Theories of early development and emerging child psychopathology suggest a central role for physiological responsiveness (e.g., Dadds & Frick, Reference Dadds and Frick2019). These theories posit that children’s psychological outcomes are dependent upon individual factors, such as, propensity for autonomic nervous system (ANS) arousal, especially in response to their environment, including caregiver interactions (e.g., El-Sheikh & Erath, Reference El-Sheikh and Erath2011). In this context, ANS arousal is considered a moderator of environmental influences (e.g., parenting conflict, maternal sensitivity, exposure to trauma) on child adjustment and the development of psychopathology (El-Sheikh & Erath, Reference El-Sheikh and Erath2011). Across human and animal studies, the ANS has been shown to be particularly sensitive to early caregiving (Alkon et al., Reference Alkon, Boyce, Tran, Harley, Neuhaus and Eskenazi2014; Hostinar et al., Reference Hostinar, Sullivan and Gunnar2014; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015; Shonkoff et al., Reference Shonkoff, Boyce and McEwen2009; Sroufe, Reference Sroufe2005), and has been implicated in the development of psychopathology (Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006; De Bellis & Zisk, Reference De Bellis and Zisk2014; Shonkoff et al., Reference Shonkoff, Boyce and McEwen2009). The individual differences observed in ANS responsiveness is thought to protect, or amplify, the effects of the environment in the development of psychopathology. Baseline levels of ANS arousal are thought to affect children’s readiness to respond adaptively to environmental stimulus, and ANS activity is thought to indicate sensitivity to environmental changes (El-Sheikh & Erath, Reference El-Sheikh and Erath2011). Moreover, the ANS comprises two neurological subsystems—the sympathetic nervous system (SNS) and parasympathetic nervous systems (PNS)—each of which likely plays a differential role in regulating responsivity (Berntson et al., Reference Berntson, Cacioppo and Quigley1993). To understand an infant’s potential risk for emergent psychopathology, methods are needed to measure an infant’s autonomic reactivity within important environmental contexts (e.g., parent–child interactions).
Laboratory-based stress tasks provide a means for researchers to study infant responsiveness within parent-child interactions in real time (Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018). Those paradigms that are most widely used to manipulate stress levels and examine sensitivity to environmental input in children and adults (such as the Trier Social Stress Test and Startle paradigm) are not appropriate for infants. Thus, the Face-to-Face Still-Face (FF-SF) paradigm is most commonly used to examine sensitivity to environmental input with infants (Adamson & Frick, Reference Adamson and Frick2003; Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). The FF-SF paradigm is considered a mild social stressor, which has been found to elicit an arousal response in infants (Adamson & Frick, Reference Adamson and Frick2003; Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). The FF-SF is thought to elicit infant distress because it involves a violation of social expectations within the caregiver-infant dyad (Tronick et al., Reference Tronick, Als, Adamson, Wise and Brazelton1978). Rather than providing the infant with the expected regulatory emotional scaffolding through movement and changes in facial expressions (Beeghly & Tronick, Reference Beeghly and Tronick1994; Stack & Muir, Reference Stack and Muir1990; Weinberg et al., Reference Weinberg, Beeghly and Olson2008; Weinberg & Tronick, Reference Weinberg and Tronick1996), mothers become nonresponsive to their child, interrupting the infant’s goals for connectedness and social engagement which are typically realized with their mothers (Tronick et al., Reference Tronick, Als, Adamson, Wise and Brazelton1978). The FF-SF paradigm typically involves three phases: A Baseline Free-play where mothers are instructed to play with their infant as they would at home; the Still-Face segment where mothers are asked to become unresponsive to their infant; and a Reunion Play segment where mothers are instructed to resume playing with their infant (Tronick et al., Reference Tronick, Als, Adamson, Wise and Brazelton1978). To date, observational coding during the FF-SF has implied that when mothers become unresponsive to infant cues indicators of stress are evident in newborns (e.g., Bertin & Striano, Reference Bertin and Striano2006) through to children aged up to 2.5-years-old (e.g., Weinberg et al., Reference Weinberg, Beeghly and Olson2008). For instance, during the Still-Face phase children tend to show increased gaze aversion, less smiling, more negative affect, increased motor activity, increased tactile object- and self-stimulation, and less positive affect compared to the baseline free-play phase (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009).
A number of studies have examined whether infants also show psychophysiological indicators of arousal during the FF-SF (see Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018 for a review). These studies have mostly focused on specific PNS activity or nonspecific ANS activity (i.e., aggregating SNS and PNS activity, e.g., heart rate). This literature has reported that infants become more aroused moving from the Baseline Play to Still-Face periods, with evidence for significant increases in heart rate (e.g., Bazhenova et al., Reference Bazhenova, Plonskaia and Porges2001; Conradt & Ablow, Reference Conradt and Ablow2010; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996) and decreases in respiratory sinus arrhythmia (e.g., Bazhenova et al., Reference Bazhenova, Plonskaia and Porges2001; Conradt & Ablow, Reference Conradt and Ablow2010; Ham & Tronick, Reference Ham and Tronick2006; Moore, Reference Moore2009; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996).
Comparatively, few studies have examined SNS activity during the FF-SF (Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006, Reference Ham and Tronick2009; Suurland et al., Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2017) with researchers commonly reporting methodological challenges (e.g., difficulty in obtaining resting state recordings due to excessive movement; likelihood of recording devices being removed during the experiment; see also, Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). Bosquet Enlow et al. (Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014) found there were no groupwise variations in T-wave amplitude across the episodes of the FF-SF. Similarly, Suurland et al. (Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2017) found no groupwise variations across the episodes of the FF-SF in relation to infant preejection period. Ham and Tronick (Reference Ham and Tronick2006) and Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) measured skin conductance and found differences across the FF-SF paradigm. In their pilot study (n = 12), Ham and Tronick (Reference Ham and Tronick2006) found that skin conductance typically increased from the Baseline play to Still-face for 5-month-old infants. Similarly, Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) found that 6-month-old infants demonstrated a significant increase in skin conductance from the Baseline play to Still-face phase (n = 140).
