INTRODUCTION
Injection sites are susceptible to a wide range of bacterial infections; as result of poor hygiene injecting drug users (IDUs) are particularly vulnerable to such infections. These bacterial infections can result in illnesses ranging from localized infections of the skin and soft tissues, such as abscesses and cellulitus, to systemic and toxin-producing infections, such as endocarditis and botulism [Reference Cherubin and Sapira1, Reference Del Guidice2]. These infections can be serious, requiring costly in-patient intervention and can also lead to death [Reference Cherubin and Sapira1, Reference Del Guidice2]. The prevalence of recent or current skin and soft tissue infections in IDUs has been reported to be one in ten in Vancouver and Sydney [Reference Lloyd-Smith3, Reference Salmon4]; however, other studies from North America and in Europe indicate prevalence can be as high as one in three [Reference Takahasi5–Reference Hope8]. In North America these infections are the most common diagnoses in IDUs presenting to emergency departments [Reference Kerr9, Reference Palepu10].
Injection site infections have been associated with poor hygiene and unsafe injection practices including: inadequate cleaning of the hands or the injection sites [Reference Murphy11–Reference Dwyer13]; needle and syringe re-use [Reference Hope8, Reference Murphy11]; frequent injection [Reference Hope8, Reference Topp14–Reference Darke, Ross and Kaye16]; subcutaneous injection [Reference Biswanger6, Reference Murphy11]; the injection sites used [Reference Hope8, Reference Dwyer13, Reference Darke, Ross and Kaye16]; the drugs injected [Reference Hope8, Reference Murphy11, Reference Topp14, Reference Spijkerman, van Ameijden and Mientjes15]; and drawing blood back into the syringe repeatedly [Reference Kerr9]. Higher levels of these infections have also been reported to be associated with environmental factors, including poor housing and homelessness [Reference Lloyd-Smith3, Reference Salmon4, Reference Dwyer13], and with gender [Reference Lloyd-Smith3, Reference Salmon4, Reference Hope8, Reference Topp14] and source of income [Reference Salmon4].
Since the start of the decade there has been increasing concern in the UK about the extent of bacterial infections in IDUs [17–Reference Irish19]. There has been a marked rise in hospital admissions by drug users with skin and soft tissue infections, e.g. admissions due to cutaneous abscesses of trunk or groin increased from 92 to 613 (566%) between fiscal years 1997/1998 and 2003/2004 [Reference Irish19]. Annual reports of severe group A streptococcal infections in IDUs have increased more than tenfold from less than ten in the mid-1990s to 143 in 2004 [17], although reports have since declined [17]. There have been outbreaks of Clostridium novyi, tetanus and wound botulism in IDUs [Reference Jones20–Reference Akbulut22] in recent years. Community-acquired methicillin-resistant Staphylococcus aureus infection has also been reported in IDUs [Reference Kearns23]. A community recruited study of IDUs in 2004 found that over a third (36%) of IDUs in the seven sites sampled in England reported either an abscess or open wound at an injection site in the past year, and this study estimated a healthcare burden cost of between £15.5 and £47 million per annum [Reference Hope8]. These infections are thus likely to place a considerable burden on health services in the UK, as has been observed elsewhere [Reference Takahasi5, Reference Biswanger6, Reference Darke, Ross and Kaye16, Reference Morrison, Elliott and Gruer24], and this may be increasing [17, Reference Irish19].
In response to these concerns and the limited available data on the extent of, or the factors associated with, injection site infections in IDUs, the UK's national annual unlinked anonymous sero-behavioural survey of IDUs added in 2006 the collection of self-reported data on symptoms likely to be due to bacterial infection. Using data from this system for the period 2006–2008, this paper explores the prevalence and factors associated with self-reported symptoms of injection site infections in IDUs.
