Diet (nutrition) plays an important role in the management of diabetes, and for preventing and/or delaying the development of type 2 diabetes. Dietary approaches can be used alone (in the case of the onset of type 2 diabetes and in mild hyperglycaemia) or in combination with oral hypoglycaemic agents or insulin. Often, the success or failure of the management of diabetes depends on the knowledge of the diabetic patient regarding the dietary principles related to diabetes. Although there have been major advances in the control of diabetes through oral hypoglycaemic agents and insulin, the management of diabetes by dietary agents such as vegetables, fruits and spices is more appropriate and economical in developing countries such as Pakistan. There is an inverse association between vegetable consumption and chronic disease reduction, such as cancer, CVD and diabetes(Reference Bazzano, He and Ogden1–Reference Williams, Wareham and Cox3).
Asparagus officinalis L. is a dietary agent native to most European, African and Asian countries. Its medicinal usage has been reported in the British and Indian Pharmacopoeias and in traditional systems of medicine such as Ayurveda, Unani and Siddha. Asparagus has been used as a vegetable and medicine in many countries, owing to its delicate flavour and diuretic properties. Asparagus is frequently used in salads, vegetable dishes and soups. Green asparagus is a good source of vitamin C and its stalks are rich in antioxidants. Among the vegetables commonly consumed in the USA and Europe, asparagus has been reported to be a rich source of antioxidants, in terms of both quality and quantity(Reference Vinson, Hao and Su4, Reference Pellegrini, Serafini and Colombi5). In a comparative study among thirty-four fruits and vegetables(Reference Pellegrini, Serafini and Colombi5), asparagus has been placed 7th in the rank of radical scavengers and 13th in ferric-reducing power.
The chemical constituents of A. officinalis have been studied to some extent. The compounds so far reported include saponins(Reference Pant, Panwar and Negi6, Reference Shao, Poobrasert and Kennelly7), saccharides(Reference Shiomi, Yamada and Izawa8–Reference Fukushi, Onodera and Yamamori10), acetylenic compounds(Reference Terada, Honda and Suwa11) and sulphur-containing compounds(Reference Yanagawa, Kato and Kitahara12–Reference Matsubayashi and Sakagami15). Various reports have suggested that polysaccharides derived from this plant exhibit antioxidant properties(Reference Gang, Li and Xian16–Reference Zeng, Meng and Zhang18). To our knowledge, there are no studies reporting on any identified compound isolated from A. officinalis as an anti-diabetic.
Studies on the extracts of A. officinalis have revealed a wide range of biological activities including diabetes(Reference Said, Khalil and Fulder19). These activities include anti-tumour(Reference Shao, Chin and Ho20), antifungal(Reference Wang and Ng21), diuretic(Reference Balansard and Rayband22) and immunostimulatory effects(Reference Thatte and Dahanukar23, Reference Rege, Thatte and Dahanukar24). Asparagus has also been shown to exert potent antioxidant properties(Reference Rodríguez, Jaramillo and Rodríguez25). It has been claimed by traditional healers that the seeds of A. officinalis have anti-diabetic properties(Reference Ali and Khan26).
A. officinalis was reported for the first time for treating diabetes in a survey conducted in the West Bank region of Israel(Reference Said, Khalil and Fulder19). Recently, Zhao et al. (Reference Zhao, Zhang and Zhu27) reported that the aqueous extract of the by-products of A. officinalis exerts anti-diabetic activity in streptozotocin (STZ)-induced diabetic rats. In their study, only the blood glucose-lowering effect coupled with hypotriacylglycerolaemic activity has been shown. However, the precise anti-diabetic mechanism has not yet been elucidated. Most importantly, there has been no morphological study observing the pancreatic islets and β-cells in diabetic rats treated with A. officinalis.
STZ-induced diabetes has been widely used to study the pathophysiology of diabetes. The induction of STZ during adulthood produces severe diabetes that often needs insulin administration to ensure survival for longer periods. In the case of neonatal STZ-induced (n-STZ) rats, the development of hyperglycaemia, abnormal glucose tolerance and mild hypoinsulinaemia is an insidious process that is almost asymptomatic at the beginning and becomes manifested in the adult stage, the features that resemble the natural history of diabetes in humans(Reference Portha, Blondel and Serradas28, Reference Kodama, Iwase and Nunoi29). The n-STZ diabetic rats have similar insulin secretory characteristics to those found in non-obese type 2 diabetic subjects(Reference Arulmozhi, Veeranjaneyulu and Bodhankar30, Reference Portha, Movassat and Cuzin-Tourrel31). Thus, the n-STZ model is considered to be one of the suitable experimental animal models for non-obese type 2 diabetes for testing potential anti-diabetic agents.
