within a large study of over 650 patientsReference Koch and Heller47.
Fish oil containing parenteral nutrition has also been examined experimentally in septic patientsReference Mayer, Fegbeutel, Hattar, Sibelius, Kramer, Heuer, Temmesfeld-Wollbruck, Gokorsch, Grimminger and Seeger49, Reference Mayer, Gokorsch, Fegbeutel, Hattar, Rosseau, Walmrath, Seeger and Grimminger50. Anti-inflammatory effects including lower blood leukocyte counts, serum C-reactive protein concentration and production of inflammatory cytokines by isolated endotoxin-stimulated mononuclear cells and increased production of leukotriene B5 by stimulated neutrophils were reported. No clinical outcomes were reported in these studies. Koch and HellerReference Koch and Heller47 included 268 patients with abdominal sepsis in their study of n-3 fatty acid infusion. They found a significantly lower rate of infection, shorter lengths of intensive care unit and hospital stay and lower mortality in those patients receiving the highest amounts of fish oil.
A novel enteral formula used in patients with acute respiratory distress syndrome included n-3 fatty acidsReference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51, Reference Pacht, DeMichele, Nelson, Hart, Wennberg and Gadek52. By 4 days of treatment the numbers of total leukocytes and of neutrophils in the alveolar fluid declined significantly in the n-3 fatty acid group and were lower than in controlsReference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51. Alveolar fluid IL-8 was lower in the experimental group compared with controls and leukotriene B4 and TNF-α tended to be lowerReference Pacht, DeMichele, Nelson, Hart, Wennberg and Gadek52. Arterial oxygenation and gas exchange were also improved and the treated patients had a decreased requirement for supplemental oxygen, decreased time on ventilation support and a shorter length of stay in intensive careReference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51. Total length of hospital stay tended to be shorter in the experimental group and fewer patients developed new organ failureReference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51. Mortality was 12 % in the experimental group and 19 % in the control group, but this difference was not statistically significantReference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51. However, the experimental n-3 fatty acid formula also contained more medium chain triglycerides, β-carotene, taurine, carnitine, vitamin C and vitamin E than the control formula and hence it is not possible to ascribe the benefits to any particular nutrient. Two more recent studies also report benefits from n-3 fatty acid containing enteral formulae in acutely ill patientsReference Singer, Theilla, Fisher, Gibstein, Grozovski and Cohen53, Reference Pontes-Arruda, Aragão and Albuquerque54. In one of these studies patients with acute lung injury received a control formula or a formula enriched in n-3 fatty acids and the n-6 γ-linolenic acid for 14 daysReference Singer, Theilla, Fisher, Gibstein, Grozovski and Cohen53. By days 4 and 7 patients receiving the experimental formula showed improved oxygenation and a reduction in length of ventilation; there was no difference between the groups in mortalityReference Singer, Theilla, Fisher, Gibstein, Grozovski and Cohen53. Most recently a formula similar to that used by Gadek et al.Reference Gadek, DeMichele, Karlstad, Pacht, Donahoe, Albertson, Van Hoozen, Wennberg, Nelson and Noursalehi51 was trialed in ventilated patients with severe sepsis and septic shockReference Pontes-Arruda, Aragão and Albuquerque54. Patients receiving the experimental diet had significantly better oxygenation, more ventilator-free days, more intensive care unit-free days and less development of new organ dysfunctions. These improvements were associated with significantly lower mortality in the experimental group.
