The Mediterranean diet, which is rich in MUFA, primarily as olive oil, is associated with low CVD rates(Reference Keys, Menotti and Karvonen1–Reference Castelli, Garrison, Wilson, Abbott, Kalousdian and Kannel3). Traditionally, this beneficial effect of MUFA-rich diets has been related to their action on the lipid profile(Reference Mensink and Katan4, Reference Grundy, Nix, Whelan and Franklin5). However, with a greater understanding of the mechanisms contributing to the development of atherosclerosis, it has become clear that diets rich in MUFA may also reduce coronary risk by mechanisms other than increasing the HDL:LDL-cholesterol ratio(Reference Perez-Jimenez, Lopez-Miranda and Mata6, Reference Perez-Jimenez7).
Several lines of evidence indicate that LDL may undergo oxidative modification in vivo and that this process is critical in the initiation of atherosclerosis(Reference Witztum8). Oxidised forms of LDL (oxLDL) are taken up by the macrophage scavenger receptors (MSR) with extremely high efficiency and lead to cholesterol accumulation and foam cell formation, which in turn results in the generation of atheromatous plaque(Reference Gerrity9, Reference Gerrity10). Dietary fat is one of the most important factors determining LDL susceptibility to oxidation. Most of the studies show that LDL particles from individuals consuming a high-MUFA diet are protected from oxidative modifications as compared with individuals consuming SFA(Reference Perez-Jimenez, Lopez-Miranda and Mata6). However, the protection of the MUFA-rich diets could be effective not only at the beginning of the atherosclerotic process, by decreasing LDL oxidation, but also in a later stage. In agreement with this hypothesis, a lesser accumulation of cholesterol in the aortas of rabbits has been observed when olive oil was added to the diet(Reference Mortensen, Espensen, Hansen and Ibsen11). This phenomenon may result from reduced LDL oxidation and/or a decreased ability to take up oxLDL. oxLDL uptake is mediated via MSR(Reference Krieger and Herz12). In animal studies, an olive oil-rich diet decreased the mRNA levels of several types of scavenger receptors(Reference Miles, Wallace and Calder13). Interestingly, even though these data suggest that part of the protective effect of MUFA-rich diets could be exerted via the reduction of the macrophage uptake of oxLDL and subsequent foam cell formation, studies showing that effect have been scarce and controversial. Some studies found that supplementation in the diet with MUFA decreased macrophage uptake of oxLDL(Reference Parthasarathy, Khoo, Miller, Barnett, Witztum and Steinberg14–Reference Reaven, Parthasarathy, Grasse, Miller, Almazan, Mattson, Khoo, Steinberg and Witztum16), whereas others did not report this relationship(Reference Ramirez-Tortosa, Lopez-Pedrosa, Suarez, Ros, Mataix and Gil17). Carefully controlled studies are therefore required to definitively establish this protective effect of MUFA-rich diets. Furthermore, to our knowledge, no previous studies have compared the effect of both quantity and quality of dietary fat on macrophage uptake of oxLDL.
Currently, a decrease in the consumption of SFA is recommended for the prevention of atherosclerosis. However, it is not entirely clear whether SFA should be replaced by carbohydrates (CHO) or MUFA. The most appropriate nutritional model should be established when supported by research on biological and clinical effects of the different dietary options. Therefore, our objective was to determine the effect of both quality and quantity of dietary fat on macrophage uptake of plasma oxLDL and LDL susceptibility to oxidation in healthy young men.
