Vitamin K is a cofactor for the vitamin K-dependent carboxylase, a microsomal enzyme that facilitates the post-translational conversion of glutamyl to γ-carboxyglutamyl residues (Esmon et al. Reference Esmon, Sadowski and Suttie1975). Its classic role in this respect involves the synthesis of several coagulation factors, including plasma procoagulants, prothrombin (factor II) and factors VII, IX and X and anticoagulants (proteins C and S) (Shearer, Reference Shearer1990, Reference Shearer2000; Food & Nutrition Board, Institute of Medicine, 2001). The maintenance of plasma prothrombin concentrations was the basis for the recommended dietary intake value of 1 μg/kg body weight per d, set by the National Research Council in 1989 in the USA and by the Department of Health in 1991 in the UK. The current US recommended adequate intakes for vitamin K are 55–75 μg/d for children, which are based on median intakes for children in the third National Health and Examination Survey (Food & Nutrition Board, Institute of Medicine, 2001).
More recently, the identification of γ-carboxyglutamyl-containing proteins in bone, notably osteocalcin and matrix γ-carboxyglutamyl protein (also known as matrix Gla protein), has generated much interest in the role of vitamin K in bone metabolism and bone health (Binkley & Suttie, Reference Binkley and Suttie1995; Vermeer et al. Reference Vermeer, Jie and Knapen1995; Food & Nutrition Board, Institute of Medicine, 2001; Weber, Reference Weber2001). Furthermore, it has been suggested that dietary phylloquinone (vitamin K1) levels that are sufficient to maintain normal blood coagulation may be sub-optimal for adult bone health (Kohlmeier et al. Reference Kohlmeier, Salomon, Saupe and Shearer1996; Vermeer et al. Reference Vermeer, Gijsbers, Craciun, Groenen-Van Dooren and Knapen1996; Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997).
The circulating concentration of under-γ-carboxylated osteocalcin (ucOC), a sensitive marker of vitamin K nutritional status (Sokoll & Sadowski, Reference Sokoll and Sadowski1996), has been reported to be a marker of hip fracture risk and a predictor of bone mineral density (BMD) (Vermeer et al. Reference Vermeer, Knapen, Jie and Grobbee1992; Szulc et al. Reference Szulc, Chapuy, Meunier and Delmas1993, Reference Szulc, Arlot, Chapuy, Duboeuf, Meunier and Delmas1994, Reference Szulc, Chapuy, Meunier and Delmas1996; Jie et al. Reference Jie, Bots, Vermeer, Witteman and Grobbee1996; Booth et al. Reference Booth, Tucker and Chen2000, Reference Booth, Broe, Gagnon, Tucker, Hannan, McLean, Dawson-Hughes, Wilson, Cupples and Kiel2003; Kaneki et al. Reference Kaneki, Hedges and Hosoi2001; Sugiyama & Kawai, Reference Sugiyama and Kawai2001). Moreover, the findings of two large, prospective cohort studies: the Nurses' Health Study (Feskanich et al. Reference Feskanich, Weber, Willett, Rockett, Booth and Colditz1999) and the Framingham Heart Study (Booth et al. Reference Booth, Tucker and Chen2000), support an association between relative risk of hip fracture and phylloquinone intake. Furthermore, Booth et al. (Reference Booth, Broe, Gagnon, Tucker, Hannan, McLean, Dawson-Hughes, Wilson, Cupples and Kiel2003) recently reported that adult women (from the Framingham Heart Study) in the lowest quartile of phylloquinone intake ( < 70 μg/d) had significantly lower (P < 0·005) mean BMD of the femoral neck and spine than did those in the highest quartile of phylloquinone intake (309 μg/d), an association which was not evident in men.