Despite strong agreement on expected patterns of arousal across the phases of the FF-SF task in the behavioral literature (Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009), patterns of arousal—as indexed by psychophysiological measures—have presented a more mixed picture from the still-face to the Reunion play periods across the literature. Findings are less clear from still-face to Reunion Play periods, with some studies examining PNS and nonspecific ANS arousal finding a reregulation effect with increases, on average, in respiratory sinus arrhythmia (e.g., Ham & Tronick, Reference Ham and Tronick2006; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996) and decreases, on average, in heart rate (e.g., Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996). In contrast, other studies examining PNS and nonspecific ANS arousal have found the opposite effects, on average, for respiratory sinus arrhythmia (e.g., Bush et al., Reference Bush, Jones-Mason, Coccia, Caron, Alkon, Thomas and Adler2017; Conradt & Ablow, Reference Conradt and Ablow2010) and heart rate (e.g., Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Conradt & Ablow, Reference Conradt and Ablow2010; Ham & Tronick, Reference Ham and Tronick2006; Moore et al., Reference Moore, Hill-Soderlund, Propper, Calkins, Mills-Koonce and Cox2009). When considering studies examining SNS arousal, the two skin conductance studies found increases, on average, in tonic skin conductance activity between the still-face and Reunion play phases (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006). It is possible that findings may differ according to which measure is being used, the sample employed (e.g., composition of infants with risk status, differing attachment, greater weight-for-length gain; Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018; Rudd et al., Reference Rudd, Alkon, Abrams and Bush2020; Suurland et al., Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2017), and which part of the ANS is being measured. An examination of skin conductance may be preferable over PNS and nonspecific ANS functioning due to the specificity in measurement that skin conductance allows. In particular, skin conductance provides a direct measurement of SNS activity unlike measures of PNS functioning or measures of nonspecific ANS activity that aggregate SNS and PNS activity (i.e., where researchers cannot determine which activity is PNS or SNS functioning). SNS activity is also assumed to index infant arousal during the FF-SF task (Boucsein, Reference Boucsein2012).
Although the two skin conductance studies (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006) found an increase in arousal between the Still-face and reunion phases, on average, these results differ from the extensive behavioral coding of the FF-SF. It is possible that this consistency across the two skin conductance studies indicates that SNS arousal may be experienced even when there are fewer behavioral indications of arousal. However, the skin conductance findings reported by Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) from the Still-Face to the Reunion Play did not show the same pattern of results as their other infant psychophysiological measures that were taken at the same time. This suggests that infants were not at a higher arousal level (as indicated by the other measures). Although it is difficult to say with certainty why this has occurred (i.e., both studies have failed to mention the current circuits for the electrodes used), it is likely that build-up of counter electromotive force was not estimated in Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) or Ham and Tronick (Reference Ham and Tronick2006). Counter electromotive force (e.m.f.) refers to the polarization of cellular membrane potentials in the skin that occurs with direct current electrodes over time (even when using nonpolarizing Ag/AgCl electrode gels; Boucsein, Reference Boucsein2012). Such forces are typically regarded as difficult to estimate due to build-up being nonlinear and varying between individuals (Posada-Quintero & Chon, Reference Posada-Quintero and Chon2020). While this issue has not been explicitly investigated within the electrodermal activity (EDA) literature to-date (Boucsein, Reference Boucsein2012; Grimnes et al., Reference Grimnes, Jabbari, Martinsen and Tronstad2011), it is possible that—particularly in the FF-SF paradigm, which has such a long tradition of behavioral confirmation in the empirical literature—counter e.m.f. build-up contributes to differences between behavioral results and results from the relatively few attempts to apply skin conductance measures. If this hypothesis were true, then the confounding effects of counter e.m.f. would be maximized during the reunion phase of the FF-SF paradigm.
Collectively, research to-date using the FF-SF paradigm demonstrates four important findings: (1) It is possible to examine ANS activity in infants; (2) infants consistently demonstrate increased skin conductance arousal from Baseline play to Still-face phases of the protocol; (3) the pattern of arousal from Still-Face to Reunion phases is inconsistent across the literature; and (4) the potential build-up of e.m.f. has not been appropriately modeled in previous FF-SF studies examining skin conductance. The FF-SF paradigm therefore provides an ideal task to be able to examine ANS arousal in the context of environmental influences (i.e., changes within mother-infant interactions) on child adjustment (i.e., including infants’ degree of recovery during the Reunion phase). Further, infants’ degree of recovery from the Still-face social stressor may be an important marker for the development of psychopathology as children age (Dadds & Frick, Reference Dadds and Frick2019; El-Sheikh & Erath, Reference El-Sheikh and Erath2011).
The current study
The aim of the current study was to examine the SNS psychophysiological indicators of arousal during the FF-SF as detected by changes in skin conductance to achieve a better understanding of patterns of infant reactivity to parent-child interactions in early life. The present study will be the first FF-SF study to account for the potential build-up of nonlinear counter electromotive forces over the course of the task, which is critical to the appropriate investigation of SNS psychophysiological functioning over time when examining skin conductance with DC-based measurement.
This study investigated SNS psychophysiological functioning during the FF-SF with a sample of infants aged 5.4–10.7 (M = 7.4; SD = 0.9) months. Infants within the first year of life were of interest as emerging research has shown that SNS psychophysiological functioning may be shaped by, and regulated by, dyadic caregiver interactions (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019), which may result in cascading developmental psychopathology risk across infancy (Dadds & Frick, Reference Dadds and Frick2019; El-Sheikh & Erath, Reference El-Sheikh and Erath2011). Further, the FF-SF paradigm is typically used within the first year of life, and there is limited movement using the soles of feet during this time, which allows for collection of skin conductance data with limited interfering noise.
