A previous study from this group has demonstrated that most individuals on typical antipsychotic medications have pathologically elevated prolactin levels and, if female, are hypogonadal (Reference Smith, Wheeler and MurraySmith et al, 2002). Hyperprolactinaemia is associated with reduced bone mineral density, which is probably mediated by the inhibition exerted by prolactin on the hypothalamic–pituitary–gonadal axis and the resulting hypogonadism (Reference Schlecter, Sherman and MartinSchlecter et al, 1983). It is known that osteoporosis is associated with schizophrenia (Reference Halbreich, Rojansky and PalterHalbreich et al, 1995; Reference Bilici, Cakirbay and GulerBilici et al, 2002) and several recent review papers have pointed to the potential importance of an association between antipsychotic-induced hyperprolactinaemia and osteoporosis (Reference Naidoo, Goff and KlibanskiNaidoo et al, 2003; Reference Wieck and HaddadWieck & Haddad, 2003). The aim of this study was to examine the relationship between bone mineral density, prolactin levels and hypothalamic–pituitary–gonadal axis function using a cross-sectional design in individuals with schizophrenia.
METHOD
Study participants
Male and post-menopausal female patients with a diagnosis of schizophrenia on long-term prolactin-raising antipsychotic medication (>10 years) were eligible for inclusion in the study. Post-menopausal status was defined as absence of menstruation for at least 2 years and being >52 years of age. Participants were recruited from out-patient clinics attached to three psychiatric hospitals: St Ita's and St John of God's Hospital, Dublin and the Maudsley Hospital, London. Exclusion criteria included any medications known to be associated with the development or treatment of osteoporosis or the presence of medical disorders known to be risk factors for osteoporosis. Patients with a history of eating disorders were also excluded. Ethical approval was sought from and granted by each hospital involved in the study and written informed consent to participate in the study was obtained from each participant.
Clinical assessment
Clinical assessment included a semi-structured clinical interview with resultant DSM–IV diagnoses (American Psychiatric Association, 1994). Illness severity was measured using the Brief Psychiatric Rating Scale (BPRS; Reference Overall and GorhamOverall & Gorham, 1962) and the Schedule for Assessment of Negative Symptoms (SANS; Reference AndreasonAndreason, 1983). A medical history was obtained detailing relevant information, such as a history of fractures or polydipsia. Each participant had a full physical examination, including body mass index evaluation.
A detailed drug history was recorded. In order to have a comparable value for each patient, medication was converted to chlorpromazine equivalents (Reference Taylor, McConnell and McConnellTaylor et al, 2001). The atypical antipsychotics olanzapine and risperidone were converted according to the best pharmacological match for dopamine 2 receptor blockade to typicals: clozapine and haloperidol, respectively (Reference SeemanSeeman, 1992). A modified version of an osteoporosis questionnaire was completed for each participant. This questionnaire examines information relevant to bone mineral density, such as family history, parity, dietary and weight-bearing exercise habits.
Endocrine assessment
A blood sample was drawn from each patient to measure oestradiol, progesterone, testosterone, follicle-stimulating hormone, luteinising hormone and sex hormone binding globulin (a marker of gonadal hormone concentrations). Plasma prolactin, cortisol, thyroid-stimulating hormone, thyroxine and triiodothyronine were also measured in each patient. Samples were collected at random times during the day (09.00–18.00 h), spun within 1 hour and the frozen serum/plasma was batch analysed. Blood sampling was not related to the timing of meals.
Prolactin, oestradiol, luteinising hormone, follicle-stimulating hormone, thyroid-stimulating hormone and thyroxine levels were measured using time-resolved fluoroimmunoassays methods with commercial kits (AutoDELFIA, PerkinElmer, Inc.: human hormone references supplied by Wallac Oy, Turku, Finland). Progesterone, testosterone and sex-hormone-binding globulin were measured using radioimmunoassay kits (17x-Hydroxyprogesterone 125I RIA Kit, Testosterone 125I RIA and Sex Binding Globulin 125I Immunoradiometric Assay Kit supplied by ICN Pharmaceuticals).