There has been much less research emphasis on the influence of vitamin K on bone health in earlier life. This is despite the fact that there is evidence that phylloquinone intakes in many children/adolescents are below the recommended level (Booth et al. Reference Booth, Pennington and Sadowski1996; Thane et al. Reference Thane, Pyrnne, Ginty, Bolton-Smith, Stear, Jones and Prentice2002; Pyrnne et al. Reference Pyrnne, Thane, Prentice and Wadsworth2005; Collins et al. Reference Collins, Cashman and Kiely2006). Gaining an understanding of the role of vitamin K in bone metabolism and bone mass in early life is important, because finding new strategies to maximize the accretion of bone during growth may help reduce the risk of osteoporosis in later life (Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). Recently, Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004) investigated the effect of vitamin K intake and status on bone turnover and bone mass in healthy young girls (aged 3–16 years) in the USA. This is the first study to examine the relationship between vitamin K nutritive status and bone health in children. Their findings suggest that better vitamin K status (as assessed by plasma phylloquinone and serum percentage of undercarboxylated osteocalcin (%ucOC)) was associated with decreased bone turnover, even though it was not associated with baseline BMC. Furthermore, while serum %ucOC was not associated with 4-year changes in BMC of the hip, total body, or total body minus the head, somewhat surprisingly, serum %ucOC was positively (P = 0·001) associated with 4-year changes in lumbar spine BMC. The girls in the Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004) study, however, were aged 3–16 years, representing a broad span in terms of skeletal and sexual maturity.
Therefore, the objective of the present study was to investigate the relationship between serum %ucOC, as an index of vitamin K status, and BMC and biochemical indices of bone turnover in 223 Danish girls, aged 11–12 years. This peri-pubertal stage is a dynamic period of bone development, and as such, may represent an important window of opportunity for vitamin K status to modulate childhood bone health.
Subjects and methods
Subjects
A total of 225 Danish girls, aged between 10·9 and 11·9 years, were recruited for participation in a 12-month vitamin D intervention trial on bone health (as part of the Optimal Strategy for Vitamin D Fortification (OPTIFORD) project; www.optiford.org) and the BMC and urinary pyridinium crosslink data reported in the present work were the baseline values from that intervention study. Subjects were recruited using information from the Danish National Central Offices of Civil Registrations, which allowed us identify the names and addresses of all girls born in Denmark with Danish citizenship, aged 10·0–11·0 years, living in the municipals of Copenhagen and Frederiksberg (n 1755). In order to minimize age differences at inclusion, the oldest subjects were recruited first and we continued until 225 had agreed to participate, corresponding to 22 % of the 1009 who were asked to participate by mail. Of the 225 girls participating in the intervention trial, serum %ucOC data (as determined by assessment of serum total osteocalcin and ucOC) were successfully derived for 223 girls and these were included in the present work. The study was approved by the Research Ethical Committee of Copenhagen and Frederiksberg (J.nr (KF) 01-129/01). Informed consent was obtained from the parent/guardian of each participant. Girls were excluded on the basis of the following criteria: chronic diseases; intake of drugs that could influence bone metabolism; daily intake of calcium supplements or vitamin/mineral supplements
Design
This was an association study which examined the relationship between bone status indices (BMC and biochemical markers of bone turnover) and %ucOC, as a widely used marker of vitamin K status (Sokoll et al. Reference Sokoll, Booth, O'Brien, Davidson, Tsaioun and Sadowski1997), in a cohort of healthy young Danish girls, aged 10·9–11·9 years. Subjects were instructed to collect urine samples between 08.00 and 10.00 hours after an overnight fast. In addition, after an overnight fast, a blood sample (10 ml) was also taken between 08.00 and 10.00 hours from each subject. On the same day as blood and urine sampling, each girl, with the help of their parent/guardian and a trained researcher, completed various questionnaires, including a general health and lifestyle questionnaire, a FFQ, a physical activity questionnaire and a pubertal status questionnaire. Anthropometric measurements (weight and height) were also taken at this time.
Assessment of dietary calcium and vitamin D
The subjects filled in, together with their parents/guardians, a standardized FFQ with twelve questions covering nine food items that ascertained the foods (including fortified foods) contributing to 95 % of the vitamin D intake and 75 % of the calcium intake determined from the most recent dietary intake studies in Denmark. The questionnaire had nine predetermined possible frequencies (ranging from ‘less than one time per month’ to ‘4–5 times per day or more’). A trained dietitian checked the completed FFQ, and requested additional information or clarification on entries, if necessary. The Danish Institute for Food and Veterinary Research performed the intake calculations using the General Intake Estimation System, a system developed at the Institute (Christensen, Reference Christensen2001). The FFQ and calculations used have been reported earlier (Andersen et al. Reference Andersen, Molgaard and Skovgaard2005). Unfortunately, as the FFQ was tailored for calcium and vitamin D, it could not be used to estimate phylloquinone intakes.
Pubertal status
The stage of puberty was assessed in each subject ad modum Tanner (Marshall & Tanner, Reference Marshall and Tanner1969) by a self-assessment protocol based on the evaluation of breast development by pictures and timing of menarche (J. Müller et al. unpublished results).