In accordance with the extensive FF-SF literature using infant behavioral coding (Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009) and two prior skin conductance studies (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006), it was hypothesized that infants’ skin conductance response will significantly increase between the Baseline play and Still-face phases. In accordance with our novel statistical design, it was hypothesized that infants would show a significant decrease in skin conductance during the Reunion play relative to the Still-face phase, where the impact of potential e.m.f. is expected to be maximized. Support for this second hypothesis would provide evidence that infants’ skin conductance data is consistent with the extensive FF-SF behavioral coding literature. Moreover, such support would highlight the importance of designing and applying statistical models that can flexibly adjust for potential e.m.f. build-up as the only two FF-SF skin conductance studies previously conducted (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006) both found a significant increase in skin conductance during the Reunion Play relative to the Still-Face phase, and, notably, do not employ statistical designs that adjust for potential e.m.f. build-up over the course of the task.
Method
Participants
Mother-infant dyads were drawn from the “Watch Me Grow for REAL” longitudinal birth-cohort study (Doyle et al., Reference Doyle, Mendoza Diaz, Eapen, Frick, Kimonis, Hawes and Dadds2020). The “Watch Me Grow for REAL” study follows 788 mother-infant dyads from a low socioeconomic urban area prospectively from mother’s antenatal appointments (i.e., T0 assessment). This sample was a normative community sample, but was recruited from this area as they were more likely to experience risk factors that might be associated with the development of child psychopathology (e.g., low socioeconomic status). Three hundred and ninety-six dyads attended the Time point 1 (T1) assessment (185 girls, 46.7%). Infants’ age ranged from 5.4 to 10.7 (M = 7.4; SD = 0.9) months. All children were born in Australia. Infants were of European (n = 114, 28.8%), Middle-Eastern (n = 85, 21.5%), South Asian (n = 66, 16.7%), South-East Asian (n = 59, 14.9%), African/African American (n = 22, 5.6%), Polynesian/Melanesian (n = 19, 4.8%), Hispanic/Latino (n = 15, 3.8%), East Asian (n = 10, 2.5%), and Aboriginal/Torres Strait Islander (n = 5, 1.3%) descent, and one child had missing ethnicity data (0.3%). Mothers were aged between 19.0 and 55.5 years (M = 32.0; SD = 5.2). Three hundred and fifty-eight mothers (91.3%) were in a romantic relationship with the infant’s father at T1, with 305 mothers (77.8%) reporting that they were married. Only 155 (39.1%) mothers were born in Australia. Eighty mothers (20.2%) lived in Australia for 0–5 years, 67 mothers (16.9%) lived in Australia for 6–10 years, 34 mothers (8.6%) lived in Australia for 11–15 years, 28 mothers (7.1%) lived in Australia for 16–20 years, and 182 mothers (46%) lived in Australia for 21 years or more (with 5 mothers, 1.3%, having missing data). Mothers’ highest level of completed education (as self-reported at T1) was: Never attending school (n = 1, 0.3%), primary school (n = 6, 1.5%), Grade 10 or equivalent (n = 21, 5.3%), high school (i.e., Year 12 or equivalent; n = 61, 15.4%), technical or vocational qualification (n = 94, 23.7%), undergraduate university degree (n = 145, 36.6%), postgraduate university degree (n = 64, 16.2%), and 4 mothers (1.0%) had missing data. Prior to their child’s birth, mothers reported their typical employment was full time work (n = 169, 42.7%), part time work (n = 47, 11.9%), casual work (n = 48, 12.1%), studying (n = 11, 2.8%), stay-at-home mother (n = 84, 21.2%), unemployed (n = 35, 8.8%), and 2 mothers had missing data (0.5%). According to the Australian Bureau of Statistics’ socioeconomic indices for areas (Index of Relative Socio-Economic Disadvantage), which combines Australian census data on household income, education, and unemployment by postcode, it was found that 209 (52.8%) of the sample lived in an area in the lowest decile; indicating greater disadvantage. Mothers received a $40 gift voucher to thank them for their time while infants were given a “Baby Scientist” certificate for their participation.
Procedure
Mother–infant dyads participated in the T1 laboratory visit for approximately 2 hours (for a full outline of measures at T1 see Doyle et al., Reference Doyle, Mendoza Diaz, Eapen, Frick, Kimonis, Hawes and Dadds2020). During the T1 assessment, two gelled silver–silver chloride (Ag/AgCl) ‘Skintact’ electrodes were applied to both soles of each infant’s foot. Infants’ feet were selected for placement of electrodes because eccrine sweat glands are typically most dense on the hands and feet (Mendes, Reference Mendes2009), and placement on infants’ hands would be more likely to interfere with the FF-SF task, and were not tolerated by infants during pilot testing. Electrodes were then connected to ‘Consensys GSR’ sensors (Shimmer, MA, USA), and a pair of socks was placed over the sensors and infant’s feet to prevent the infants from detaching the electrodes either deliberately or due to artifact. Infants were seated in a raised bouncer chair with their mothers seated on a chair opposite their child. The FF-SF (Tronick et al., Reference Tronick, Als, Adamson, Wise and Brazelton1978) task was commenced to elicit infant’s physiological responses to a mild social stressor involving the following sequence of events: The face-to-face Baseline play (“Baseline Play” phase) was commenced for 240 s for which toys were provided; the researcher then returned to the room gave the instructions to commence the face-to-face “Still-Face” then left the room, and the Face-to-Face “Still-Face” phase was commenced for 90 s; finally, the researcher again returned to the room and a further face-to-face play (“Reunion Play”) phase was commenced for 240 s.