Bone mineral density assessment
Osteoporosis is defined by the World Health Organization as a bone mineral density of more than 2.5 standard deviations below the mean value for peak bone mass in young adults when measured by dual-energy X-ray absorptiometry (DEXA) (World Health Organization Study Group, 1994). The bone mineral density was determined in lumbar vertebrae L1–L4 and in the femoral neck, trochanteric and intertrochanteric regions of the left hip using a DEXA scan, which is currently the most precise and widely used method of assessing bone mineral density. Bone density is measured in relation to two sets of values generated by the DEXA scanner: t scores compare the patients’ results with standardised peak bone mass of females/males 20–30 years old; and Z scores compare the bone mineral density with the average for her/his age group. Only Z scores are reported in this paper.
Statistical analysis
The SPSS for Windows (1999 version) was used to analyse the data. Results were analysed both for the whole group and separately for each gender. Independent t-tests (two-tailed) were used to identify differences between groups, and cross-tabulations were performed using χ2 analyses when appropriate. Multiple regression analyses were performed to examine associations between the multiple variables hypothesised to be correlated with bone mineral density. Results are expressed as means and standard deviations.
RESULTS
Demographic data
Demographic data are shown in Table 1. Twenty-five post-menopausal women and 30 men were recruited into the study. The subjects were either of Caucasian (n=46) or African–Caribbean (n=9) ethnicity. There was a significant difference between male and female groups in terms of age (P<0.01), number of cigarettes smoked per day (P<0.05) and daily exercise taken (P<0.001). Females had been exposed to medication for significantly longer periods than males (P=0.04).
Table 1 Demographic and clinical details of male and female participants
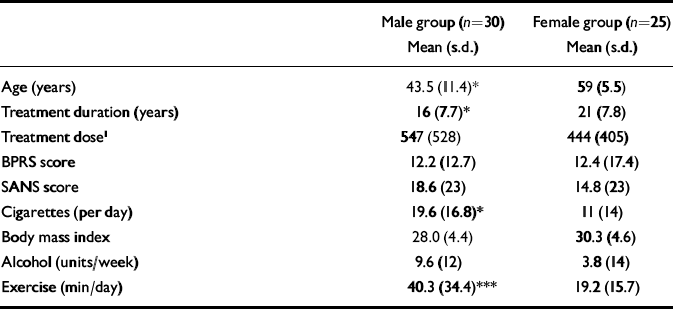
| Male group (n=30) Mean (s.d.) | Female group (n=25) Mean (s.d.) | |
|---|---|---|
| Age (years) | 43.5 (11.4)* | 59 (5.5) |
| Treatment duration (years) | 16 (7.7)* | 21 (7.8) |
| Treatment dose1 | 547 (528) | 444 (405) |
| BPRS score | 12.2 (12.7) | 12.4 (17.4) |
| SANS score | 18.6 (23) | 14.8 (23) |
| Cigarettes (per day) | 19.6 (16.8)* | 11 (14) |
| Body mass index | 28.0 (4.4) | 30.3 (4.6) |
| Alcohol (units/week) | 9.6 (12) | 3.8 (14) |
| Exercise (min/day) | 40.3 (34.4)*** | 19.2 (15.7) |
Clinical data
No associations were found between bone mineral density values and clinical measures of psychiatric illness (BPRS or SANS), or the presence of polydipsia. Among the lifestyle variables, only cigarette smoking was significantly correlated with reduced Z scores in L4 for the combined group (r=-0.27, P=0.04).
Endocrine data (see Table 2)
Both gender groups had a mean prolactin level of approximately three times the upper limit of normal, with no gender or ethnicity differences in mean values between groups. Hyperprolactinaemia was present in 62% of the overall group (18 (60%) of males and 16 (64%) of females) and the mean dose of medication was higher in those who were hyperprolactinaemic (602 (s.d.=477) mg/day) compared with those who were normoprolactinae normoprolactinaemic (267 (s.d.=336) mg/day: t=2.8, d.f.=53, P<0.01).