METHODS
IDUs have been recruited since 1990 into an annual voluntary unlinked-anonymous survey across England, Wales and Northern Ireland, methodological details of which have been previously published [Reference Noone25]. Briefly, drug agencies (both statutory and non-statutory providers of advice, needle exchange, opiate substitution therapy, or addiction treatment) invite clients who have ever injected illicit drugs to participate in the annual surveys. Those drug users who agree to take part provide an oral fluid sample and self-complete a brief surveillance questionnaire. The oral fluid samples are tested for antibodies to HIV (anti-HIV), hepatitis C (anti-HCV) and hepatitis B core antigen (anti-HBc). The agency selection reflects the range of services provided for IDUs as well as reported geographic variations in the extent of injecting drug use, with the sampling structure reviewed regularly. The survey received multi-site approval from the London Research Ethics Committee.
The survey's questionnaire is reviewed regularly, and since 2006 it has collected information on self-reported symptoms of injection site infections. The following question was added: ‘In the last year have you had a swelling containing pus (abscess), sore, or open wound at an injection site’. These symptoms are most likely to be due to a bacterial infection, although they could have other causes.
Those taking part in the survey between 2006 and 2008 inclusive, who completed the questionnaire and reported injecting illicit opiates (e.g. heroin) or stimulants (e.g. crack-cocaine, powder cocaine, or amphetamines) in the last year were included in the analyses. All analyses were undertaken in SPSS version 17 (SPSS Inc., USA). Univariate associations between the reporting of symptoms and the following variables were examined using the χ2 test: demographic, service use and environmental characteristics; the drugs used; region of recruitment; and survey year. Those characteristics found to be associated in the univariate analysis were then entered using the forward stepwise procedure in SPSS into a logistic regression model with inclusion assessed using the likelihood ratio (with the stepwise probability for inclusion of 0·05 and exclusion of 0·1). As information on injecting practice such as frequency of injection, equipment sharing, and the body sites used for injection was collected only from those who reported injecting in the 4 weeks prior to taking part in the survey (current IDUs) a second analysis examining associations between injecting practice and reported symptoms was undertaken using this subgroup.
RESULTS
Over the 3-year period there were 5209 participations by drug users who had injected illicit opiates or stimulants in the preceding year. Of these, 25% (1313) were female, 16% (821) were aged <25 years (mean age 32·5, median 32 years), and 24% (1230) had been injecting for <5 years (mean number of years since first injected 10·6, median 9). Just over two-thirds (69%, 3570) reported currently receiving prescribed medication for their drug use (such as opiate substitution therapy), and 89% (4643) reported using a needle exchange service in the previous 12 months. Over half (56%, 2898) had injected both opiates and stimulants during the last year, with two fifths (40%, 2105) having injected only opiates and 4% (206) having injected only stimulants. During the year preceding participation 42% (2194) reported being homeless and 35% (1315) reported having been imprisoned.
Overall, 36% (1863) report having a ‘swelling containing pus (abscess), sore, or open wound’ at an injection site during the previous 12 months. This varied little over the 3-year period with 35%, 37% and 34% reporting this in 2006, 2007 and 2008, respectively (Table 1). Univariate associations between characteristics and the reporting of injection site symptoms are shown in Table 1. In the multivariable analysis a higher level of reported symptoms was found in 2007 compared to the other 2 years, suggesting a fluctuating level, but no trend, over time. Reporting symptoms was less common in the North East region compared to the other regions of England, Wales and Northern Ireland (Table 1). The reporting of symptoms increased with number of years injecting, and was higher in women, those recently homeless, those having recently used a needle exchange service, and those reporting injecting both opiates and stimulants, but lower in those only injecting stimulants (Table 1).