Physiological assessments
Physiological EDA data were continuously collected throughout all phases of the experiment using a sampling frequency of 5 Hz. Phases corresponding with those of the FF-SF paradigm (described above) were time-stamped by researchers using the event marking feature of the ConsensysPRO software package (Shimmer Research Ltd., Dublin, Ireland). EDA data were preprocessed using the Frog (v.0.1.1) package in the R language developed specifically for the “Watch Me Grow for REAL” study. A range of psychophysiological features have been identified in the literature for EDA including skin conductance levels (SCL) and skin conductance peaks per epoch (PPE; see Boucsein, Reference Boucsein2012 for a review). These features were extracted using an unsupervised process provided by the Affective Computing Lab at MIT (Taylor et al., Reference Taylor, Jaques, Chen, Fedor, Sano and Picard2015). The threshold for a skin conductance peak was set to 0.005 μSiemens. Artifacts were identified in 5-s epochs upon the basis of concomitant accelerometer data using a state-of-the-art multiclass classification algorithm from the same toolkit (Hemmelmann, Reference Hemmelmann2018; Taylor et al., Reference Taylor, Jaques, Chen, Fedor, Sano and Picard2015).
Missing data
Of the 396 infant–mother dyads who attended the T1 assessment, 34 (8.6%) dyads did not attempt the FF-SF paradigm because the infant was too fussy, fell asleep before attempting the paradigm, or mothers ran out of time to complete all aspects of the broader T1 assessment. Of the 362 dyads who attempted the FF-SF paradigm, EDA data was not collected for 24 (6.1%) infants (e.g., computer program was not working; application of EDA sensors aborted due to infant distress/fussiness). From those who had data available, a further 83 (20.1%) infants were excluded: 34 infants (66 channels; [16.6% of the full sample]) for whom data were missing (e.g., event markers missing [14%] or erroneous [47%]; accelerometer data missing [39%]); 49 infants (49 channels; [12.3% of full sample]) with mean conductance < 5 μS or > 120 μS by device, indicating defective channels. In total, 255 infants (509 channels; [64.2% of the T1 sample]) were retained for statistical analysis, which is similar to, or better than, rates of data loss reported by past studies measuring skin conductance in infants (Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019).
Sociodemographic differences between those who attended the T1 assessment (n = 396) and those included in the final sample (n = 255; 52.5% girls) were assessed. Infants in the final sample were of Caucasian (n = 80, 31.4%), Middle-Eastern (n = 56, 22.0%), South Asian (n = 42, 16.5%), South-East Asian (n = 34, 13.3%), African/African American (n = 10, 3.9%), Polynesian/Melanesian (n = 12, 4.7%), Hispanic/Latino (n = 11, 4.3%), East Asian (n = 6, 2.4%), and Aboriginal/Torres Strait Islander (n = 3, 1.2%) descent, and one child had missing ethnicity data (0.4%). A generalized linear regression model (GLM) was fit to the data with birth weight, gestation duration, and the number of years the infant’s mother has lived in Australia included as predictors. Although the GLM was found to fit the data well (Hosmer−Lemeshow goodness of fit test, χ2(8) = 13.45, p = .097), all predictors were found to be nonsignificant (all ps > .05). Similarly, no differences were found between groups in child gender using Pearson’s Chi-squared test with Yates’ continuity correction, χ2(1) = 2.42, p = .120, Cohen’s ω = .06. Finally, no differences were found between groups for mothers’ age, Welch’s two-sample t test, t(200.94) = .19, p = .850. These tests were repeated to examine whether there were differences between those initially recruited (N = 788) and those included in the final sample in this study (n = 255); however, again, nonsignificant differences were found (all ps > .05).
Data preparation
For each participant, data were extracted based upon the first event marker (i.e., transition in phase between Baseline Play and Still-Face); truncating recordings for Baseline Play and Reunion Play phases to uniform bin-widths. Data were resampled from 5 to 8 Hz in preparation for peak and artifact detection classification. Preparatory visualizations indicated the data did not conform to linear expectations at the extremes of the data set. To determine whether cohort effects or outlier behavior drove this tendency, individual plots were built for each participant leading to the identification and further exclusion of eight participants (3.1% of inclusions; 2.0% of full sample).
Devices (IDs CD1C and CD2B) were randomly placed on infants’ feet and recorded EDA data throughout the FF-SF. At the time of data collection, it was not recorded which device ID was on each foot for each infant. Further, chirality data was not collected for each participant. Chirality data was not collected as handedness does not stabilize in infants until 18–24 months of age (Michel et al., Reference Michel, Campbell, Marcinowski, Nelson and Babik2016; Nelson et al., Reference Nelson, Campbell and Michel2013) and was not expected to drive the target effects of this study. Thus, for each participant, we had two streams of EDA data which were associated a device ID (i.e. CD1C and CD2B). At the time of data analysis, to examine whether there would be a possibility of an interaction between handedness (unknown) and device ID (known), a simulated exploratory analysis was run. Naïve linear models were used to simulate (n = 10,000) the possible interaction between chirality and the specific instrument applied to the infant for the purposes of recording EDA (i.e. device IDs CD1C and CD2B). During each run of the simulation a single channel from one of the devices was randomly selected from each participant; thus, this approach counterbalances for device ID and controls for sampling bias. Results of this simulation indicated the presence of an instrumental difference of device ID. Device ID was therefore taken into account in subsequent analyses (see Supplementary File 1 for full details).
Statistical Analyses
Statistical analyses were conducted using the R language v3.6.1 (R Core Team, 2021) within the RStudio IDE (RStudio Team, 2020). Full reproducible code and session information are available in Supplementary Files. As range of psychophysiological measures have been identified in the literature for EDA (Boucsein, Reference Boucsein2012), the EDA data from this study were analyzed in two ways to examine whether the pattern of effects was consistent across these two measures (i.e. peak events by phase and tonic SCLs).
First, a one-way ANOVA was employed to analyze PPE data. To examine peak events by phase, post hoc comparisons using Tukey’s HSD test were run.