Table 2 Endocrine data of male and female participants
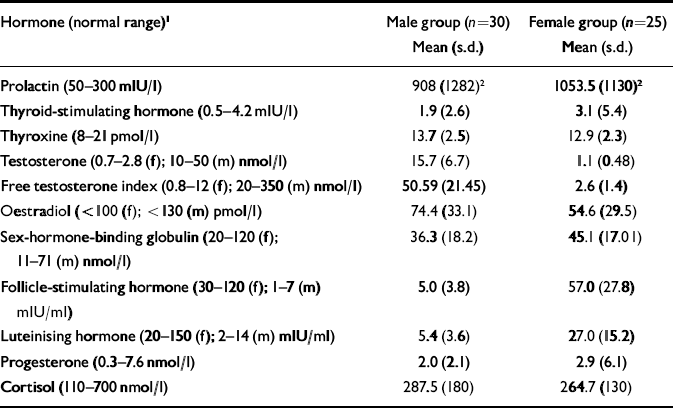
| Hormone (normal range)1 | Male group (n=30) Mean (s.d.) | Female group (n=25) Mean (s.d.) |
|---|---|---|
| Prolactin (50–300 mlU/l) | 908 (1282)2 | 1053.5 (1130)2 |
| Thyroid-stimulating hormone (0.5–4.2 mlU/l) | 1.9 (2.6) | 3.1 (5.4) |
| Thyroxine (8–21 pmol/l) | 13.7 (2.5) | 12.9 (2.3) |
| Testosterone (0.7–2.8 (f); 10–50 (m) nmol/l) | 15.7 (6.7) | 1.1 (0.48) |
| Free testosterone index (0.8–12 (f); 20–350 (m) nmol/l) | 50.59 (21.45) | 2.6 (1.4) |
| Oestradiol (<100 (f); <130 (m) pmol/l) | 74.4 (33.1) | 54.6 (29.5) |
| Sex-hormone-binding globulin (20–120 (f); 11–71 (m) nmol/l) | 36.3 (18.2) | 45.1 (17.01) |
| Follicle-stimulating hormone (30–120 (f); 1–7 (m) mlU/ml) | 5.0 (3.8) | 57.0 (27.8) |
| Luteinising hormone (20–150 (f); 2–14 (m) mlU/ml) | 5.4 (3.6) | 27.0 (15.2) |
| Progesterone (0.3–7.6 nmol/l) | 2.0 (2.1) | 2.9 (6.1) |
| Cortisol (110–700 nmol/l) | 287.5 (180) | 264.7 (130) |
Medication (see Table 3)
Within the total group, 17 (31%) were receiving more than one antipsychotic agent. All patients taking the prolactin-sparing antipsychotic olanzapine were also on a conventional depot antipsychotic drug. Participants were divided into two groups: those receiving more than the upper limit of the British National Formulary range for ‘usual maintenance dose’ of chlorpromazine (300 mg/day) and those receiving less than this. There were no significant differences between these groups in terms of age, gender, treatment duration, lifestyle variables or scores on clinical rating scales.
Table 3 Frequency of antipsychotic use in the study population
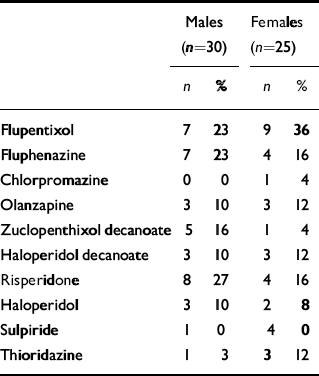
| Males (n=30) | Females (n=25) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Flupentixol | 7 | 23 | 9 | 36 |
| Fluphenazine | 7 | 23 | 4 | 16 |
| Chlorpromazine | 0 | 0 | 1 | 4 |
| Olanzapine | 3 | 10 | 3 | 12 |
| Zuclopenthixol decanoate | 5 | 16 | 1 | 4 |
| Haloperidol decanoate | 3 | 10 | 3 | 12 |
| Risperidone | 8 | 27 | 4 | 16 |
| Haloperidol | 3 | 10 | 2 | 8 |
| Sulpiride | 1 | 0 | 4 | 0 |
| Thioridazine | 1 | 3 | 3 | 12 |
Significantly more patients had hyperprolactinaemia and bone loss in the high-compared with the low-dose group: 20 (81%) v. 11 (45%) for hyperprolactinaemia (χ2=7.505, d.f.=1, P<0.01) and 15 (62%) v. 7 (27%) for bone loss (χ2=6.42, d.f.=1, P=0.01), respectively. Similarly, patients in the high-compared with the low-dose group had significantly more severely reduced bone mineral density scores (<2 s.d. below normative data): 9 (35%) and 3 (11.5%), respectively (χ2=4.73, P=0.03).