Methods
Subjects
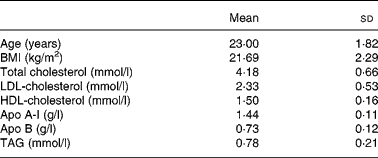
A group of twenty men were recruited from among students at the University of Cordoba. The baseline anthropometric characteristics and plasma lipid and apolipoprotein concentrations of the healthy young men are shown in Table 1. Informed consent was obtained from all participants. All subjects underwent a comprehensive medical history, physical examination, and clinical chemistry analysis before enrolment. Subjects showed no evidence of any chronic disease (hepatic, renal, thyroid or cardiac dysfunction), obesity or unusually high levels of physical activity (for example, sports training). None of the subjects had a family history of premature coronary artery disease or had taken medication or vitamin supplements in the 6 months before the study. Physical activity and diet, including alcohol consumption, were recorded in a personal diary for 1 week and the data were used to calculate individual energy requirements. Mean BMI was 21·7 (sd 2·3) kg/m2 at the onset of the study and remained constant throughout the experimental period. Subjects were encouraged to maintain their regular physical activity and lifestyle and were asked to record in a diary any event that could affect the outcome of the study, such as stress, change in smoking habits and alcohol consumption or intake of foods not included in the experiment design. The study protocol was approved by the Human Investigation Review Committee at the Reina Sofia University Hospital.
Table 1 Baseline anthropometric characteristics and plasma lipid and apolipoprotein concentrations of the healthy young men
(Mean values and standard deviations)
Diets
The study design included an initial 28 d period during which all subjects consumed an SFA-rich diet, with 15 % protein (% of energy in the total diet), 47 % CHO and 38 % fat (20 % SFA, 12 % MUFA and 6 % PUFA). After this period, volunteers were randomly assigned to one of two diet sequences. Ten subjects received a MUFA-rich diet containing 15 % protein, 47 % CHO and 38 % fat ( < 10 % SFA, 22 % MUFA, 6 % PUFA) for 28 d. This diet was followed for 28 d by consumption of a CHO-rich diet containing 15 % protein, 55 % CHO and < 30 % fat ( < 10 % SFA, 12 % MUFA, 6 % PUFA). The other ten subjects consumed the CHO diet before the MUFA diet. The cholesterol intake was constant (under 300 mg/d) during the three periods. During the MUFA diet period, 80 % of the MUFA content was provided by virgin olive oil, which was used for cooking, salad dressing, and as a spread. CHO intake of the CHO diet was based on the consumption of biscuits, jam and bread. Butter and palm oil were used during the SFA dietary period.
The composition of the experimental diets was calculated using the United States Department of Agriculture(18) food tables, and the Spanish food composition tables for local foodstuffs(Reference Varela19). All meals were prepared in the hospital kitchen and were supervised by a dietitian. Lunch and dinner were consumed in the hospital dining room, whereas breakfast and an afternoon snack were eaten in the medical school cafeteria. Fourteen menus were prepared with regular solid foods and rotated during the experimental period. Duplicate samples from each menu were collected, homogenised and stored at − 70°C. Protein, fat and CHO contents of the diet were analysed by standard methods(20). Dietary compliance was verified by analysing the fatty acids in LDL-cholesteryl esters (CE) at the end of each dietary period(Reference Ruiz-Gutierrez, Prada and Perez-Jimenez21). The study took place from January to March to minimise seasonal effects and academic stress.
Lipid analysis and biochemical determinations
Venous blood samples were collected into EDTA-containing (1 g/l) tubes from all subjects after a 12 h overnight fast at the beginning of the study and at the end of each dietary period. Plasma was obtained by low speed centrifugation (1500 g) for 15 min at 4°C within 1 h of venepuncture. To reduce inter-assay variation, plasma was stored at − 80°C and analysed at the end of the study. Plasma cholesterol and TAG levels were determined by enzymic techniques(Reference Allan, Taylor and Taylor22, Reference Bucolo and David23). HDL-cholesterol was measured after precipitation with phosphotungstic acid(Reference Assmann, Schriewer, Schmitz and Hagele24). Apo A-I and B were determined by immunoturbidimetry(Reference Riepponen, Marniemi and Rautaoja25). LDL-cholesterol concentration was calculated using the Friedewald formula(Reference Friedewald, Levy and Fredrickson26).