Second, tonic SCLs were examined. As noted above, our design attempts to estimate the impact of e.m.f. as a potential confound within the tonic SCL data. By treating e.m.f. as an individual difference factor that increases over the duration of the experiment (i.e., as a random effect within a linear mixed effects model), it may be possible to partition variance associated with linear build-up of e.m.f. into a dummy variable whose impact is dynamically subtracted from fixed effects of interest. Accordingly, an adjustment variable was created such that the final sample per phase per person was centered upon zero with preceding samples approaching linearly from the negative direction. An interaction was specified between this adjustment variable and each phase, ranging over the full duration of the experiment. Moreover, by centering these data, intercepts were therefore fit to the end of each phase. This was done to ensure that differences between phase recordings would be maximized and also not be affected by potential SNS latency. Expectations of differences between devices were accounted for by introducing simple contrasts on device ID with the global intercept suppressed. Random intercepts were introduced for each participant with correlated random slopes expressing the same interaction between the adjustment variable and phase as defined in the fixed effects. This model was fit under restricted maximum likelihood (Harville, Reference Harville1977) using the “nlme” package v3.1 (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2007).
Results
Analysis of peak events per epoch
Summary statistics for peak event data are presented in Table 1. Significant differences in mean peak event frequencies by phase were found using a 2 x (Device ID: CD1C, CD2B) between-subjects by 3 x (Phase: Baseline Play, Still-Face, Reunion Play) within-subjects mixed ANOVA with Hyunh−Feldt correction; F(2, 1185) = 220.16, p < .001, partial η2 = .38. Post hoc comparisons using Tukey’s HSD test indicated that the mean peak frequency for the Still-face phase was significantly higher than both the Baseline Play phase (M d = 4.96, p < .001; 95% CI [4.26, 5.66]) and the Reunion Play phase (M d = 3.81, p < .001; 95% CI [3.10, 4.52]). Likewise, the mean peak frequency for the Reunion Play phase was significantly higher than the Baseline Play phase (M d = 1.15, p < .001; 95% CI [0.45, 1.85]). These differences are visualized in Figure 1.
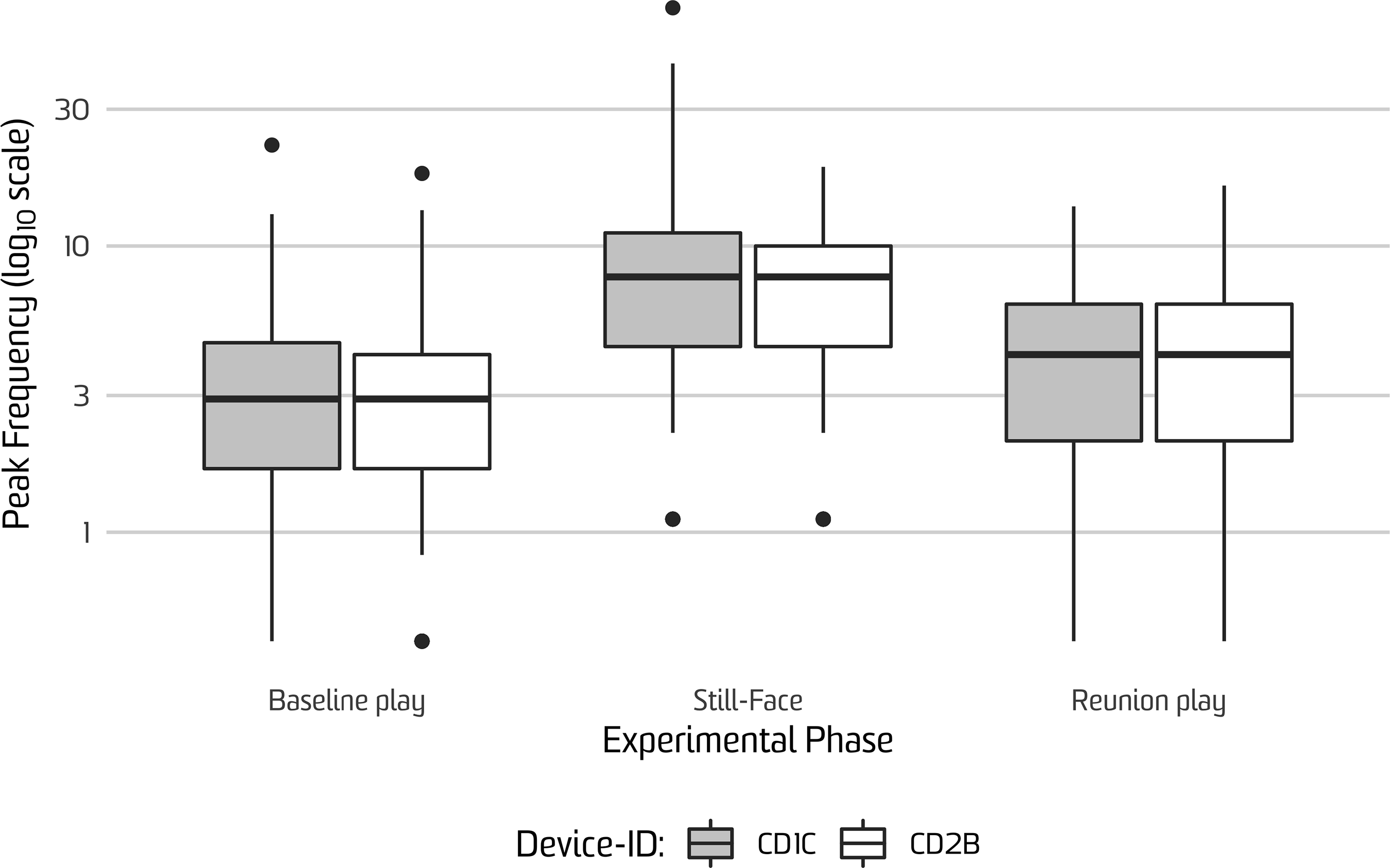
Figure 1. Frequency of infant peak events detected during the Face-to-Face Still-Face paradigm factored by device ID. y-axis reflects the number of peak events detected by phase on log10 scale. “Device ID” refers to the two Shimmer sensors that were each randomly assigned to infants’ feet (CD1C, CD2B).