Bone mass density measures
Overall, 17 (57%) of the men and 8 (32%) of the women had reduced bone mineral density values on at least one bone measure.
Correlational measures
Hormone levels and bone mineral density
The free testosterone index (testosterone/sex-hormone-binding globulin) was correlated with the bone mineral density Z summary lumbar score (see Fig. 1). The summary lumbar score is a DEXA-generated calculation of bone mineral density scores from L1 to L4 for each individual, and thus reflects the total bone density in the lumbar spine. This correlation was not found in the hip region. There was no statistical relationship between prolactin, follicle-stimulating hormone, luteinising hormone, oestradiol, progesterone or thyroid hormones and bone mineral density values.
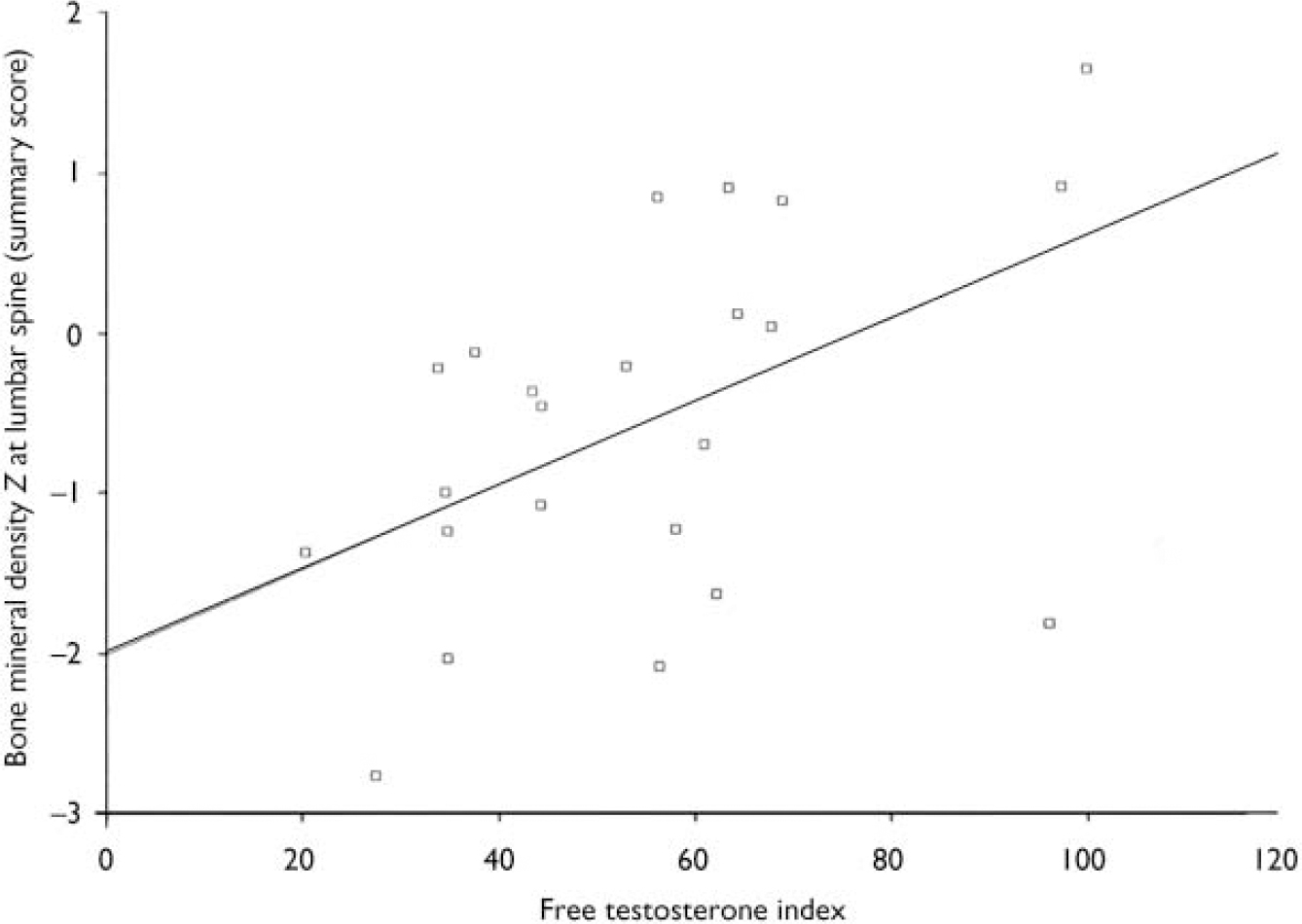
Fig. 1 Scatterplot of summary lumbar bone mineral density Z scores and free testosterone index levels in males. There is a significant correlation between free testerosterone indices in males and the summary lumbar Z scores (r=0.5, P=0.01). Free testosterone index: normal reference range 20-350 nmol/l.