Determination of low-density lipoprotein oxidation susceptibility
LDL were isolated from fresh plasma samples by sequential ultracentrifugation (2 h at 405 000 g and 4°C) immediately after separation of plasma at density between 1·019 and 1·063 and after VLDL isolation at density < 1·019 using a Beckman model LE-70 ultracentrifuge with a type NVT65 rotor (Beckman, Palo Alto, CA, USA). The LDL fraction was dialysed for 24 h at 4°C with three changes of PBS (Sigma, St Louis, MO, USA), containing 0·01 % EDTA the first time and only PBS the two other times. The PBS was maintained O2-free by purging it with pure N2. LDL protein content was determined by using the method of Bradford(Reference Bradford27). LDL oxidation was carried out by incubating 100 μg LDL protein with 5 μm-CuSO4 in 1·0 ml PBS medium. Absorbance at 234 nm was measured continuously every 5 min for 4 h at 37°C in a spectrophotometer as previously described(Reference Esterbauer, Striegl, Puhl and Rotheneder28). Lag time, rate of oxidation and total amount of conjugated dienes formed per 100 μg LDL were calculated.
Cell culture
The human monocyte line U937 was obtained from the American Tissue Culture Collection (ATCC, Rockville, MD, USA). U937 cells were grown in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10 % (v/v) heat-inactivated (56°C for 30 min) fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mm-glutamine and 12 mm-sodium carbonate in a humidified atmosphere containing 5 % (v/v) CO2 at 37°C. Differentiation of U937 monocytes into macrophages was induced by adding phorbol-myristate-acetate to the culture medium at a concentration of 12·5 μg and incubating the cells for 2 h at 37°C. They were then plated into twelve-well dishes at 0·5 × 106 cells/ml and allowed to adhere and differentiate into macrophages for 4 d(Reference Okada, Kimura, Kameoka, Kishi, Azuma and Ikuta29). In experiments, macrophages were incubated with 5 μg/ml moderately oxidised [3H]CE-LDL in RPMI-1640 medium with 10 % lipoprotein-deficient serum for 24 h at 37°C. Cell viability was determined by Trypan Blue exclusion and only cultures with viability >90 % were used in experiments.
Labelling and cellular uptake of moderately oxidised low-density lipoprotein
LDL was labelled by incubating 1·5 ml LDL-enriched fraction (1·019 < density < 1·063) with 1·0 ml of fresh lipoprotein-deficient serum and 0·74 MBq [3H]cholesterol for 24 at 37°C. The resulting [3H]CE-LDL particle was dialysed, sterilised and quantified as described above. [3H]CE-LDL (100 μg/ml protein) was moderately oxidised by incubation with 5 μm-CuSO4 in 1 ml PBS at 37°C for 2 h (instead of 4 h used to determine LDL susceptibility to oxidation). The reaction was stopped by adding 100 μm-EDTA at 4°C and immediately dialysed again to eliminate EDTA and the [3H]cholesterol released by the LDL particle during oxidation. Before experiments, moderately oxidised [3H]CE-LDL were sterilised by passage through a 0·22 mm Millipore filter. The moderately oxidised [3H]CE-LDL (5 μg/ml) were then added to a monolayer of U937-derived macrophages cultured in 1 ml RPMI-1640 medium with 10 % lipoprotein-deficient serum for 24 h. After incubation, cells were collected, washed with PBS and re-suspended in 500 μl of 5 % SDS. Radioactivity in the cells and supernatant fractions were measured with a liquid scintillation counter. The unspecific adsorption was determined of the same form, but moderately oxidised [3H]CE-LDL was incubated at 4°C instead of 37°C. The percentage of macrophage uptake of moderately-oxLDL was calculated from: ((pellet/supernatant fraction) unspecific radioactivity) − ((pellet/supernatant fraction) specific radioactivity) × 100.
Statistical analysis
We used the ANOVA for repeated measures to test the effects of the diets on plasma lipids, LDL susceptibility to oxidation and macrophage uptake of oxLDL for each dietary phase. Differences were considered significant when P < 0·05. When statistical significance was found, Tukey's post hoc comparison test was used to identify between-group differences. To determine whether plasma LDL-cholesterol concentration or macrophage uptake of moderately-oxLDL was correlated with LDL susceptibility to oxidation, we used the Pearson correlation test. An independent sample t test was made between the two groups that consumed the MUFA then CHO v. CHO then MUFA diet to test whether the MUFA–CHO differences depended on whether MUFA or CHO was first (order effects). Plasma TAG were log-transformed before statistical analyses. Experiments were performed in duplicate and replicated three times for each incubation period. The duplicates were averaged and this mean was used in the statistical analyses. Differences were considered significant at P < 0·05. Data are presented as mean values and standard deviations. Statistical analyses were conducted using SPSS statistical software (version 9.0; SPSS, Inc., Chicago, IL, USA).