Table 1. Factors associated with a self-reported abscess, sore or open wound at injecting site in the previous 12 months: injecting drug users, 2006–2008
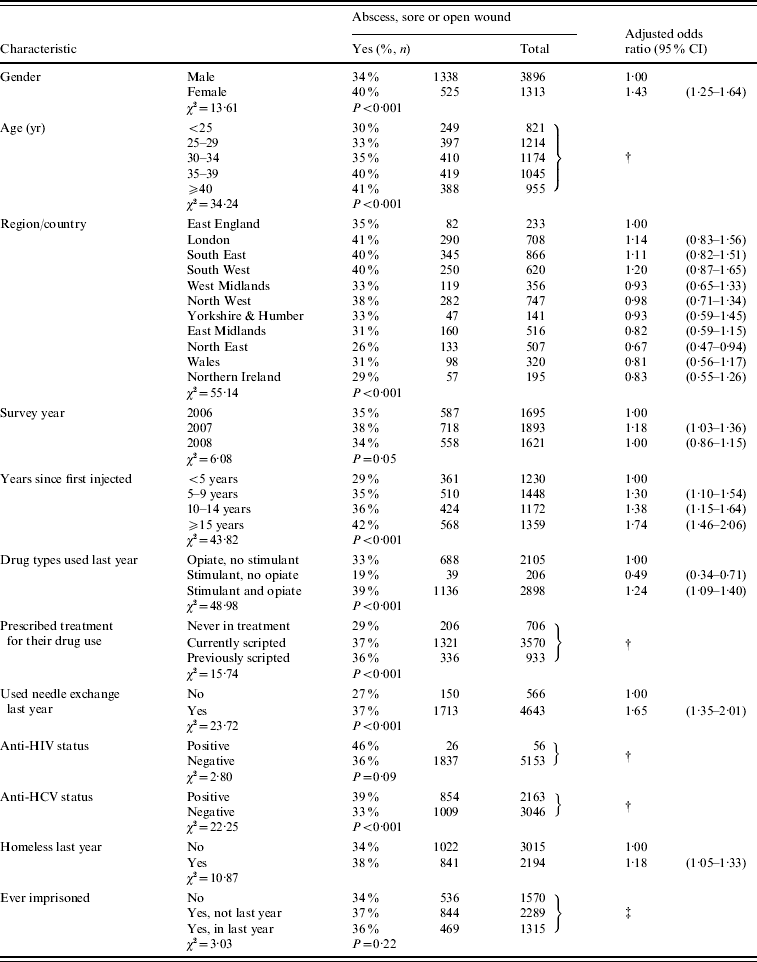
CI, Confidence interval.
† Not in final multivariable model.
‡ Not entered into multivariable analysis.
There were 3733 participations by those who reported last injecting during the preceding 4 weeks, representing 72% of those who had injected during the 12 months prior to participation. Of these current IDUs, 24% (913) were female, 17% (623) were aged <25 years (mean age 32·3, median 32 years), and 24% (893) had been injecting for <5 years (mean number of years since first injected 10·4, median 9). Those who had injected in the last month had similar levels of imprisonment and homelessness as those who had not. However, more of them had used a needle exchange (93% vs. 80%, χ(1)2=179·5, P<0·001), fewer of them were currently receiving prescribed treatment for their drug use (66% vs. 75%, χ(2)2=51·9, P<0·001), and more of them were injecting both opiates and stimulants (58% vs. 49%, χ(2)2=36·1, P<0·001).
Of those who had injected during the preceding 4 weeks 37% (1375) reported having a ‘swelling containing pus (abscess), sore, or open wound’ at an injection site during the previous 12 months. The associations between reporting a symptom in the last year and reported injecting practice in the last month are shown in Table 2. In the multivariable analysis higher levels of reported symptoms were associated with the following injecting practices: injecting daily; injecting ⩾10 times a day; injecting into the hand, groin, or legs; sharing filters; and reusing water to flush syringes (Table 2).