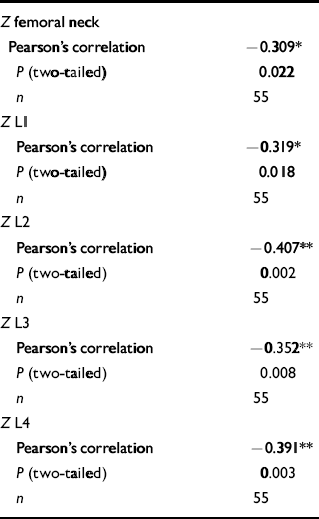
Chlorpromazine equivalents and bone mineral density (see Table 4 and Fig. 2)
There was a significant correlation between chlorpromazine equivalence scores and both lumbar-weighted scores (r=0.4, P=0.002) and combined lumbar- and hip-weighted scores (r=0.35, P=0.009).
Table 4 Correlations between age-matched bone mineral density values and chlorpromazine equivalence scores in the male group
| Z femoral neck | |
| Pearson's correlation | -0.309* |
| P (two-tailed) | 0.022 |
| n | 55 |
| Z L1 | |
| Pearson's correlation | -0.319* |
| P (two-tailed) | 0.018 |
| n | 55 |
| Z L2 | |
| Pearson's correlation | -0.407** |
| P (two-tailed) | 0.002 |
| n | 55 |
| Z L3 | |
| Pearson's correlation | -0.352** |
| P (two-tailed) | 0.008 |
| n | 55 |
| Z L4 | |
| Pearson's correlation | -0.391** |
| P (two-tailed) | 0.003 |
| n | 55 |
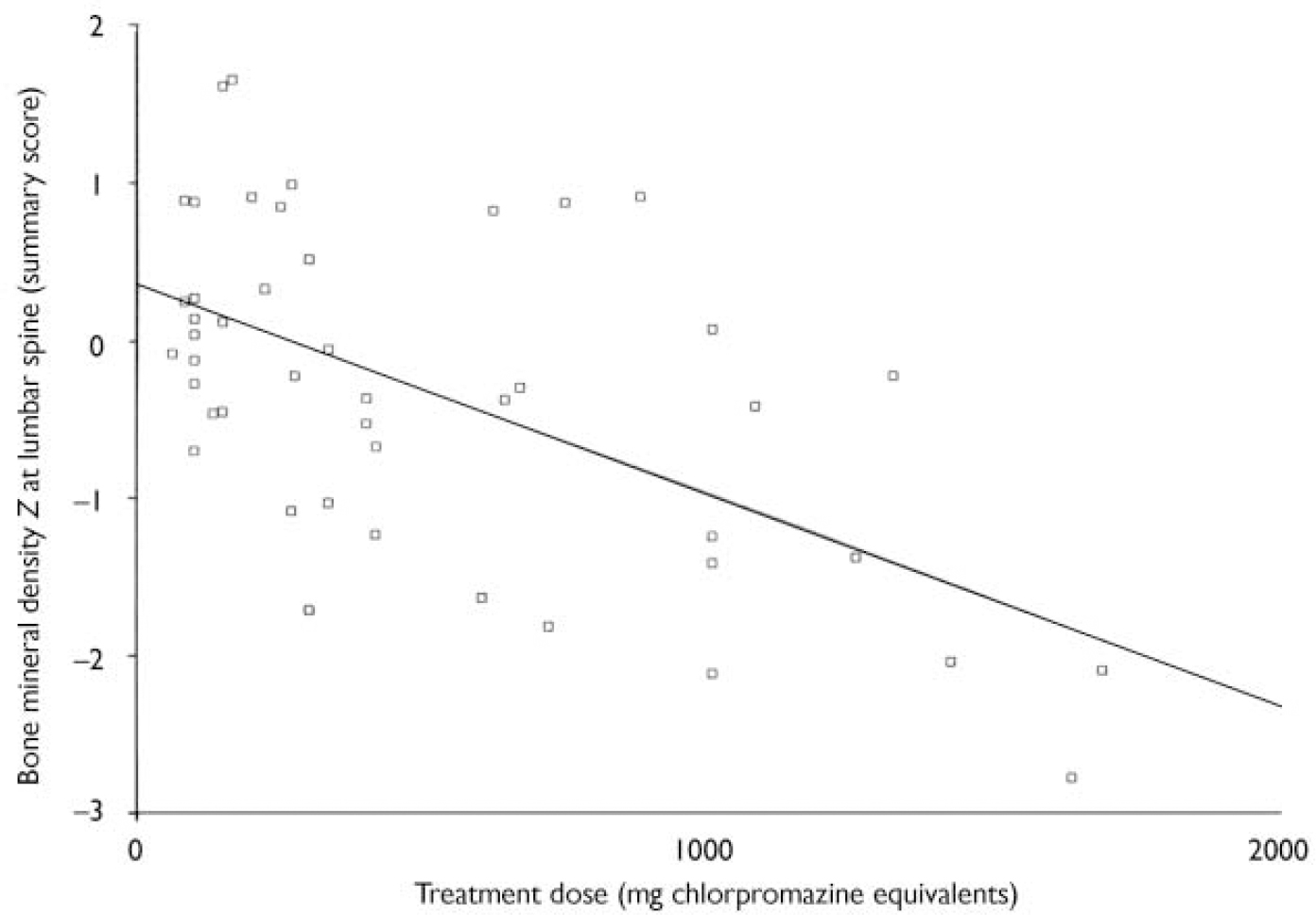
Fig. 2 Scatterplot of summary lumbar bone mineral density Z scores and the dose of antipsychotic in chlorpromazine equivalents. There is a significant correlation between summary lumbar Z scores and the dose of antipsychotic prescribed (r=0.5, P=0.01).
Multivariate regression analysis
In a multivariate regression analysis for both genders combined examining clinical, endocrine and lifestyle variables, only the chlorpromazine equivalence scores were statistically predictive of reduced bone mineral density scores at L1–L4 (P<0.0001). When genders were analysed separately, no further factors were predictive of bone mineral density loss in females. In the male group the free testosterone index was also predictive of low bone mineral density in L1–L4 (P=0.03); and sex-hormone-binding globulin almost reached significance as a predictive value (P=0.06).
DISCUSSION
Main findings
This study was designed to evaluate the long-term effects of prolactin-raising antipsychotic medication on bone metabolism in patients with a diagnosis of schizophrenia. Both male and female groups had high rates of hyperprolactinaemia (62%) and had a range of osteopenic and osteoporotic measures that fell outside the normal age-related values. Age-consistent osteopenia or osteoporosis was found in 57% and 32% of the male and female groups, respectively. High levels of medication were associated with high prolactin levels and reduced bone mineral density scores. In a multivariate analysis for both genders combined, only treatment dose remained associated with bone loss but low free testosterone index values were additionally correlated with bone loss in the male group.