The present study was conducted to investigate the effect of A. officinalis on insulin secretion and pancreatic β-cell function in n-STZ non-obese type 2 diabetic rats by measuring blood glucose, serum insulin and total antioxidant status (TAS). In addition, morphological examination of the pancreas was also performed to further substantiate the beneficial effect of A. officinalis on pancreatic β-cell function. The immunohistochemical method for the triple staining of insulin, glucagon and the nuclei was combined with morphometrical analysis. The efficacy of A. officinalis was compared with a standard anti-diabetic drug, glibenclamide.
Materials and methods
Materials
STZ and glibenclamide were obtained from Sigma; Insulin ELISA kit from Crystal Chem, Inc.; TAS kit from Randox. Insulin (guinea-pig anti-insulin) antibody was from Abcam and glucagon (mouse anti-glucagon, Clone K79bB10) from Sigma. The fluorescent-labelled secondary antibodies, Texas Red-donkey anti-guinea-pig IgG and Cy2-donkey anti-mouse IgG were obtained from Jackson ImmunoResearch. 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Wako Pure Chemical.
Preparation of the extract
Seeds of A. officinalis (1 kg) were bought from a local market in Karachi, Pakistan. The plant material belongs to Drosh City, Chitral District, Khyber-Pakhtunkhwa, Pakistan. The seeds were authenticated by a taxonomist at the University of Karachi, and a voucher specimen (no. 73 960) was deposited at the Karachi University Herbarium, Pakistan. The powdered seeds were soaked twice in 3 litres of 80 % aqueous methanol for 72 h at room temperature. The pooled extracts were filtered, concentrated and evaporated to dryness under vacuum by using a rotary evaporator. Finally, the crude extract was freeze-dried to obtain the experimental extract (76·5 g). The extract was dissolved in distilled water before its administration to the diabetic rats. The extract yield was 7·6 %.
In vitro studies
1,1-Diphenyl-2-picrylhydrazyl radical-scavenging activity assay
The radical-scavenging property of the extract was evaluated by assessing the 1,1-diphenyl-2-picrylhydrazyl (DPPH)-scavenging activity according to Fenglin et al. (Reference Fenglin, Ruili and Bao32). Propyl gallate was used as a positive control.
α-Glucosidase inhibitory assay
An in vitro assay for α-glucosidase inhibitory activity was performed according to Atta-ur-Rahman et al. (Reference Atta-ur-Rahman, Zareen and Choudhary33). Acarbose was used as a positive control.
In vivo studies
Animals
Wistar rats of both sexes obtained from the animal house of the International Center for Chemical and Biological Sciences, University of Karachi, Pakistan were used throughout the study. The animals were kept in a temperature- and humidity-controlled environment (25 ± 2°C; 50–55 % humidity, respectively) with a 12 h light–12 h dark cycle. The rats were maintained in clean cages with ad libitum access to water and food. The experimental design of the present study was conducted according to the guidelines for care and management of laboratory animals, and the animal experimental protocol was approved by the institutional animal ethical committee.
Induction of type 2 diabetes
A freshly prepared solution of STZ (90 mg/kg) in 10 ml citrate buffer (0·1 m, pH 4·5) was intraperitoneally injected to 2-d-old Wistar rat pups to obtain type 2 diabetic rat models(Reference Bonner-Weir, Trent and Honey34). After 12 weeks of STZ induction, an oral glucose tolerance test at a dose of 3 g/kg glucose was performed and blood glucose was measured at 0, 30, 60 and 120 min, respectively. The rats with fasting blood glucose levels of 7·6–10·9 mmol/l at 0 min and the highest rise to 12·5–22·1 mmol/l at 30 min were included in the study. Blood was withdrawn from the tail tip every week to measure blood glucose levels using a glucometer (Accu-Chek® Go; Roche Diagnostics).
Chronic extract treatment
The experimental rats were divided into five groups of six animals each: age-matched non-diabetic control that did not receive STZ (group I); diabetic rats without any treatment (Db, group II); diabetic rats treated with A. officinalis at a dose of 250 mg/kg (Db+AO250, group III); diabetic rats treated with A. officinalis at a dose of 500 mg/kg (Db+AO500, group IV); diabetic rats treated with 5 mg glibenclamide/kg (Db+GB, group V). The extract and GB were administered orally once daily via syringe for 28 d to the diabetic rats. The control rats were given an equivalent volume of water. Animal weights were measured every week throughout the experiment and the dose was adjusted accordingly.