Physical activity
Physical activity was recorded using a 24 h recall questionnaire (Habitual Activity Estimation Scale; Boucher et al. Reference Boucher, Lands, Hay and Hornby1997), as used previously in young Danish girls (Molgaard et al. Reference Molgaard, Thomsen and Michaelsen2001). In brief, the Habitual Activity Estimation Scale questionnaire records the hours per day spent on each of four activity levels corresponding to supine position (I), sitting (II), walking (III) and running (IV). In the current study, hours per day spent on high activity (level IV) was used as a measurement for activity level, as this has been shown to correlate well with mean daily activity computer (Caltrac) measurement (counts/d) during a week (7 d; Molgaard et al. Reference Molgaard, Thomsen and Michaelsen2001). Activity was recorded during 2 week days and one weekend day and the mean of the 3 d activity (level IV) was used in the analyses.
Bone mineral density and bone mineral content
Bone mineral content (BMC; measured in g hydroxyapatite), bone size (expressed as anterior–posterior projected bone area (BA); measured in cm2) and BMD (BMD = BMC/BA in g/cm2) as well as percentage fat and lean body mass content were measured by dual-energy X-ray absorptiometry using the Hologic 1000/W scanner (Hologic Inc., Waltham, MA, USA). The skeletal sites assessed were the whole body and the lumbar spine (L2–L4). Subjects wore only underpants and a cotton T-shirt during the scan. For whole body and lumbar analyses, software version V5.73 and V4.76P, respectively, were used. For quality control, a spine phantom was scanned daily. The CV for the BMC, BMD and BA measurements on the spine phantom over a period of 2 years (n 429) was 0·35, 0·32 and 0·32 %, respectively. In adults examined at 8-week intervals reproducibility, expressed as CV, was 1·6, 2·2, 0·9, 2·3 and 1·9 % for whole body BMC, BA, BMD, percentage fat and lean body mass, respectively (D Hansen & A Astrup, unpublished results). The effective dose for a whole body and a lumbar dual-energy X-ray absorptiometry scan was not more than 10 μSv in total, equal to about daily background radiation in Denmark.
Collection and preparation of samples
Subjects were supplied with suitable collection containers for urine samples and asked to collect morning void urine samples between 08.00 and 10.00 hours after an overnight fast. Portions of urine were stored at − 20°C from the morning of collection until required for analysis. Blood was collected by venepuncture into vacutainer tubes with no additive and were processed to serum, which was immediately stored at − 80°C until required for analysis.
Experimental techniques
Serum total and undercarboxylated osteocalcin
Total (intact) osteocalcin levels, a biomarker of bone formation, were measured in serum samples using an ELISA (Metra™ Osteocalcin EIA Kit, Quidel Corporation, CA, USA). The intra- and inter-assay CV were 6·0 and 7·6 %, respectively. ucOC (a measure of vitamin K status) was measured using the method of Yan et al. (Reference Yan, Zhou and Greenberg2004), which is a modification of that described by Gundberg et al. (Reference Gundberg, Nieman, Abrams and Rosen1998). In brief, 60 μl of each serum sample was treated with 30 μl hydroxyapatite (15 mg/ml; Calbiochem, Merck Biosciences, Bestor, Nothingham, UK). Samples were shaken for 1 h at room temperature and then centrifuged for 5 min. ucOC in the supernatants was quantified using the Metra™ Osteocalcin EIA Kit. ucOC was expressed as the percentage of total osteocalcin (%ucOC). The intra- and inter-assay CV were 9·5 and 12·8 %, respectively.
Serum 25-hydroxyvitamin D
Serum 25-hydroxyvitamin D (25 (OH) D) levels were measured by a HPLC-based method, as described in detail elsewhere (Andersen et al. Reference Andersen, Molgaard and Skovgaard2005).
Urinary creatinine, pyridinoline and deoxypyridinoline
Creatinine was determined in urine samples using a diagnostic kit (Metra™ Creatinine Assay Kit, catalogue no. 8009, Quidel Corporation). Urine samples were analysed in duplicate for total pyridinoline (Pyr) and total deoxypyridinoline (Dpyr), biochemical markers of bone resorption, using an automated HPLC system, as described in detail elsewhere (Doyle et al. Reference Doyle, Jewell, Mullen, Nugent, Roche and Cashman2005). Urinary Pyr and Dpyr were expressed on a creatinine basis.