Table 2. Injecting practices is last month associated with a self-reported abscess, sore or open wound at injecting site during the previous 12 months, injecting drug users 2006–2008
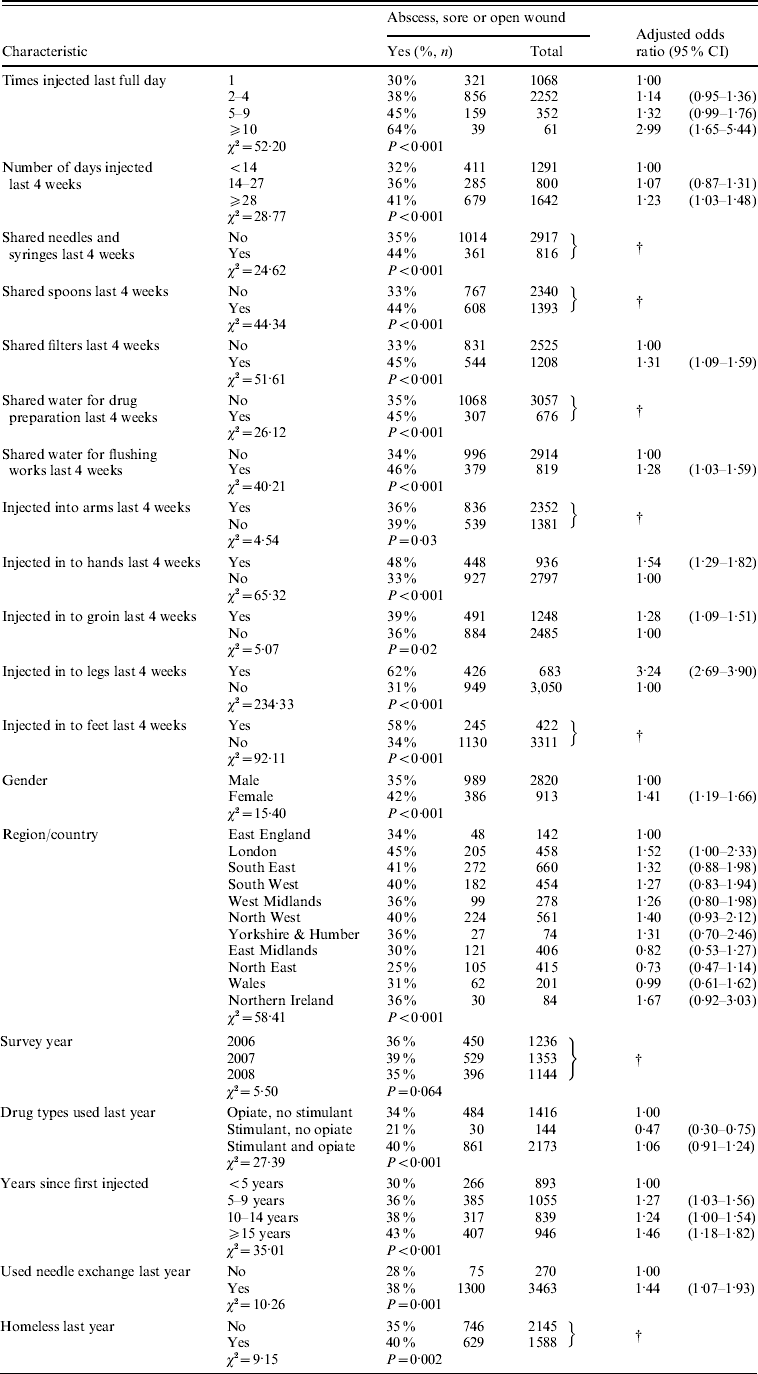
CI, Confidence interval.
† Not in final multivariable model.
DISCUSSION
Symptoms of injection site infections are common in IDUs throughout England, Wales and Northern Ireland. These symptoms were reported by just over a third of IDUs, and while the level fluctuated over the 3 years examined, there was no clear trend. These symptoms were associated with injecting both opiates and stimulants, having been injecting for a long time, being female, homeless, and with recent use of a needle exchange. Symptoms were also associated with more frequent injection, use of particular body sites for injection, and the reuse of filters and flushing water.