Collection of blood samples and estimation of biochemical parameters
At 13 weeks after the administration of STZ, blood samples from overnight fasted rats of each group were collected from the tail vein and the day was designated as day 1 (start day of the treatment). After 28 d of the extract treatment, i.e. on day 29, rats were anaesthetised (sodium thiopental, 60 mg/kg) and killed, and their venous blood was collected. The blood samples were centrifuged, and serum was separated within 30 min, divided into aliquots and kept at − 80 °C for biochemical assay. Thereafter, the pancreas was carefully and rapidly excised, trimmed of fat and adipose tissue, and fixed in 10 % neutral buffered formalin overnight. Then, all the formalin-fixed tissues were dehydrated in graded 2-propanol series and cleared with xylene. This was immediately followed by paraffin embedding using an embedding system. The tissues were cut into 5 μm serial sections with a microtome for haematoxylin–eosin (H&E) and immunohistochemical staining, and mounted on slides coated with gelatin.
Fasting serum glucose was measured by the glucose oxidase method (%CV 5·43 %) and fasting serum insulin was measured using the Ultra Sensitive Rat Insulin ELISA kit (%CV 3·95 %) on days 1 and 29, according to the manufacturer's protocol. TAS was measured using the ABTS® substrate assay kit (%CV 2·88 %) according to the manufacturer's instruction (Randox). Serum creatinine, alanine aminotransferase and aspartate aminotransferase were measured by standard techniques using the Reflotron® Plus Dry Chemistry Analyzer (Roche Diagnostics).
Microscopic examination
Haematoxylin and eosin staining
For H&E staining, sections were deparaffinised in xylene, rehydrated in graded 2-propanol series and washed in water. The sections were then stained with H&E. Pancreatic sections were visualised using a Nikon 90i microscope (Nikon) and the images were acquired with a Nikon DXM 1200C camera using NIS-Elements image analysis software AR 3.0 (Nikon).
Immunohistochemical staining
For immunohistochemical staining, six sections in different planes from each rat were triple stained for insulin, glucagon and nuclei. For this purpose, the sections were deparaffinised, rehydrated, washed in water and subjected to antigen retrieval (90°C for 30 min) in 0·1 m-citrate buffer (pH 6·0). After cooling to room temperature, each section was incubated with a blocking solution (2 % normal donkey serum in PBS) for 10 min at room temperature. Then, each section was incubated with a mixture of guinea-pig anti-insulin (1:100) and mouse anti-glucagon (1:1500) for 1 h. After washing with PBS, the sections were incubated with a mixture of Texas Red-conjugated donkey anti-guinea-pig IgG (1:100) and Cy2-conjugated donkey anti-mouse IgG (1:100) for 30 min. The nuclei were stained with DAPI, washed with PBS and mounted in Fluoromount solution (Sigma). The fluorescent images were visualised using a Nikon TE2000E fluorescent microscope equipped with a Nikon DS-2MBWc camera in DAPI, fluorescein isothiocyanate and TxRed channels. The images were acquired using NIS-Elements image analysis software AR 3.0 (Nikon). Finally, image processing was performed with Adobe Photoshop CS2. As a negative control, primary antibodies were excluded and no specific immunostaining was observed.
Morphometry
Morphometric measurements were performed using NIS-Elements image analysis software AR 3.0. The β-cell area was measured by acquiring at least five to eight non-overlapping images from each DAPI/insulin-stained section (six sections per rat) using a TE2000E microscope with a 10 × objective. DAPI/insulin-stained images were acquired from all visible multicellular islets as small as ten cells per section and as large as 160 cells per section. The total β-cell area was determined on sections stained with insulin antibody and the number of β-cell nuclei was counted. The total β-cell area was divided by the total number of nuclei to calculate individual β-cell size.
Toxicity evaluation
The extract of A. officinalis was tested for acute toxicity (if any) in rats. To determine acute toxicity, a single oral administration of A. officinalis, at different doses of the extract (0·25, 0·5, 1·0, 1·5 and 2·0 g/kg body weight), was administered orally to the different groups of Wistar rats (n 6) of both sexes. Mortality and general behaviours of the animals were observed continuously for the initial 4 h and intermittently for the next 6 h, and then again at 24, 48 and 72 h following extract administration. After 28 d of the extract treatment, autopsy was performed for examining any abnormality in the liver, kidney, gastrointestinal tract, spleen and heart. Serum creatinine, alanine aminotransferase, aspartate aminotransferase were also determined to examine whether there was any sign of toxicity in the kidney and liver, respectively.