Results
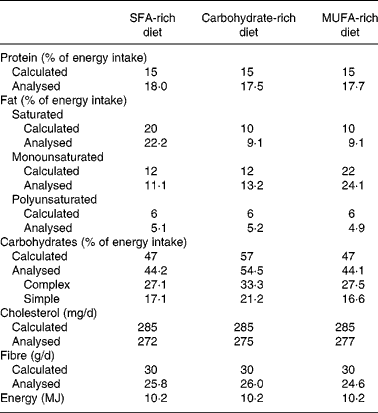
The composition of the mean daily intake of the participants is shown in Table 2. Analysis of LDL-CE obtained after each dietary period showed good adherence in the different intervention stages. After the SFA diet period, we observed a significantly greater (P < 0·005) increase in palmitic acid in the LDL-CE than were observed after the CHO and MUFA diets: 27·3 (sd 1·4) % compared with 19·8 (sd 3·9) and 15·2 (sd 0·4) %, respectively. A significantly greater (P < 0·05) increase in oleic acid in the CE was also seen after the MUFA diet (50·3 (sd 4·7) %) than after the CHO diet (38·8 (sd 9·0) %), but not after the SFA diet (47·2 (sd 4·4) %).
Table 2 Daily intake during each experimental diet period
The concentrations of total cholesterol, LDL-cholesterol, HDL-cholesterol, apo A-I and apo B after the three diets are shown in Table 3. Changes in diet were associated with significantly (P < 0·05) lower concentrations of total (P < 0·001), LDL- (P < 0·001) and HDL-cholesterol (P < 0·05) and apo A-I (P < 0·05) and apo B (P < 0·001) after the CHO and MUFA diets. However, in comparison with the MUFA diet, the CHO diet was associated with significantly lower plasma concentrations of HDL-cholesterol (P < 0·05) and apo A-I (P < 0·01). Significant differences were not observed in TAG concentrations among the three different diets (P = 0·695). Furthermore, the shift from the MUFA diet to the SFA- or CHO-rich diets reduced the resistance of LDL particles to oxidation, decreasing the lag time period (P = 0·038) and increasing (P = 0·001) the propagation rate of the LDL oxidation curve (Table 3, Fig. 1). However, significant differences were not observed between SFA- and CHO-rich diets in the oxidation parameters. In addition, we observed a significant correlation (r − 0·27; P < 0·005) between the increase in LDL-cholesterol plasma concentration and the decrease in lag time.
Table 3 Plasma lipids, apolipoproteins and parameters of low-density lipoprotein susceptibility to oxidation at the end of each dietary period
(Mean values and standard deviations)
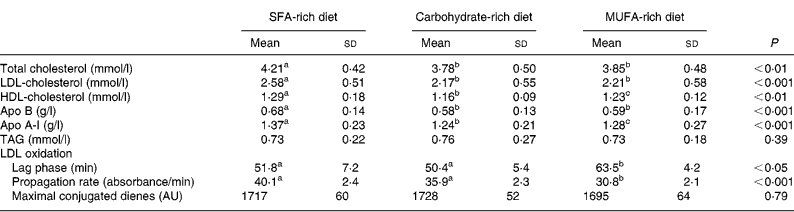
AU, arbitrary units.
a,b,c Values within a row with unlike superscript letters were significantly different (P < 0·05).

Fig. 1 Determination of LDL susceptibility to oxidation in LDL from healthy young men at the end of each dietary period. (–■–), SFA-rich diet; (–△–), carbohydrate-rich diet; (–●–), MUFA-rich diet.