The findings of associations between chlorpromazine equivalents scores and both prolactin levels and low bone mineral density values is novel. Antipsychotic medication blocks the type 2 dopamine (D2) receptors on the lactotrophs of the anterior pituitary, thus releasing these cells from dopamine inhibition and consequently suppression of prolactin secretion (Reference Dickson, Seeman and CorenblumDickson et al, 2000). One positron emission tomography study has demonstrated that hyperprolactinaemia is likely to occur when central D2 receptor occupancy exceeds 72% (Reference Kapur, Zipursky and JonesKapur et al, 2000). This physiological process may underlie the strong statistical relationship between chlorpromazine equivalence scores and prolactin levels. Any potent D2-blocking antipsychotic, whether ‘typical’ or ‘atypical’, is likely to cause prolactin secretion and thus all patients in this study taking the antipsychotic risperidone had hyperprolactinaemia, in keeping with the established prolactin-raising profile of this drug (Reference Kinon, Gilmore and LiuKinon et al, 2003).
Bone strength is largely determined by calcium content and rate of bone loss, which in turn is determined by genetic factors, weight, ethnicity, diet, exercise, hormone status and gender (Reference Hobson and EalstonHobson & Ealston, 2001). Although the physiological processes mediating bone loss are complicated, the most common cause of bone loss is sex hormone deficiency (Reference Schlecter, Sherman and MartinSchlecter et al, 1983; Reference Davies, Gulekli and JacobsDavies et al, 1995). The bone loss associated with hyperprolactinaemic states, whether prolactin is elevated pathologically or physiologically, is probably mediated by secondary hypogonadism, although other mechanisms have been proposed (see Reference Wieck and HaddadWieck & Haddad, 2003). Prolactin interferes with the pulsatile secretion of gonadotrophin-releasing hormone from the hypothalamus and may also have a peripheral effect at the level of the gonads, inhibiting some luteinising hormone- and follicle-stimulating hormone-mediated effects.
The association of low free testosterone index with osteoporosis in this study supports hyperprolactinaemic-induced hypogonadism as being the relevant pathological process in the male patients because the association was present in males only in the gender-specific multivariate regression analysis. Free testosterone index values represent the biologically active, or unbound, part of the circulating testosterone. Bone loss was associated with the free testosterone index in the lumbar spine region only, and not the hip joint. The absence of bone loss in the hip joint in this relatively young male sample is in keeping with the fact that bone loss typically first occurs in the spine region (in 92% of individuals).
The female group was post-menopausal and thus uniformly hypogonadal, preventing an a priori hypothesis of an association between gonadal status and bone measures to be tested. A previous study by our group found that 75% (n=15) of a group of premenopausal females with a diagnosis of schizophrenia on prolactin-raising antipsychotics were hyperprolactinaemic and high prolactin levels were negatively correlated with both oestradiol and progesterone levels (Reference Smith, Wheeler and MurraySmith et al, 2002). A recently published study has also reported associations between high prolactin levels and low key reproductive hormone levels in males and females on antipsychotic medication (Reference Kinon, Gilmore and LiuKinon et al, 2003).
Osteoporosis and schizophrenia
Decreased bone mineral density in psychiatric patients has been recognised for some time but only recently investigated. Cases of osteoporosis have been reported in women and men with anorexia nervosa, chronic alcoholism, depression and schizophrenia (Reference Delva, Crammer and JarzyloDelva et al, 1989; Reference Abraham, Friedman and VergheseAbraham et al, 1995; Reference Halbreich, Rojansky and PalterHalbreich et al, 1995). Halbreich et al (Reference Halbreich, Rojansky and Palter1995) reported that patients, particularly males with major depressive disorder, schizophrenia, schizoaffective disorders, mania and adjustment disorders, had significantly decreased bone mineral density. Original reports of osteoporosis in people with schizophrenia suggested an association with polydipsia (Reference Delva, Crammer and JarzyloDelva et al, 1989) but we found no correlation between polydipsia and reduced bone mineral density. Bilici et al (Reference Bilici, Cakirbay and Guler2002) reported increased rates of reduced lumbar spine bone mineral density scores in a group of patients with schizophrenia taking ‘classical’ antipsychotic medication compared with a group on atypical antipsychotics and a healthy control group. That study did not examine relative prolactin levels in the groups but all the patients in the ‘classical’ antipsychotic group were on prolactin-raising agents, whereas 38% in the atypical group (n=15) were on prolactin-sparing agents. Previous studies have not specifically addressed a possible association between osteoporosis and antipsychotic-induced hyperprolactinaemia, but more recent reviews have focused on the possibility of such a relationship (Reference Naidoo, Goff and KlibanskiNaidoo et al, 2003; Reference Wieck and HaddadWieck & Haddad, 2003).