Statistical analysis
Statistical analyses were performed using the SPSS 12.0 statistical package for Windows (SPSS, Inc.). Data are expressed as means with their standard errors. The significance of the differences among the mean values was calculated using one-way ANOVA with Dunnett's and Bonferroni post hoc tests, respectively. To compare data within the groups, a paired t test (two-tailed) was performed. Morphometric data are expressed as medians with their ranges and compared using the Mann–Whitney U test. P < 0·05 was considered to be statistically significant.
Results
In vitro antioxidant and α-glucosidase inhibitory activities of Asparagus officinalis
A. officinalis was evaluated for its radical-scavenging activity using DPPH. A. officinalis at a concentration of 0·5 mg/ml exhibited 86·8 % radical-scavenging activity, as shown by a significant decrease in the absorbance of DPPH radicals. These results suggest that A. officinalis has potent antioxidant activity, as the positive control propyl gallate exhibited 91·4 % radical-scavenging activity. A. officinalis exhibited only 32 % inhibition of α-glucosidase at a concentration of 0·5 mg/ml. This inhibitory result suggests that this extract has a very little effect on delaying glucose absorption.
Effect of the 28 d treatment of Asparagus officinalis extract on fasting blood glucose
In the type 2 diabetic rats, fasting blood glucose was increased moderately after 3 months of STZ induction compared with the non-diabetic rats (8·28 (sem 0·12) mmol/l v. 4·50 (sem 0·23) mmol/l, P < 0·001; Fig. 1). These fasting blood glucose levels did not alter significantly during the experimental periods (1–29 d). After the A. officinalis extract treatment, there was a significant decrease in fasting blood glucose in a dose- and time-dependent manner. At the 250 mg/kg dose, the blood glucose levels were significantly decreased compared with the untreated diabetic rats with respect to the values of day 15 (7·64 (sem 0·11) mmol/l v. 8·39 (sem 0·20) mmol/l, P < 0·001) and day 29 (6·77 (sem 0·17) mmol/l v. 8·51 (sem 0·23) mmol/l, P < 0·001). The 500 mg/kg dose decreased the blood glucose levels more efficiently compared with the 250 mg/kg dose. This dose (500 mg/kg) significantly decreased the blood glucose levels compared with the untreated diabetic rats with respect to the values of day 15 (6·54 (sem 0·18) mmol/l v. 8·39 (sem 0·20) mmol/l, P < 0·001) and day 29 (5·44 (sem 0·13) mmol/l v. 8·51 (sem 0·23) mmol/l, P < 0·001).
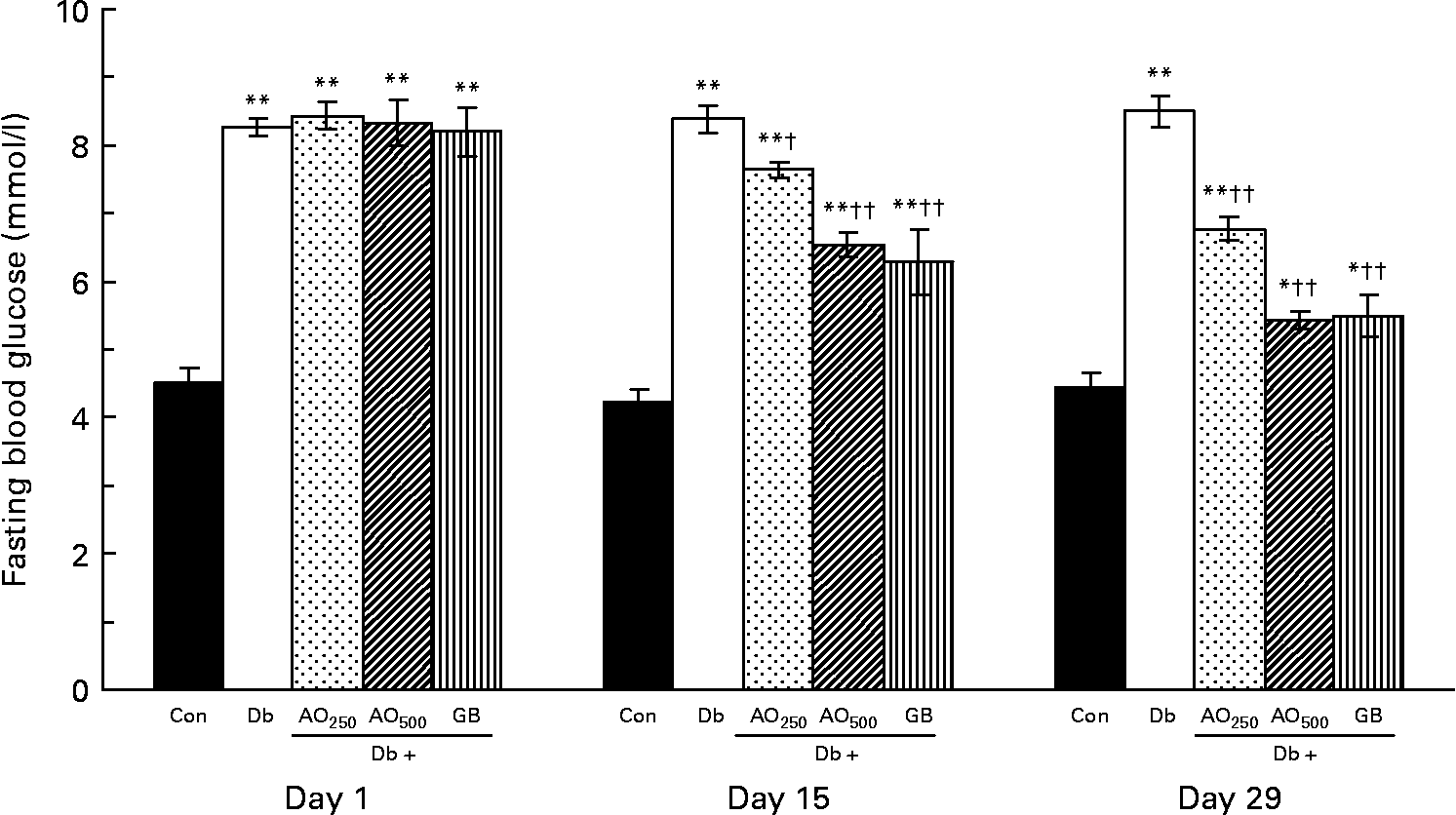