Antioxidant micronutrients
Several studies investigating parenteral or enteral (mainly parenteral) antioxidant micronutrients in post-surgery, burned or critically ill patients have been conducted and eleven were included in a recent meta-analysis looking at clinical outcomesReference Heyland, Dhaliwal, Suchner and Berger55. The nutrients studied were zinc, copper, selenium, vitamin E, vitamin C, and N-acetyl cysteine alone or in various combinations. Most studies did not evaluate immune markers although some did. One study showed that parenteral zinc, copper and selenium did not influence blood lymphocyte subsets, neutrophil chemotaxis or T lymphocyte proliferation in burned patients in the ICU, although there was a decrease in IL-6 levelsReference Berger, Spertini, Shenkin, Wardle, Wiesner, Schindler and Chiolero56. Several studies show improved clinical outcome with antioxidant micronutrients including fewer infections in burned patientsReference Berger, Spertini, Shenkin, Wardle, Wiesner, Schindler and Chiolero56, Reference Porter, Ivatury, Azimuddin and Swami57 and fewer infections and organ failures in trauma patientsReference Tanaka, Matsuda, Miyagantani, Yukioka, Matsuda and Shimazaki58. The meta-analysisReference Heyland, Dhaliwal, Suchner and Berger55 showed reduced mortality (RR = 0·65) with antioxidants, but no effect on infectious complications. Parenteral antioxidants reduced mortality (RR = 0·56) but enteral did not, and effects on mortality were only seen when selenium was administered either alone or in combination (RR = 0·59). Although the lack of effect of antioxidant micronutrients on infectious complications suggests that they do not act via immune modulation, their anti-inflammatory actions might contribute to improved survival since hyper-inflammation is linked to organ failure. It is worth noting however that some individual studies do report reduced infectionsReference Berger, Spertini, Shenkin, Wardle, Wiesner, Schindler and Chiolero56–Reference Tanaka, Matsuda, Miyagantani, Yukioka, Matsuda and Shimazaki58 an effect confirmed by a recent study in burned patients, which also found improved wound healing and reduced requirement for regraftingReference Berger, Baines, Raffoul, Benathan, Chiolero, Reeves, Revelly, Cayeux, Sénéchaud and Shenkin59.
Mixtures of nutrients
Several enteral formulas using a combination of nutrients have been developed, typically including arginine, nucleotides and long-chain n-3 fatty acids. The majority of trials in surgical and critically ill patients have used the commercially-available product IMPACT® and a number of these studies reported immune and/or inflammatory outcomes (see 60 for references). Most studies reporting circulating lymphocyte numbers and subsets, and circulating immunoglobulin concentrations showed little difference between IMPACT® treated patients and controls, although some studies reported benefits on phagocytosis, respiratory burst, lymphocyte proliferation, HLA-DR expression on monocytes and cytokine production (see 60). These effects could be due to any single specified nutrient (i.e. arginine, nucleotides, long-chain n-3 fatty acids) or to a combination.
Meta-analyses of controlled, randomized clinical studies using IMPACT or similar immunonutrition formulae have identified significant reductions in infections and length of hospital stay but these effects are more evident in surgical rather than critically ill patientsReference Beale, Bryg and Bihari61–Reference Waitzberg, Saito, Plank, Jamieson, Jagannath, Hwang, Mijares and Bihari65 and none of the meta-analyses shows a significant effect on mortality. Despite some clear statements to the contrary in the earlier meta-analysesReference Beale, Bryg and Bihari61–Reference Heyland, Novak, Drover, Jain, Su and Suchner63, concern has been raised that these formulae may actually be detrimental in the seriously illReference Heyland, Dhaliwal, Drover, Gramlich and Dodek66–Reference Suchner, Heyland and Peter68. This is because some studies of immunonutrition mixtures in critically ill patients reported increased mortalityReference Beale, Bryg and Bihari61–Reference Heyland, Novak, Drover, Jain, Su and Suchner63. The source of the concern is the high arginine content, which is thought to drive excessive production of nitric oxideReference Zhou and Martindale43, Reference Suchner, Heyland and Peter68. A consensus recommendation for the use of enteral immunonutrition mixtures was made following a US summit69. The recommendations are as follows:
Clearly established benefit in elective gastrointestinal surgery and in blunt or penetrating torso trauma
Probable benefit in elective major surgery, severe head injury, burns > 30 % body surface area, ventilator-dependent non-septic intensive care unit patients
No benefit in patients able to resume oral intake within 5 days or in patients in intensive care unit for monitoring only
More recently the European Society for Clinical Nutrition and Metabolism (ESPEN) has established guidelines for use of enteral nutrition that included a consideration of “immunonutrient” mixesReference Weimann, Braga, Harsanyi, Laviano, Ljungqvist, Soeters, Jauch, Kemen, Hiesmayr, Horbach, Kuse and Vestweber70, Reference Kreymann, Berger, Deutz, Hiesmayr, Jolliet, Kazandjiev, Nitenberg, van den Berghe, Wernerman, Ebner, Hartl, Heymann and Spies71. The guidelines for surgical patientsReference Weimann, Braga, Harsanyi, Laviano, Ljungqvist, Soeters, Jauch, Kemen, Hiesmayr, Horbach, Kuse and Vestweber70 included:
Use enteral nutrition with immuno-modulating substrates (arginine, nucleotides and long-chain n-3 fatty acids) perioperatively in:
patients undergoing major neck surgery for cancer
patients undergoing major abdominal surgery for cancer.