The MUFA diet reduced macrophage uptake of plasma moderately-oxLDL (P = 0·031) as compared with the SFA diet (Fig. 2). Significant differences were not observed between the CHO-rich diet and the SFA- or MUFA-rich diets on oxLDL uptake. In order to know the possible mechanisms by which MUFA diets exert this effect, conjugated dienes were determined in moderately-oxLDL before incubation with macrophages. We observed that MUFA diets reduced the amount of conjugated dienes in moderately-oxLDL as compared with SFA or CHO diets (1384 (sd 49) v. 1635 (sd 53) or 1619 (sd 61) arbitrary units respectively; P = 0·026). Finally, macrophage uptake of plasma oxLDL was correlated (r 0·45; P = 0·040) with total amount of conjugated dienes after LDL oxidation.
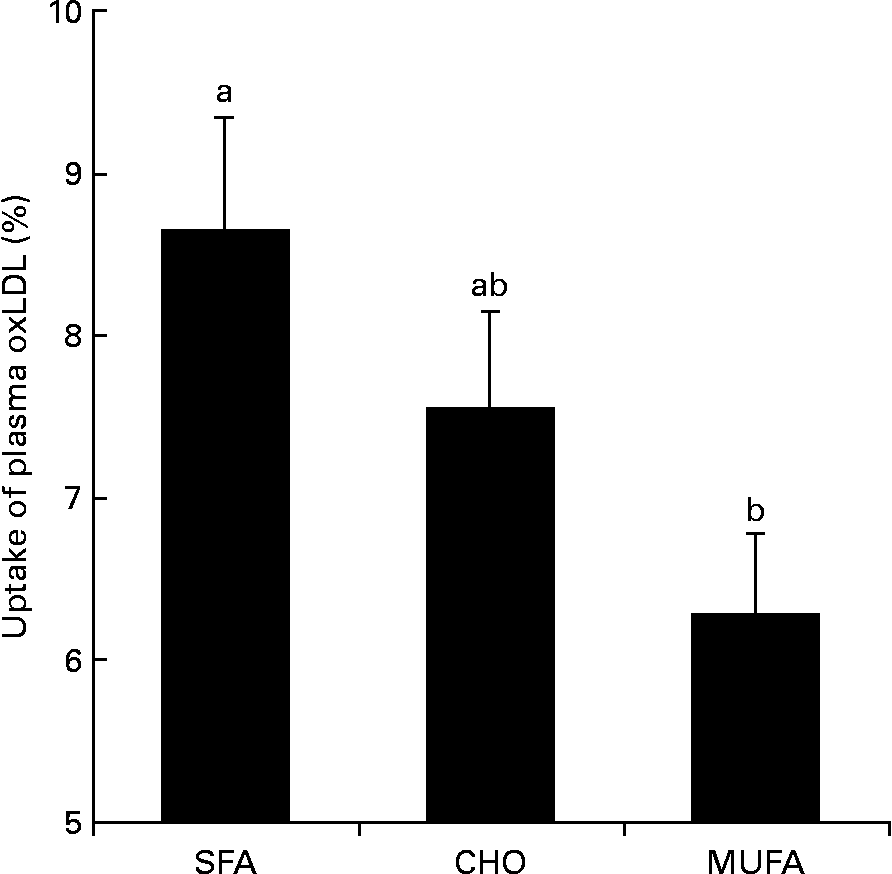
Fig. 2 Percentage of macrophage uptake of plasma oxidised LDL (oxLDL) at the end of each dietary period in healthy young men. Values are means, with their standard deviations represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05). SFA, SFA-rich diet; CHO, carbohydrate-rich diet; MUFA, MUFA-rich diet.
Discussion
The present results show that the shift from an SFA to a MUFA diet decreases macrophage uptake of moderately-oxLDL in healthy young men. However, when the CHO diet was compared with the SFA- or MUFA-rich diets no differences in moderately-oxLDL uptake were observed. Furthermore, we found that a MUFA-rich diet reduces LDL susceptibility to oxidation in comparison with SFA- and CHO-rich diets.