Table 1. Summary statistics for the time-adjusted frequency of peak events detected by phase and by device ID
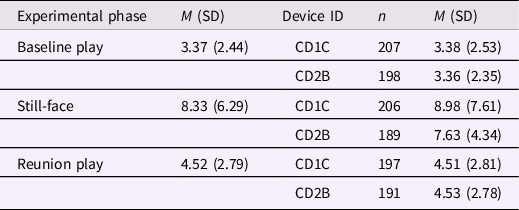
Note. M = mean; SD = standard deviation.
Analysis of tonic skin conductance
Linear mixed-effects modeling was used to examine tonic conductance by phase. Fixed effects for this model are reported in Table 2, with random effects and associated variance-covariance matrix reported in Table 3. A significant positive change in the gradient was observed between the Baseline free-play and still-face phases. Conversely, a significant negative change in the gradient was observed between the Still-Face and Reunion Play phases. The change observed from the Baseline Play to the Still-Face phase supports our experimental predictions that infants experience greater SNS arousal, as measured in skin conductance, with the still-face manipulation, in accordance with conventional results from the behavioral FF-SF paradigm. Concomitant decreases in SNS activity between still-face and Reunion Play phases suggest that mothers play an important role in externally regulating psychophysiological stress in infants. Predictions are further supported by significant differences observed between intercept values between the Reunion Play and Baseline Play phases. Fixed effects for device ID were small but nonetheless significant, vindicating the use of contrasts. Finally, significant interactions between the adjustment variable and both the still-face and Reunion Play phases—though, notably, not the Baseline Play phase—were identified, validating the inclusion of the adjustment variable for the purpose of adjusting for potential electromotive force build-up over the duration of the experiment. It is notable that SEs for the random effects of phase decrease for each consecutive phase while SEs for their interactions with the adjustment variable increase (see again, Table 2). Taken together, these features indicate a strong increase in SCL variability over the course of the experiment.
Table 2. Estimated model fixed effects for infant skin conductance responses during the Face-to-Face Still-Face paradigm at 6 months
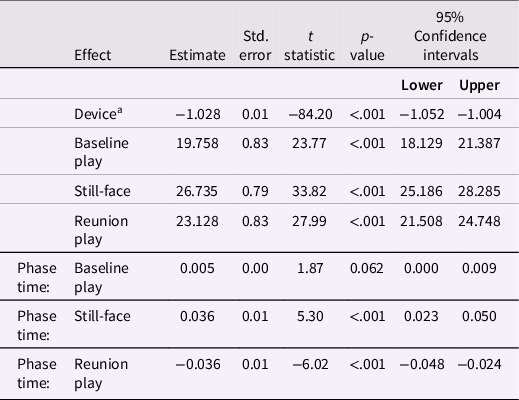
Note. aReference category for device is CD1C. “Phase time” refers to the within-phase adjustment variable.
Table 3. Estimated random effects accompanying with associated variance–covariance matrix

Note. Random effects fitted with positive-definite structure using Log-Cholesky parametrization (Pinheiro & Bates, Reference Pinheiro and Bates1996). “Phase time” refers to the within-phase adjustment variable.
Discussion
We mapped the topography and temporal sequencing of SNS psychophysiological indicators of arousal during the FF-SF as detected by changes in skin conductance with a large and diverse sample of infants. In accordance with our first hypothesis, on average, infants showed a significant increase in SCL between the Baseline Free-Play phase and the Still-Face phase of the FF-SF paradigm. This result held across both the peak events and tonic skin conductance analyses. These findings align with the two previously conducted studies of skin conductance arousal during FF-SF paradigm (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006), as well as what tends to be seen behaviorally in the extensive FF-SF behavioral coding literature (Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). Thus, it would appear that there is robust evidence that during infancy the Still-Face manipulation acts as a universal mild social stressor by disrupting reciprocal engagement between infant and caregiver, as indicated by increased SNS activity. These findings also complement findings on PNS and nonspecific ANS psychophysiological arousal indicators (e.g., respiratory sinus arrhythmia, heart rate) showing increased arousal during the FF-SF (see Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018 for a review).
Supporting our second hypothesis, it was shown that, on average, there was a significant decrease in skin conductance during the Reunion Play when compared to the Still-Face phase. Although we did not examine coded infant behavior in this study, our skin conductance finding aligns with the extensive literature that has coded infants’ behavior during the FF-SF in showing that mothers’ re-engagement in play has a buffering effect in reducing infants’ arousal (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). In particular, behaviorally coded FF-SF data has shown that infants tend to display increased gaze toward their mother, less negative affect, and more positive affect during the reunion phase compared with the still-face phase (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). However, when using various psychophysiological measures (i.e., measures of SNS; PNS; and, nonspecific ANS, which include measures that do not differentiate between SNS and PNS functioning), there are more mixed findings in the literature for changes between the still-face to Reunion Play phases. Consistent with the present study, while some studies have found a re-regulation effect as evidenced by increases in respiratory sinus arrhythmia (e.g., Ham & Tronick, Reference Ham and Tronick2006; Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996) and decreases in heart rate (e.g., Moore & Calkins, Reference Moore and Calkins2004; Weinberg & Tronick, Reference Weinberg and Tronick1996), other studies have found opposite patterns for respiratory sinus arrhythmia (e.g., Bush et al., Reference Bush, Jones-Mason, Coccia, Caron, Alkon, Thomas and Adler2017; Conradt & Ablow, Reference Conradt and Ablow2010), heart rate (e.g., Bosquet Enlow et al., Reference Bosquet Enlow, King, Schreier, Howard, Rosenfield, Ritz and Wright2014; Conradt & Ablow, Reference Conradt and Ablow2010; Ham & Tronick, Reference Ham and Tronick2006; Moore et al., Reference Moore, Hill-Soderlund, Propper, Calkins, Mills-Koonce and Cox2009), and skin conductance (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006) response.