Other established risk factors, such as high alcohol intake and cigarette smoking, could possibly account for the high rates of osteopenia/osteoporosis found in this sample with schizophrenia. We did find a correlation between bone mineral density scores in the lumbar spine and smoking, but this significance was lost in the multivariate analysis, suggesting that the medication-related risk factors were more significant. There was no association between alcohol intake and any of the bone measures. Immobility is an unlikely cause of reduced bone mineral density even in individuals with negative symptoms of schizophrenia because activity levels involved in routine daily living are sufficient to retard bone mineral density loss in weight-bearing bones. It is unknown to what degree decreased exposure to sunshine and dietary deficiency contribute to reduced bone mineral density in patients with chronic mental illnesses, but all participants in our study indicated on the osteoporosis questionnaire that they had adequate intake of calcium in their diet. Low body mass index is another established risk factor for bone mineral loss, but all participants in this study were overweight with a mean body mass index bordering on obese.
It is conceivable that bone loss found in these people may be related to a metabolic disorder that is part of the syndrome of schizophrenia rather than the endocrine consequences of antipsychotic medication. This is unlikely, given the findings of significant associations between bone loss measures and medication and endocrine variables, and the absence of associations between clinical symptom scores and bone mineral density measures.
Osteoporosis: morbidity and mortality
According to the World Health Organization, osteoporosis affects 30% of women over the age of 50 years, with the risk of hip fracture increasing 2.6-fold for each standard deviation decrease in hip bone mineral density. Hip fracture complications include a mortality of 20% in one year, with only 25% of patients recovering full function and 25% requiring long-term institutional care (Reference Hobson and EalstonHobson & Ealston, 2001).
The absence of evidence associating prolactin-raising antipsychotic drug use with bone loss may explain why drugs such as glucocorticoids, lithium, anticonvulsants, thyroxine and gonadotrophin-releasing hormone antagonists are listed by the World Health Organization and the Royal College of Physicians as being associated with the development of osteoporosis but antipsychotic drugs are not (World Health Organization Study Group, 1994).
Limitations of this study
The study is limited by the absence of a control group of patients with schizophrenia who were receiving prolactin-sparing antipsychotics. We were unable to recruit the latter group because prolactin-sparing antipsychotics have not been widely in use for the time duration required for this study.
A potential limitation of the study was the collection of blood samples at random times during working hours, given that the secretion of prolactin is subject to a diurnal rhythm. This is not important, however, in the context of either low- or high-grade hyperprolactinaemia because the diurnal rhythm is largely obliterated in both states (Reference Veldman, Frolich and PincusVeldman et al, 2001). In states of normal prolactin secretion, the nocturnal surge accounts for the diurnal variation and levels between 09.00 h and 24.00 h are generally stable.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Patients with schizophrenia on long-term typical antipsychotic medication have high rates of hyperprolactinaemia and BMD loss.
-
▪ Higher doses and dopamine 2 blocking potency of antipsychotic medication predict both hyperprolactinaemia and BMD loss.
-
▪ Patients on long-term prolactin-raising antipsychotics are at high risk of developing osteoporosis, suggesting that these drugs are an unidentified risk factor for this disease.
LIMITATIONS
-
▪ The study did not have a comparison group: either individuals with schizophrenia who had been exposed to prolactin-sparing antipsychotic medication, or a healthy group without psychiatric illness.
-
▪ The high rates of bone loss in this group could have been contributed to by other risk factors prevalent in this population, such as smoking, poor diet and inactivity, or could be related to a metabolic disorder that is part of the syndrome of schizophrenia rather than the endocrine consequences of antipsychotic medication.
-
▪ The female group were post-menopausal, preventing any associations between gonadal status and other variables from being assessed.









eLetters
No eLetters have been published for this article.