The high prevalence of self-reported injection site infections found in this study, supports previous finding from England [Reference Hope8] and also elsewhere [Reference Lloyd-Smith3–Reference Lloyd-Smith7]. The costs associated with injection site infections are considerable, with conservative estimates for England suggesting total costs of at least £15.5–19.5 million per annum, but possibly as high as £47 million in 2006. Overall healthcare costs related to problematic drug use, both injecting and non-injecting, in England have been estimated to be around £500 million per annum in the financial year 2003/2004 [Reference Gordon, Singleton, Murray and Tinsley26], with £25 million of this due to bloodborne viruses (HIV, hepatitis B and C) in IDUs.
Injection site infections thus place a considerable burden on the healthcare system that may be greater than that due to infections by bloodborne viruses. The high costs associated with injection site bacterial infections are in part likely to be due to delays in seeking healthcare. Studies suggest that IDUs tend not to seek timely medical care for their injecting-related health problems, often resulting in emergency treatment at considerable cost [Reference Hope8, Reference Palepu10, Reference Morrison, Elliott and Gruer24, Reference Ciccarone27, Reference French28]. The failure to seek earlier treatment probably reflects obstacles, e.g. barriers to accessing care and poor compliance with medication and follow-up care, and competing priorities such as obtaining money and acquiring and using drugs [Reference Palepu10, Reference Morrison, Elliott and Gruer24, Reference French28, Reference van Beek, Dwyer and Malcom29]. Thus, the high levels of reported symptoms found here are a concern and highlight the need for interventions. Targeted prevention and healthcare have been shown to reduce emergency department attendances, the need for surgery, and in-patient days [Reference Harris, Young and Organ30, Reference Grau31].
The current study found that frequent injection, the use of certain injection sites (hand, groin, legs), and the reuse of injecting paraphernalia were all associated with reporting symptoms of injection site infections during the last year. Previous studies have found similar associations [Reference Hope8, Reference Murphy11, Reference Vlahov12, Reference Topp14, Reference Spijkerman, van Ameijden and Mientjes15], and also associations with inadequate washing of hands or cleaning of the injection sites [Reference Biswanger6, Reference Murphy11, Reference Vlahov12], and the use of multiple injection sites [Reference Murphy11, Reference Vlahov12]. The associations with particular injection sites needs further examination, but may reflect some of these sites being more difficult to keep clean or the need to use certain sites due to others becoming unusable due to vascular damage, injury, or infection. The association with femoral (‘groin’) injection in the current study is of particular concern as this practice has become more common in the UK over the last decade [Reference Rhodes32], with indications of increased hospital admissions related to femoral injection in IDUs [Reference Irish19].
The association with injecting both opiates and stimulants is a concern. The mostly widely injected stimulant in the UK is crack-cocaine, with this often being injected either in combination or in parallel with heroin. A previous study in England found that those reporting crack-cocaine use also reported higher levels of injection site infections, and studies elsewhere have observed similar associations with injection of cocaine [Reference Lloyd-Smith7, Reference Palepu10] and heroin and cocaine combinations [Reference Murphy11, Reference Spijkerman, van Ameijden and Mientjes15]. There is also evidence that suggests that crack-cocaine use, which has been associated with risky behaviours [Reference Rhodes32, Reference Rhodes33], has become more common in the UK in recent years [Reference Hope, Hickman and Tilling34].
Women were more likely to report an injection site infection, and this has also been found in other studies [Reference Lloyd-Smith7, Reference Hope8, Reference Murphy11, Reference Spijkerman, van Ameijden and Mientjes15]. This could reflect a greater awareness of infections, and/or a greater vulnerability to injection site infections in female injectors [Reference Topp14]. As women are more likely to be injected by others, or to need assistance with injecting, it has been argued that as a result they may be at increased risk of exposure to contaminants [Reference Salmon4]. The reason for the difference in prevalence with gender needs further investigation.