Fig. 1 Effect of Asparagus officinalis extract on fasting blood glucose (mmol/l) in type 2 diabetic rats. Values are means, with standard errors represented by vertical bars (n 6). Mean values were significantly different from those of the non-diabetic control rats: *P < 0·01, **P < 0·001 (one-way ANOVA with Bonferroni post hoc test). Mean values were significantly different from those of the untreated diabetic rats: †P < 0·01, ††P < 0·001 (one-way ANOVA with Bonferroni post hoc test). Con, age-matched non-diabetic control; Db, untreated diabetic rats; AO250, diabetic rats treated with 250 mg/kg A. officinalis extract; AO500, diabetic rats treated with 500 mg/kg A. officinalis extract; GB, glibenclamide.
In A. officinalis-treated rats, comparison of the blood glucose values with their baseline values (day 1) showed that both the doses of A. officinalis extract significantly decreased the fasting blood glucose levels in a time-dependent manner. The blood glucose levels of the Db+AO250 rats significantly decreased on day 15 as well as on day 29 with respect to their value of day 1 (day 1, 8·44 (sem 0·20) mmol/l; day 15, 7·64 (sem 0·11) mmol/l; day 29, 6·77 (sem 0·17) mmol/l). The blood glucose levels of the Db+AO500 group also significantly decreased on day 15 as well as on day 29 with respect to their value of day 1 (day 1, 8·33 (sem 0·33) mmol/l; day 15, 6·54 (sem 0·18) mmol/l; day 29, 5·44 (sem 0·13) mmol/l). The standard drug GB also lowered the blood glucose levels significantly in a dose- and time-dependent manner.
Effect of the 28 d treatment of Asparagus officinalis extract on fasting serum insulin
Fasting serum insulin levels were significantly (P < 0·001) decreased in the untreated diabetic rats (49·28 (sem 7·19) pmol/l) when compared with the control group (120·22 (sem 18·02) pmol/l; Fig. 2). There was no significant change in serum insulin levels in the control and untreated diabetic rats during the experimental periods. When the diabetic rats were treated with A. officinalis extract at a dose of 250 mg/kg for 28 d, there was a slight, statistically non-significant, increase in serum insulin levels compared with the untreated diabetic rats (62·23 (sem 6·30) pmol/l v. 46·91 (sem 6·92) pmol/l, P = 0·133). However, the dose of 500 mg/kg significantly increased the serum insulin levels compared with the untreated diabetic rats (77·55 (sem 9·40) pmol/l v. 46·91 (sem 6·92) pmol/l, P < 0·05). GB also significantly increased the serum insulin levels (81·47 (sem 7·62) pmol/l).

Fig. 2 Effect Asparagus officinalis extract on fasting serum insulin (pmol/l) in type 2 diabetic rats. Values are means, with standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the non-diabetic control rats (P < 0·001; one-way ANOVA with Bonferroni post hoc test). † Mean values were significantly different from those of the untreated diabetic rats (P < 0·01; one-way ANOVA with Bonferroni post hoc test). Con, age-matched non-diabetic control; Db, untreated diabetic rats; AO250, diabetic rats treated with 250 mg/kg A. officinalis extract; AO500, diabetic rats treated with 500 mg/kg A. officinalis extract; GB, glibenclamide.