Given that a Westernised diet enriched in SFA increases LDL-cholesterol plasma levels and contributes to the development of coronary artery disease, it is widely accepted that a healthy diet should contain a limited amount of this nutrient. However, it is not entirely clear whether SFA should be replaced by CHO or MUFA. In accordance with the present results, previous studies have indicated that both MUFA and CHO diets reduce total cholesterol and LDL-cholesterol as compared with an SFA-rich diet(Reference Mensink and Katan4, Reference Moreno, Perez-Jimenez, Marin, Gomez, Perez-Martinez, Moreno, Bellido, Fuentes and Lopez-Miranda30). Nevertheless, when subjects consumed a MUFA diet the levels of HDL-cholesterol and apo A-I were higher compared with those consuming a CHO diet. Thus, diets rich in MUFA may be a better nutritional option than the low-fat, high-CHO diets. However, no evidence exists that the reduction of HDL-cholesterol, related to low-fat diets, favours the onset of CHD(Reference Connor and Connor31). Therefore, it is necessary to identify other non-lipid beneficial effects of MUFA-rich diets, since new findings could be an important argument in favour of performing general dietary advice for populations that consume SFA-rich diets.
LDL oxidation has been shown to be involved in the initiation and progression of atherosclerosis(Reference Witztum and Steinberg32). Numerous studies have shown that dietary fat determines the susceptibility of LDL particles to oxidative modification(Reference Visioli, Bellomo, Montedoro and Galli33). As shown previously, we observed that a shift from an SFA-rich diet or a diet rich in CHO to a MUFA-rich diet increases the resistance of LDL particles to oxidation(Reference Okada, Kimura, Kameoka, Kishi, Azuma and Ikuta29, Reference Moreno, Perez-Jimenez, Marin, Gomez, Perez-Martinez, Moreno, Bellido, Fuentes and Lopez-Miranda34). In the present study, 80 % of the MUFA content during the MUFA diet was provided by virgin olive oil. This fact could explain the present results since virgin olive oil contains a range of micronutrients with antioxidant properties, the phenolic compounds, which beneficially increase LDL oxidation resistance(Reference Moreno, Lopez-Miranda, Gomez, Benkhalti, El Boustani and Perez-Jimenez35–Reference Ruano, Lopez-Miranda, Fuentes, Moreno, Bellido, Perez-Martinez, Lozano, Gomez, Jimenez and Perez Jimenez37). The mechanism of the increased oxidative stress of LDL when subjects consume an SFA diet, given that these fatty acids are not prone to oxidation, probably involves other factors, such as the LDL-cholesterol concentration and the particle residence time in the circulation. The elevation in plasma levels of LDL-cholesterol and the prolonged circulation of these atherogenic lipoproteins in the plasma cause an oxidative stress which is associated with an increased susceptibility to oxidation. Thus, the SFA diet was associated with significantly higher plasma LDL-cholesterol concentrations than was either of the hypolipidaemic diets. Furthermore, we observed a correlation between increases in LDL-cholesterol plasma concentration and decreases in lag time.