Statistical methods
The distribution of %ucOC and concentrations of urinary pyridinium crosslinks were skewed to the right, so the data were converted to the natural logarithm prior to analysis. Multiple regression analysis was used to assess the association between the biochemical indicator of vitamin K status (serum %ucOC) and biochemical markers of bone resorption (urinary Pyr and Dpyr) and bone formation (serum total osteocalcin). Covariates that were considered in these models included age, Tanner stage, height, weight, dietary calcium intake, vitamin D status and physical activity because these factors could theoretically affect the concentrations of the markers of bone turnover. Covariates were kept in the regression models for a given marker if they were significantly associated (P < 0·05) with that bone marker for serum %ucOC. Multiple regression analysis was used to assess the association between serum %ucOC and BMC of the total body and lumbar spine. Because of the known size-related effects on estimates of bone density by dual-energy X-ray absorptiometry, particularly in children (Nelson & Koo, Reference Nelson and Koo1999), we chose to use BMC as the outcome variable and statistically adjust for BA, by using the natural logarithm of both, as recommended by Prentice et al. (Reference Prentice, Parsons and Cole1994). Potential covariates that were considered in the regression models of BMC included BA, age, Tanner stage, height, weight, dietary calcium intake, vitamin D status and physical activity. For all BMC analyses, regression models were first run with all relevant covariates, and then a reduced model that included only those variables that were associated with the BMC outcome at P < 0·05 was fitted. The relationships between serum %ucOC and serum total osteocalcin, between serum %ucOC and 25 (OH) D, as well as between urinary pyridinium crosslinks and serum total osteocalcin, were assessed using Pearson's correlation coefficients. Differences in serum %ucOC and 25 (OH) D among seasons were assessed using one-way ANOVA. P < 0·05 was considered significant.
Results
Descriptive characteristics of the girls and values for biochemical measures of vitamin K and D status as well as bone turnover are given in Table 1. The median (mean) serum 25 OH D concentration and %ucOC were 41·9 (mean 43·2) nmol/l and 21·9 (mean 22·2) %, respectively, but there was a wide variation in both status measures (8·5–93·1 nmol/l and 4·5–58·4 %, respectively).
Table 1 Subject characteristics and values for biochemical measures of vitamin K status and for bone turnover
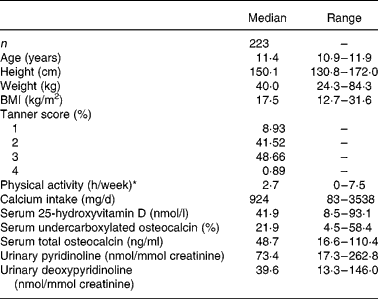
* Hours per day spent on high activity (level IV) was used as a measurement for activity level, as this has been shown to correlate well with mean daily activity computer (Caltrac) measurement (counts/d) during a week (7 d).
Serum %ucOC was not significantly correlated with serum total osteocalcin concentration (r − 0·085, P = 0·206). Serum %ucOC was inversely correlated with serum 25 OH D concentration (r − 0·143, P = 0·035). There was a significant seasonal variation evident in serum 25 OH D concentration (P < 0·0001) and serum %ucOC (P < 0·05). Serum total osteocalcin was positively correlated with urinary Pyr (r 0·119, P = 0·049) and urinary Dpyr (r 0·155, P < 0·026). As expected, urinary Pyr and Dpyr were strongly correlated (r 0·768, P < 0·0001).
We examined the relation between %ucOC and markers of bone turnover and BMC of lumbar spine and total body (Table 2). There was no association between serum %ucOC and serum total osteocalcin, and between serum %ucOC and urinary Pyr and Dpyr, even after adjusting for covariates. Serum %ucOC was inversely associated with BMC of the lumbar spine and total body, when adjusted for BA, pubertal maturation, weight, vitamin D status or dietary calcium.
Table 2 Associations between serum percentage undercarboxylated osteocalcin (%ucOC) and biochemical markers of bone turnover and bone mineral content (BMC) (n 223)*
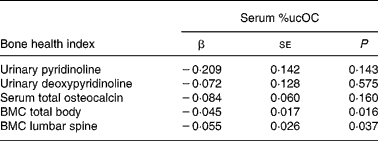
* β, Regression coefficients were generated from natural log-transformed data. Direct interpretation of the coefficients requires back transformation to original units. All regression models included pubertal maturation, weight, vitamin D status, dietary calcium and bone area (for BMC relationships).