The positive association with needle exchange use in the last year is an interesting, if counter-intuitive, finding. However, this needs to be interpreted with care as those reporting needle exchange use in the last year will include not only those making extensive regular use of needle exchange services but also those making irregular or limited use of these services. Studies suggest the consistent high coverage of interventions like needle exchange is needed for them to be effective against bloodborne viruses [Reference Van Den Berg35–Reference Craine37], and it is likely that such coverage issues would similarly affect their impact on injection site infections. It should also be noted that not all needle exchanges in the UK provide a full range of injecting equipment, and that some IDUs may also have accessed a needle exchange service either as a route to wound care or as a preventive measure following an injection site infection. However, this association requires further examination.
In our study homelessness was associated with higher rates of reporting of injection site symptoms, while a previous study in England did not find any association with homelessness [Reference Hope8]. Studies elsewhere have found similar associations with poor housing and homelessness [Reference Lloyd-Smith3, Reference Salmon4, Reference Dwyer13]. Homelessness is possibly an indicator of increased rates of injection in public or semi-public environments, which has been related to poor injection hygiene [Reference Murphy11, Reference Vlahov12].
Preventive interventions should thus focus on further reducing the re-use of injecting equipment, and target frequent injectors, those injecting into groin, legs or hand, using both opiates and stimulants, and female injectors; these interventions should also aim to reach those who are homeless. Development of community-based interventions, such as targeted wound clinics, may be effective [Reference Harris, Young and Organ30, Reference Grau31]. While further work needs to identify, develop, and evaluate suitable interventions, our findings suggest a need to focus on improving injection hygiene, the better management of the body sites used for injection, and access to services, including the provision of paraphernalia and sterile water.
The 3 years' data presented here did not indicate any overall upwards or downwards trend in symptoms associated with injection site infection, with the level much the same as that found in a study of current IDUs recruited in the community at seven sites in England conducted 2 years earlier [Reference Hope8]. This suggests that the recent level of symptoms may have been relatively stable; however, this may not remain so. The concerns expressed earlier this decade about the extent of infections in IDUs [17–19, 21–Reference Kearns23] have resulted in increased efforts to reduce infections, both viral and bacterial. The National Institute for Health and Clinical Excellence has recently issued guidance on optimizing needle exchange provision [38] and a national awareness and information campaign has recently been launched in England [39]. Ongoing surveillance of symptoms of injection site infections may shed light on the impact of these initiatives.
It is important to consider the limitations and generalizability of these findings. Self-reported symptoms of injection site infections were used in this study. While some may question the accuracy of these, studies have shown good agreement between self-reported symptoms and clinical diagnosis [Reference Morrison, Elliott and Gruer24]. The comparative rarity, marginalization and illegal nature of injecting drug use impedes the recruitment of a representative sample of injectors. This study aimed to minimize sampling biases and maximize representativeness by using an established survey utilizing widespread service provision as a sampling structure. However, bias might arise if those using services where the sampling occurred were either at higher or lower risk than the overall IDU population. Those not in contact with services might be those most marginalized and so at highest risk but, alternatively, they may be those most stable and integrated with well-controlled drug use and so at low risk. Both of these groups are possibly under-represented; however, surveys recruiting independently of services through community settings, using a range of sampling approaches, find that most IDUs recruited in the UK have, or have recently had, service contact [Reference Hickman40].
Taken together these findings suggest injection site infections are a common experience in IDUs in England, Wales and Northern Ireland, and that the resultant healthcare burden is substantial. Further research is also needed to explore issues around the robustness of self-reports and the relationship between reported symptoms and actual infections. Moreover, interventions need to be developed and piloted to reduce the level of infections. Continued surveillance should provide the means to shed light on the impact of new initiatives to reduce infections in IDUs.
ACKNOWLEDGEMENTS
This work was support by core funding to the Health Protection Agency. We are grateful to all of the injecting drug users who took part in the survey and to the staff of many services that assisted with their recruitment. We thank those who undertook the bloodborne virus testing on the samples, in particular Bharati Patel, and those who have assisted with the running survey, particularly Merrington Omakalwala and Jacquelyn Njoroge.
DECLARATION OF INTEREST
None.