In regards to the specific skin conductance findings, our Still-face to Reunion Play results differ to findings from Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) and Ham and Tronick (Reference Ham and Tronick2006), which found that skin conductance increased, on average, across the FF-SF task. These prior findings, however, may be explained by the lack of explicit statistical control for e.m.f. build-up when using DC electrodes, which this study aimed to overcome (for comparison between models with and without our adjustment variable, see Supplementary File 2). While the overall variance accounted for by our solution was modest (see Table 2), it was nonetheless significant and aligns with the results of the simulation analysis (see Supplementary File 1) indicating a pattern of increased variance across the FF-SF task. Furthermore, the absence of explicit controls for potential e.m.f. build-up in Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) may explain why their skin conductance findings between Still-face and Reunion play did not show the same pattern of results with their other infant psychophysiological measures taken at the same time. It is likely, but less clear, that potential effects of counter e.m.f. explain the pattern of results in Ham and Tronick’s (Reference Ham and Tronick2006) pilot work. Due to the small sample size, it is more challenging to deduce whether sample composition (e.g., composition of infants with risk status, differing attachment, greater weight-for-length gain; Jones-Mason et al., Reference Jones-Mason, Alkon, Coccia and Bush2018; Rudd et al., Reference Rudd, Alkon, Abrams and Bush2020; Suurland et al., Reference Suurland, van der Heijden, Smaling, Huijbregts, van Goozen and Swaab2017) and/or measurement problems (e.g., counter e.m.f. build-up) are influencing the group-wise and sub-group findings. Our present findings highlight the importance of adjusting for potential e.m.f. build-up across time when employing skin conductance measures with direct current circuits in future studies.
Although infants’ arousal reduced from the Reunion Play when compared to the Still-Face phase, arousal remained significantly higher than during the Baseline Play phase. These findings indicate that infants recover once the stressor (i.e., Still-Face) has been removed, and highlights the likely role of self-regulation and maternal co-regulation behaviors (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). It is possible that increasing missingness in the data may have artificially deflated Reunion Play estimates as infants at times fidgeted or “kicked off” electrodes. That is, each successive phase over time had fewer electrodes with active recordings (i.e., infants were more likely to fidget/“kick off” the electrodes over time simply as a matter of opportunity) and therefore there were a greater proportion of channels with missing active recordings and/or poor skin contact during the Still-Face and Reunion Play phases of the experiment, which could have negatively impacted fixed effects estimated for these phases. However, we believe it is unlikely that this issue wholly accounted for our results for two reasons: First, the random effects structure applied in the analysis of SCLs adjusts for the contribution of high variance channels via partial pooling within the random effects structure of our analytic models; second, the tonic skin conductance dynamics match those identified in the analysis of PPE for which no partial pooling was applied by participant or by phase. Nonetheless, it is notable that, to our knowledge, the confound associated with increased data loss over time has not been considered within the EDA literature, particularly in relation to infants for whom the collection of psychophysiological data is especially challenging and artifact and data loss are ineluctable. Indeed, when examining missing data trends across this literature, Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) reported 40%–58% of infants in their sample had missing skin conductance data during each phase of the FF-SF; which was the highest level of missingness across all of the infant psychophysiological measures examined. Similar to the present study, Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) also collected skin conductance data from infants’ feet. Missingness for SCLs in the present study was markedly lower when compared to Busuito et al. (Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) across all phases of the FF-SF paradigm: Baseline Play: 5 (2.0%); Still-Face: 6 (2.4%); Reunion Play: 12 (4.7%). Future research should take care to ensure that infants are fitted with EDA sensors in locations that are least likely to result in disconnection due to fidgeting, infant interference, and/or removal due to expected movement during the FF-SF paradigm (e.g., increased motor activity or increased tactile object- and self-stimulation; Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009). Future research might also seek to identify and include data for missing samples due to such disconnection issues to better allow statistical models to adjust in relation to other parameters (e.g., participant; phase; device ID).
The current findings should be considered in light of some methodological limitations. First, because EDA provides a measure of emotion-related arousal—but not discrete emotional states or associated valence—we are unable to differentiate which discrete emotional states or associated valence were captured with EDA (Collet et al., Reference Collet, Vernet-Maury, Delhomme and Dittmar1997). This is because EDA provides a measure of emotion-related arousal, but not discrete emotional states or associated valence. Second, resting state EDA recordings could not be taken with this infant sample. The primary reason for this was that infants appeared to be mildly uncomfortable following the application of the electrodes associated with the Shimmer sensors (see Figure 2). This meant that any resting state recordings might have been confounded due to infants’ differing capacity to tolerate the application of the electrodes; a limitation shared by most infant studies. Although EDA measures with adult participants typically uses differences between resting state EDA recordings and experimental EDA recordings as a within-subjects dimension of genuine skin conductance responding, in this study differences between the three phases of the FF-SF paradigm were examined. Third, as outlined earlier, a measurement of chirality was not collected because handedness does not stabilize in infants until 18–24 months of age (Michel et al., Reference Michel, Campbell, Marcinowski, Nelson and Babik2016; Nelson et al., Reference Nelson, Campbell and Michel2013). Nonetheless, future research may wish to empirically test the possibility of an interaction between infants’ handedness and the devices used or ensure that devices are counterbalanced across recording site. Finally, this study did not include a measure of infants’ behavior. Extensive previous research, however, has been conducted on infants’ behavioral responding during the FF-SF (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009), with which our psychological findings aligned with typical behavioral patterns. As some studies (e.g., Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019) have not shown concordance between psycho-physiological measures and coded behavior, it is important to note that it is possible that behavior in the present sample may not align with our skin conductance arousal findings nor expected behavioral patterns; for example, some infants in the current sample may have discordant behavioral responses. Future research is needed to clarify if the current sample’s skin conductance aligns with coded behavioral measures within the present sample.