Discussion
Interest in the role of vitamin K nutritive status in childhood bone health has heightened in recent times with the findings that better vitamin K status (plasma %ucOC and phylloquinone concentrations), if not dietary intake, was related to decreased bone turnover in healthy girls, aged 3–16 years (Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). A reduction in the rate of bone turnover, arising from better vitamin K status, would usually correspond to greater bone mass; however, this was not found to be the case in the study by Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004). In fact, that study showed inconsistent, and somewhat unexpected, associations between vitamin K status and BMC. For example, while plasma %ucOC or phylloquinone were, in general, not associated with baseline BMC, plasma phylloquinone was inversely (P = 0·03) associated with lumbar spine BMC (Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). Furthermore, while plasma %ucOC was not associated with 4-year changes in BMC of the hip, total body, or total body minus the head, it was positively (P = 0·001) associated with 4-year changes in lumbar spine BMC (Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). In contrast, the present study showed that better vitamin K status, as indicated by lower %ucOC, was indeed positively related to BMC of the total body and lumbar spine in a group of 11–12-year-old girls.
The reasons for the divergent findings between the two studies are unclear, but may be related to the differences in age-profile of the study participants. The subjects in the study by Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004) were aged 3–16 years, representing girls in a number of distinct phases of sexual and bone maturity, whereas the girls in the present study were 11–12 years, and, for the most part, were peri-pubertal. The rate of increment in BMD can be very pronounced during this phase of early life (Theintz et al. Reference Theintz, Buchs and Rizzoli1992), and, as such, might represent a period during which the impact of differences in vitamin K status on childhood bone mass may be more evident. Another notable distinction between the two studies was the inclusion and omission of data on vitamin D status of the girls in the regression models in the present study and that of Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004), respectively. Of note, we found that serum %ucOC was inversely correlated with serum 25 (OH) D levels, the most widely used marker of vitamin D status. This is in line with a similar relationship reported in elderly women (Szulc et al. Reference Szulc, Chapuy, Meunier and Delmas1993). Whether the relationship between vitamin D status and serum %ucOC is causal has not been investigated; however, Szulc et al. (Reference Szulc, Chapuy, Meunier and Delmas1993) showed that vitamin D plus calcium supplementation significantly reduced the %ucOC in elderly women. Furthermore, serum %ucOC as well as serum 25 OH D concentrations exhibited clear seasonal variation (with lowest and highest values, respectively, evident in late summer/early autumn) in these elderly women and in the young adolescent girls in the present study. Thus, while more research is needed to define better the relationship between vitamin D and K status, vitamin D status could be an important confounder in studies which investigate the role of vitamin K in bone health. Vitamin D status has important effects on bone health. Moreover, data from a number of studies in adolescents provide evidence of a possible adverse effect of low vitamin D status for bone health in early life (Outila et al. Reference Outila, Karkkainen and Lamberg-Allardt2001; Lehtonen-Veromaa et al. Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari2002; Cheng et al. Reference Cheng, Tylavsky and Kroger2003). For example, in a 3-year longitudinal study of Finnish girls aged 9–15 years, Lehtonen-Veromaa et al. (Reference Lehtonen-Veromaa, Mottonen, Nuotio, Irjala, Leino and Viikari2002) found that baseline 25 (OH) D levels were positively correlated with unadjusted 3-year change in BMD at the lumbar spine (r 0·35; P < 0·001) and femoral neck (r 0·32; P < 0·001). Viljakainen et al. (Reference Viljakainen, Natri, Kärkkäinen, Huttunen, Palsae, Jakobsen, Cashman, Molgaard and Lamberg-Allardt2006) recently showed that vitamin D supplementation of adolescent Finnish girls, mean age 11·4 years, for 12 months significantly increased BMC of femora and vertebrae. Indeed, there may be an interaction between vitamin K and D in relation to production of fully functional osteocalcin (Shearer, Reference Shearer1997), with possible consequences for bone health. Additionally, while the present study assessed indices of vitamin K and bone turnover in fasting serum samples, Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004) used non-fasting serum samples.