The guidelines for patients in intensive careReference Kreymann, Berger, Deutz, Hiesmayr, Jolliet, Kazandjiev, Nitenberg, van den Berghe, Wernerman, Ebner, Hartl, Heymann and Spies71 included:
Immune modulating formulae (formulae enriched with arginine, nucleotides and long-chain n-3 fatty acids) are superior to standard enteral formulae in:
Elective upper gastrointestinal surgical patients
Patients with mild sepsis
Patients with trauma
Patients with acute respiratory distress syndrome (formulae containing omega-3 fatty acids and antioxidants)
No recommendation for immune-modulating formulae can be given in burned patients due to insufficient data.
ICU patients with very severe illness who do not tolerate more than 700 ml enteral formula per day should not receive an immune-modulating formula enriched with arginine, nucleotides and omega-3 fatty acids.
Glutamine should be added to standard enteral formula in:
Burned patients
Trauma patients
Conclusion
Surgery, trauma, burns and injury are insults that can induce an excessive inflammatory response, which may be associated with a later immunosuppressed state. Hyperinflammation can lead to organ damage and failure while immunsuppression increases susceptibility to infection. The resulting septic syndromes are associated with significant morbidity and mortality. A range of nutrients are able to modulate inflammation and its partner oxidative stress and to maintain or improve immune function and the intestinal barrier. These include several amino acids, antioxidant vitamins and minerals, long-chain n-3 fatty acids and nucleotides. Experimental studies support a potential role for each of these nutrients in surgical, injured or critically ill patients. There is good evidence that parenteral or enteral glutamine influences immune function in such patients and that this is associated with clinical improvement. This conclusion is supported by meta-analyses and recent guidelines. Evidence is also mounting for the use of long-chain n-3 fatty acids in surgical and septic patients, but more evidence of efficacy is required in these groups and there is a lack of studies in other patient groups that might benefit. Mixtures of antioxidant vitamins and minerals are also clinically effective, especially if they include selenium. Their action appears not to involve improved immune function, although an anti-inflammatory mode of action has not been ruled out. Enteral immunonutrient mixtures, usually including arginine, nucleotides and long-chain n-3 fatty acids have been used widely in surgical and critically ill patients. Evidence of efficacy is good in surgical patients; this conclusion is supported by meta-analyses and recent guidelines. However whether these same mixtures are beneficial, or should even be used, in critically ill patients remains controversial. While some studies show decreased mortality with such mixtures, several show increased mortality. There is a view that this is due to a high arginine content driving nitric oxide production. It is interesting that these mixtures do not typically include glutamine, which is clearly of benefit. It seems likely that novel immunonutrient mixtures will be developed in the future. Clearly more research using larger, better designed trials will be needed to see whether these benefit immune function, with an improved clinical benefit in vulnerable patients.
Conflict of interest statement
The author has research funding from B. Braun Melsungen, receives consultancy fees from Royal Dutch Numico and speaking fees from B. Braun Melsungen and Fresenius Kabi.