Figure 2. Exemplar EDA recording by experimental phase and device ID, with fixed and random effects overlaid. μS = micro Siemens. Experimental phase axes are measured in seconds. Fixed effects are represented by the red lines; random effects by the blue lines. “Device ID” refers to the two Shimmer sensors that were each randomly assigned to infants’ feet (CD1C, CD2B).
Despite these limitations, this study features some noteworthy strengths. This study was the first study to examine SNS functioning, and specifically SCR, during the FF-SF paradigm with a heterogeneous sample (i.e., low SES; varied ethnicity; larger infant age range; families from culturally and linguistically diverse backgrounds) of mother-infant dyads constituting the largest sample of infants to date (N = 255). This is a notable study strength as the psychological literature is typically dominated by studies of predominantly Caucasian samples, as is previous studies investigating skin conductance during the FF-SF (Busuito et al., Reference Busuito, Quigley, Moore, Voegtline and DiPietro2019; Ham & Tronick, Reference Ham and Tronick2006). Consideration of both autonomic reactivity and environmental context is needed to understand infants’ potential risk for emergent psychopathology. Thus, in order to ensure that SNS responses to the FF-SF paradigm are robust, it is essential that researchers examine infants with a range of risk profiles. A second study strength unique to the present study was that statistical analyses were designed to adjust for the potential e.m.f. build-up across the duration of the paradigm. This statistical approach provides confidence that instrumental factors with respect to the choice of skin conductance electrodes do not account for our findings. Moreover, our results also appear internally reliable as they were consistent across multiple psychophysiological measures of EDA (i.e., PPE and SCLs), and align with what tends to be seen behaviorally in the large body of literature that has examined the FF-SF using behavioral coding (Adamson & Frick, Reference Adamson and Frick2003; Mesman et al., Reference Mesman, van IJzendoorn and Bakermans-Kranenburg2009).
Our results stand to inform conceptualizations of the role that the ANS, particularly its sub-component of the SNS, plays in early life. Across human and animal studies, the ANS has been shown to be particularly sensitive to early caregiving (Alkon et al., Reference Alkon, Boyce, Tran, Harley, Neuhaus and Eskenazi2014; Hostinar et al., Reference Hostinar, Sullivan and Gunnar2014; McLaughlin et al., Reference McLaughlin, Sheridan, Tibu, Fox, Zeanah and Nelson2015; Shonkoff et al., Reference Shonkoff, Boyce and McEwen2009; Sroufe, Reference Sroufe2005), and has been implicated in the development of psychopathology (Anda et al., Reference Anda, Felitti, Bremner, Walker, Whitfield, Perry and Giles2006; De Bellis & Zisk, Reference De Bellis and Zisk2014; Shonkoff et al., Reference Shonkoff, Boyce and McEwen2009). In this study, the FF-SF paradigm provided the ideal context in which to examine infants’ SNS functioning during maternal interactions with a diverse community birth-cohort sample of infants. It was shown that for all infants, on average, the Still-face manipulation was a mild social stressor; yet infants also differed in their relative Baseline free-play skin conductance, as well as the extent to which they experienced SNS arousal to the social stressor. There was strong variability over the duration of the paradigm that might be explained by individual difference factors, for which future research attention is needed. By establishing theoretically important and generalized responses to social interactions, it allows the paradigm to measure individual differences in arousal that may provide insight into which infants are predisposed to mental health problems later in life (Dadds & Frick, Reference Dadds and Frick2019). The strong variability observed over the duration of the paradigm may well be explained by individual difference factors (such as infants’ patterns of reactivity and recovery time), and therefore warrants future research attention. By further examining infants’ physiological responsiveness within dyadic caregiver interactions, researchers may better understand the extent to which infants’ responsiveness protects, or amplifies, the effects of the environment on the development of child psychopathology (Dadds & Frick, Reference Dadds and Frick2019).
In conclusion, an infant’s potential risk for emergent psychopathology, is linked to both autonomic reactivity (e.g., skin conductance) and environmental contexts (e.g., parent-child interactions). EDA data from 7-month-old infants showed that tonic SCLs —as a measure of SNS functioning—significantly increased, on average, during the Still-face and then significantly reduced, on average, during the Reunion Play phase. Infants’ skin conductance during the Reunion Play phase still remained significantly higher than during the Baseline Play phase; indicating that they had not fully recovered from the mild social stressor. Moreover, an identical pattern in SNS responding was found when separately analyzing peak events data. Unlike previous examinations of infants’ skin conductance during the FF-SF paradigm, these findings align with what tends to be seen the extensive behavioral coding literature on the Face-to-Face Still-Face paradigm.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421001553
Acknowledgments
This study was approved by the Human Research Ethics Committees of the South Western Sydney Local Health District and The University of Sydney. We would like to express our gratitude to our “Watch Me Grow for REAL” research assistants (Charlotte Burman, Annie Chapman, Sophie Dickson, Isabel Lopez, Bronte Morgan, Scott O’Loughlin, and Sinia Sareen), our research and clinical students, our “Watch Me Grow for REAL” volunteers, the team at the Ingham Institute, as well as the antenatal and postnatal staff at Liverpool Hospital in Sydney, NSW Australia. The authors acknowledge the technical assistance provided by Sara Taylor from the Massachusetts Institute of Technology’s Affective Computing Lab, Marius Mather from the University of Sydney’s Informatics Hub (SIH), and Peter Humburg from the University of New South Wales’ StatsCentral.
Author contributions
Louis Klein and Frances L. Doyle hold joint first authorship. LK, FD, and JN wrote the first and successive drafts of this manuscript. LK was responsible for data preparation and analysis. All authors contributed to conception and design of the study, and critically revised the manuscript for intellectual content. All authors have read and approved the final manuscript.
Funding statement
This publication is an outcome of the “Watch Me Grow for REAL” study, which is funded by the Australian Government’s National Health and Medical Research Council (NHMRC; APP1127952). The funding body had no role in the study design, interpretation, writing the manuscript, or the decision to submit the paper for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.