Effect of the 28 d treatment of Asparagus officinalis on fasting insulin:glucose ratio
The insulin:glucose ratio was calculated from the fasting insulin and glucose values. It was found that 25·01 (sem 0·66) pmol insulin/mmol glucose was available in the control rats; however, only 5·9 (sem 0·39) pmol insulin/mmol glucose was available in the diabetic rats (Fig. 3). After the 28 d treatment of A. officinalis extract, interestingly, there was a 1·7- and 2·6-fold increase in insulin:glucose ratio in the AO250- and AO500-treated diabetic rats compared with the untreated diabetic rats (Db, 5·40 (sem 0·33) pmol/mmol; Db+AO250, 9·15 (sem 0·40) pmol/mmol; Db+AO500, 14·15 (sem 0·73) pmol/mmol). GB also increased the insulin:glucose ratio (14·72 (sem 0·77) pmol/mmol) comparable to the value of AO500.
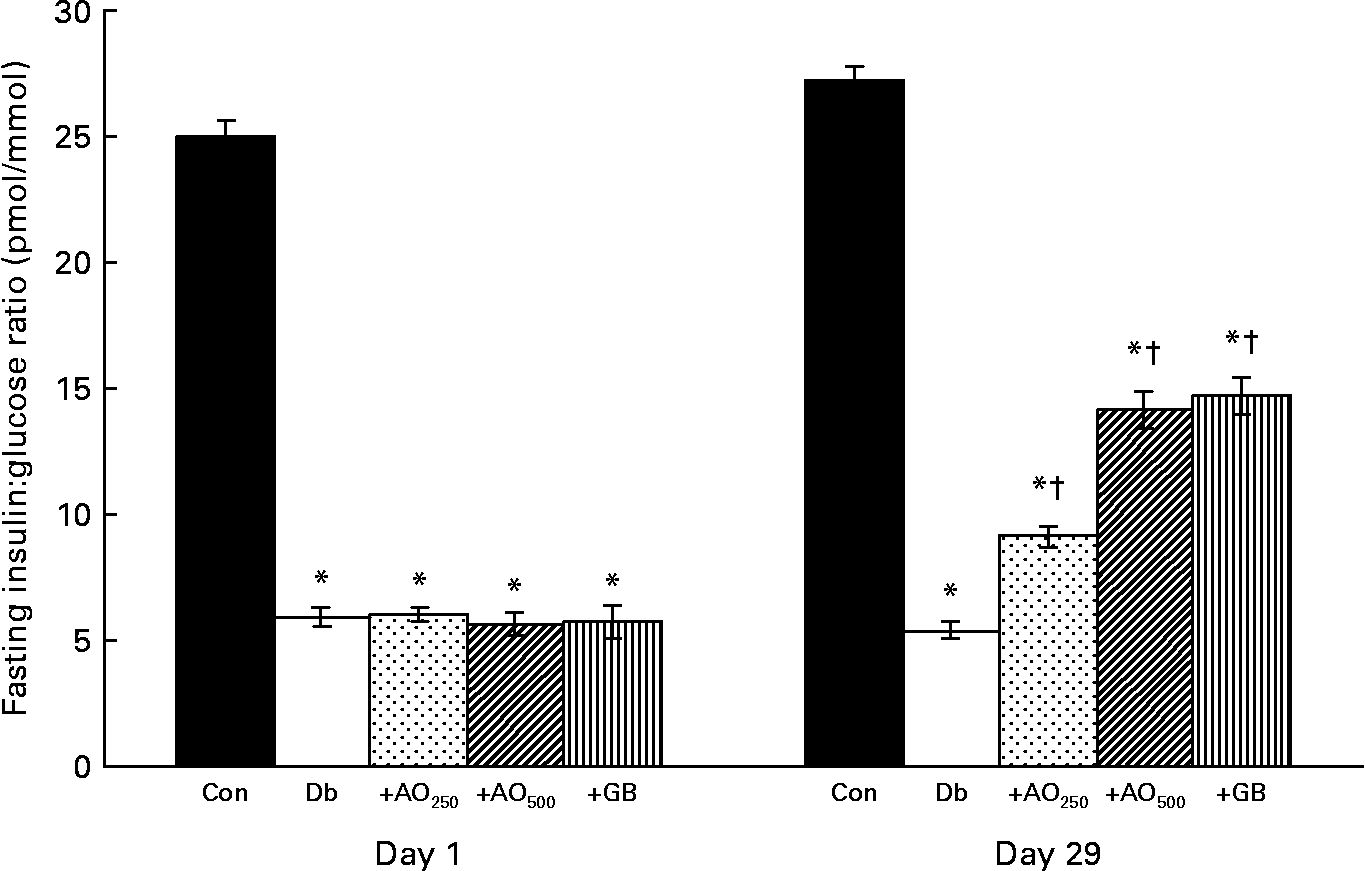
Fig. 3 Effect of Asparagus officinalis extract on fasting insulin:glucose ratio (pmol/mmol) in the experimental rats. Values are means, with standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the non-diabetic control rats (P < 0·001; one-way ANOVA with Bonferroni post hoc test). † Mean values were significantly different from those of the untreated diabetic rats (P < 0·001; one-way ANOVA with Bonferroni post hoc test). Con, age-matched non-diabetic control; Db, untreated diabetic rats; AO250, diabetic rats treated with 250 mg/kg A. officinalis extract; AO500, diabetic rats treated with 500 mg/kg A. officinalis extract; GB, glibenclamide.