The present study is the first to examine the effects of both quality and quantity of dietary fat on macrophage uptake of oxLDL. Our data indicate that a MUFA-rich diet reduces macrophage uptake of plasma oxLDL as compared with an SFA diet. Nevertheless, no differences in oxLDL macrophage uptake were observed between CHO- and SFA-rich diets. Considering that a reduction in the cellular uptake of oxLDL may prevent foam cell formation, the key event in atherosclerosis(Reference Gerrity9, Reference Gerrity10), these findings confirm that a MUFA-rich diet may be a better nutritional option than low-fat, high-CHO diets for substituting saturated fat energy in the diet. The present results are in agreement with previous studies in healthy subjects, which have demonstrated that MUFA dietary supplementation from olive oil or an oleate-enriched variant of sunflower-seed oil leads to a reduction in macrophage uptake of oxLDL(Reference Aviram and Eias15, Reference Reaven, Parthasarathy, Grasse, Miller, Almazan, Mattson, Khoo, Steinberg and Witztum16). However, Ramirez-Tortosa et al. (Reference Ramirez-Tortosa, Lopez-Pedrosa, Suarez, Ros, Mataix and Gil17) did not observe this relationship in patients suffering from peripheral vascular disease consuming an olive oil supplement. The lack of effect in that study could be due to different reasons: one possibility is that the unhealthy status of their patients negated or minimised the impact of olive oil. Other possibilities include the small sample size, type and amount of olive oil, etc. Currently, the intrinsic mechanisms by which MUFA consumption decreases macrophage uptake of oxLDL are not well understood. OxLDL uptake is mediated via MSR(Reference Krieger and Herz12). The important role of MSR type A and other LDL scavenger receptors, such as CD36, in plaque formation has been determined in diet-induced atherosclerosis in mice(Reference Sakaguchi, Takeya and Suzuki38). Miles et al. (Reference Miles, Wallace and Calder13) demonstrated that mRNA levels of CD36 and MSR-A type I and type II were reduced after olive-oil feeding as compared with SFA supplementation. It is probable that the lower expression of MSR could be also associated with a lower number of these receptors in the cell surface, thus decreasing the uptake of modified LDL. This phenomenon could partially explain the present results since virgin olive oil was used as the principal source of fat during the MUFA diet. Another possible explanation of the present results may be related to the effect of oxidised LDL on MSR expression. Some studies have demonstrated the presence of oxidised LDL in atherosclerotic lesions and this co-localises with MSR expression(Reference Yla-Herttuala, Rosenfeld, Parthasarathy, Sigal, Sarkioja, Witztum and Steinberg39). It has been observed that oxidative modifications in LDL up regulate the transcription and expression of MSR(Reference Nagy, Tontonoz, Alvarez, Chen and Evans40–Reference Yoshida, Quehenberger, Kondratenko, Green and Steinberg42). Thus, LDL particles would be taken up by macrophages according to their degree of oxidation: with extremely high efficiency when these particles are highly oxidised, and with a lesser affinity when they are minimally oxidised. In the present study, we determined the amount of conjugated dienes in moderately-oxLDL before incubation with macrophage. We observed that when LDL particles obtained after the MUFA diet were partially oxidised, a lower formation of conjugated dienes was observed as compared with SFA- or CHO-rich diets. Therefore it is possible that the MUFA diet, by decreasing oxidative modifications in LDL, could attenuate MSR gene expression, thus reducing macrophage uptake of these atherogenic lipoproteins, as we have observed. In agreement with this hypothesis, we observed a positive correlation between macrophage uptake of oxLDL and the amount of conjugated dienes in these particles. Although all these observations would explain the present results, it is important to note that we have not determined them directly in the present study. New studies are needed, therefore, to confirm the present results and to characterise the molecular markers of LDL uptake that underpin the potential anti-atherogenic effect of LDL generated after MUFA consumption.
In conclusion, the present study further supports the anti-atherogenic properties of high-MUFA diets enriched in virgin olive oil and demonstrates important beneficial effects of this diet on two important linked pathways in atherogenesis, i.e. LDL oxidation and macrophage cholesterol accumulation.
Acknowledgements
J. A. M. was responsible for the collection of data, analysis of data, and writing of the manuscript. C. M. and P. G. contributed to the collection and analysis of data. P. P. M. provided statistical advice and contributed to the writing of the manuscript. R. M. contributed to the collection of data and the writing of the manuscript. J. A. P. was responsible for the design of the study and analysis of data. F. P. J. and J. L. M. were responsible for the conception and design of the study, analysis of data and writing of the manuscript. None of the authors had any conflict of interest.
The present study was supported by research grants from the CICYT (SAF 01/2466-C05 04, SAF 01/0366 and SAF 2003-05770), the Spanish Ministry of Health (FIS 98/153, FIS 01/0449 and FIS 99/0949), Fundación Cultural ‘Hospital Reina Sofía-Cajasur’, Consejería de Salud, Servicio Andaluz de Salud (99/116, 99/165, 00/212, 00/39, 01/243, 01/239, 02/64, 02/65, 02/77, 03/73, 04/191, 03/75, 04/237 and 04/238) and Consejería de Educación, Plan Andaluz de Investigación, Universidad de Córdoba.