The association between vitamin K status and BMC in adolescent girls is in line with similar findings in adults. For example, the circulating concentration of ucOC has been reported to be a marker of hip fracture risk and a predictor of BMD in adults (Vermeer et al. Reference Vermeer, Knapen, Jie and Grobbee1992; Szulc et al. Reference Szulc, Arlot, Chapuy, Duboeuf, Meunier and Delmas1993, Reference Szulc, Chapuy, Meunier and Delmas1994, Reference Szulc, Chapuy, Meunier and Delmas1996; Jie et al. Reference Jie, Bots, Vermeer, Witteman and Grobbee1996; Booth et al. Reference Booth, Tucker and Chen2000, Reference Booth, Broe, Gagnon, Tucker, Hannan, McLean, Dawson-Hughes, Wilson, Cupples and Kiel2003; Kaneki et al. Reference Kaneki, Hedges and Hosoi2001; Sugiyama & Kawai, Reference Sugiyama and Kawai2001). Improving vitamin K status has been shown in some (Binkley et al. Reference Binkley, Krueger, Engelke, Foley and Suttie2000; Booth et al. Reference Booth, Lichtenstein, O'Brien-Morse, McKeown, Wood, Saltzman and Gundberg2001), but not all (Braam et al. Reference Braam, Knapen, Geusens, Brouns, Hamulyak, Gerichhausen and Vermeer2003), intervention studies to reduce the levels of markers of bone turnover in adults, which might explain the effects of vitamin K status on bone mass. However, serum %ucOC was not related to biochemical markers of bone turnover in the present study, in contrast to the findings of Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004). The reasons for these divergent findings are unclear. One possibility is that, as suggested by Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004), bone markers may reflect relatively acute effects of vitamin K, whereas the observed relations between serum %ucOC and BMC may reflect more longer-term effects on bone.
Unfortunately, we were unable to estimate the habitual phylloquinone intake in the adolescent girls in the present study due to limitations in the dietary assessment tool used in the study. However, dietary phylloquinone has not been found to be a good predictor of bone turnover or BMC in healthy girls (Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004). Not withstanding the lack of dietary intake data, the girls in the present study had a relatively high degree of undercarboxylation of serum osteocalcin (median 21·9 %), which is within the range reported for healthy girls from other studies [13·6 % in the USA (aged 3–16 years; Kalkwarf et al. Reference Kalkwarf, Khoury, Bean and Elliot2004) and 33·9 % in Ireland (aged 11–12 years; unpublished results from our laboratory)]. There is a major difference, however, between estimates of circulating %ucOC levels in healthy children and in children at risk of vitamin K deficiency. For example, Conway et al. (Reference Conway, Wolfe, Brownlee, White, Oldroyd, Truscott, Harvey and Shearer2005) recently showed that 11-year-old children with cystic fibrosis had a median %ucOC of 71 %, which may be more related to vitamin K malabsorption. However, some caution is warranted when comparing the results of %ucOC from studies which use different methods of assessing ucOC. For example, assays based on antibodies against ucOC tend to overestimate %ucOC compared with its measurement with hydroxyapatite binding assays (Gundberg et al. Reference Gundberg, Nieman, Abrams and Rosen1998). While our studies (of Danish and Irish adolescent girls) and that of Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004) used the hydroxyapatite binding assay, that of Conway et al. (Reference Conway, Wolfe, Brownlee, White, Oldroyd, Truscott, Harvey and Shearer2005) used the immunoassay.
In conclusion, there is evidence that phylloquinone intakes in many children/adolescents are below the recommended level (Booth et al. Reference Booth, Pennington and Sadowski1996; Thane et al. Reference Thane, Pyrnne, Ginty, Bolton-Smith, Stear, Jones and Prentice2002; Pyrnne et al. Reference Pyrnne, Thane, Prentice and Wadsworth2005; Collins et al. Reference Collins, Cashman and Kiely2006). The findings of the present study, and that of Kalkwarf et al. (Reference Kalkwarf, Khoury, Bean and Elliot2004), suggest potentially adverse effects of low vitamin K status for bone health indices, however, they do not provide evidence of cause and effect. Therefore, there is a need for well-designed, randomized phylloquinone supplementation trials in children and adolescents to confirm these epidemiological findings of an association between vitamin K status and bone health.
Acknowledgements
This work was supported in part by funding made available under The European Commission Fifth Framework Programme (OPTIFORD, contract no. QLK1-CT-2000-00 623) and the National Development Plan 2000–2006, Dublin, Ireland with assistance from the European Regional Development Fund. The authors thank Birgitte Hermansen for performing most of the practical work in the study. There are no conflicts of interest.