Morphology of rat pancreas
The results of typical H&E staining obtained upon histological examination are shown in Fig. 4. In the control rats (Fig. 4(a)), islets of different sizes (small, medium and large) and shapes were found in all the pancreatic sections throughout the study period. On the contrary, diabetic pancreatic sections showed a reduction in pancreatic islet area and number (Fig. 4(b)). In the 250 and 500 mg/kg A. officinalis-treated diabetic groups, the pancreatic area and number was more than that observed in the untreated diabetic group (Fig. 4(c) and (d), respectively). The GB-treated group also showed improved islet size (Fig. 4(e)) similar to the AO500-treated group.

Fig. 4 (a–e) Light microscopic study of the pancreatic islets (10 × ) in the different experimental groups: (a) non-diabetic control; (b) untreated diabetic; (c) Asparagus officinalis 250 mg/kg, (d) A. officinalis 500 mg/kg; (e) glibenclamide-treated. White dotted lines represent the islet area and the arrow indicates the small islets.
Immunohistochemical studies on the pancreas
Pancreatic islets stained for insulin, glucagon and DAPI fluorescence in the experimental rats are shown in Fig. 5. Insulin-positive cells were found abundantly in the islets of the control rats, and were located mostly in the centre of the islet (Fig. 5(a)). Different sizes of the total β-cell area were seen in the pancreas of the control rats. The ratio of the β- and α-cells was found to be 4:1. In the untreated diabetic rats, the insulin-positive cells were also found in the centre of the islet; however, the total number and the area of the β-cells were reduced significantly (Fig. 5(b)). In the diabetic rats, the large islets were absent and only a few β-cells containing the islets were visible. In contrast, when the diabetic rats were treated with A. officinalis extract for 28 d, the average size of the total β-cell area and β-cell number was increased in both AO250- and AO500-treated groups (Fig. 5(c) and (d), respectively). The GB-treated diabetic group also showed an increased total β-cell area and number of the β-cells (Fig. 5(e)) in the islets.

Fig. 5 (a–e) Mutichannel fluorescence microscopic study of insulin-positive β-cells (red), glucagon-positive α-cells (green) and nuclei (blue) of the pancreatic islets (20 × ) of the different experimental groups: (a) non-diabetic control; (b) untreated diabetic; (c) Asparagus officinalis 250 mg/kg, (d) A. officinalis 500 mg/kg; (e) glibenclamide-treated.
The glucagon-positive cells were found around the periphery of the insulin-positive cells in the control islets (Fig. 5(a)). In the diabetic rats, the distribution pattern of the glucagon-positive cells was similar to that of the control group (Fig. 5(b)); however, in some cases, the glucagon-stained cells were also seen scattered within the centre of the islets (data not shown). Interestingly, the number of the α-cells relatively increased in the diabetic rats. The A. officinalis extract had no effect on the distribution of the glucagon-positive cells in the diabetic rats (Fig. 5(c) and (d)). GB also had no effect on the distribution of the glucagon-positive cells in the diabetic rats (Fig. 5(e)).
A representation of the nuclei staining of the non-diabetic control (Fig. 5(a)), diabetic control (Fig. 5(b)), A. officinalis 250 and 500 mg/kg (Fig. 5(c) and (d), respectively), and GB-treated rats (Fig. 5(e)) is also shown for comparison.
Morphometric studies on the pancreas
In the control rats, the total β-cell area/islet, expressed as medians, was 18 420 (range 5375–37 543) μm2, whereas in the diabetic rats, it was decreased significantly (6099 (range 3165–15 391) μm2, P < 0·001; Table 1). The β-cell area was 8918 (range 3123–15 068) and 10 205 (range 3065–17 575) μm2 in the AO250- and AO500-treated rats, respectively. These results indicate that A. officinalis can markedly (P < 0·001) increase the total β-cell area. In the control rats, the number of β-cells per islet, expressed as medians, was 111 (range 22–160). However, the β-cell number decreased significantly (P < 0·001) in the diabetic rats (28 (range 11–64) v. 111 (range 22–160), P < 0·001). There was a significant increase in β-cell number in the AO250-treated (35 (range 18–58)) and AO500-treated (44 (range 14–98)) rats, respectively. Although the β-cell area and number decreased significantly in the diabetic rats, very interestingly, the individual β-cell size, expressed as medians, was increased in the diabetic rats compared with the control rats (221 (range 85–699) μm2v. 202 (range 44–475) μm2, P < 0·060). The P value is just outside the border of the significance level, suggesting that hyperplasia of the β-cell may have occurred in this type 2 diabetic rat model. The individual β-cell size was increased (very close to the significance level) in the AO250-treated rats compared with the control rats (222 (range 79–460) μm2v. 202 (range 44–475) μm2, P < 0·065). There was no significant change in individual β-cell size in the AO500- or GB-treated diabetic rats. These results suggest that β-cell hypertrophy is the phenomenon of this rat model; however, it is the hyperplasia, not hypertrophy, of the β-cell in the A. officinalis-treated diabetic rats that might have contributed to the anti-diabetic effect.
Table 1 Morphometric analysis of the pancreatic islets in the experimental rats (Median values with their ranges; sixty islets/rats; n 6–9)
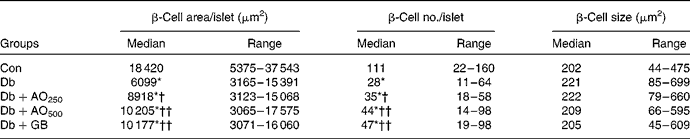
Con, age-matched non-diabetic control; Db, untreated diabetic rats; AO250, diabetic rats treated with 250 mg/kg Asparagus officinalis extract; AO500, diabetic rats treated with 500 mg/kg Asparagus officinalis extract; GB, glibenclamide.
* Median values were significantly different from those of the non-diabetic control (P < 0·001; Mann–Whitney U test). Median values were significantly different from those of the untreated diabetic: †P < 0·05, ††P < 0·01 (Mann–Whitney U test).
Effect of the 28 d treatment of Asparagus officinalis extract on total antioxidant status
Changes in TAS in each group of rats are shown in Fig. 6. A significant decrease in TAS was found in the untreated diabetic rats compared with the control rats (1·19 (sem 0·06) mmol/l v. 1·86 (sem 0·08) mmol/l, P < 0·01). A. officinalis extract at a dose of 250 or 500 mg/kg for 28 d significantly increased the TAS in a dose-dependent manner (AO250, 1·61 (sem 0·10) mmol/l; AO500, 1·88 (sem 0·21) mmol/l). In the 500 mg/kg A. officinalis-treated rats, TAS was found even more than that of the control rats. A mild but significant increase in TAS (1·55 (sem 0·02) mmol/l) was also found in the GB-treated rats.

Fig. 6 Effect of Asparagus officinalis extract on the total antioxidant status (mmol/l) in the experimental rats. Values are means, with standard errors represented by vertical bars (n 6). * Mean values were significantly different from those of the non-diabetic control rats (P < 0·001; one-way ANOVA with Bonferroni post hoc test). Mean values were significantly different from those of the untreated diabetic rats: †P < 0·05, ††P < 0·001 (one-way ANOVA with Bonferroni post hoc test). Con, age-matched non-diabetic control; Db, untreated diabetic rats; AO250, diabetic rats treated with 250 mg/kg A. officinalis extract; AO500, diabetic rats treated with 500 mg/kg A. officinalis extract; GB, glibenclamide.
Discussion
The present study demonstrated that administration of a methanolic extract of A. officinalis seeds to n-STZ non-obese type 2 diabetic rats decreased blood glucose levels in a dose- and time-dependent manner (Fig. 1). Preliminary in vitro experiments for α-glucosidase inhibitory activity showed that A. officinalis extract has a very little effect on α-glucosidase, suggesting that A. officinalis may have a very little to no effect on delaying glucose absorption.
Serum insulin levels were significantly decreased in the untreated diabetic rats (Fig. 2). Pathological changes in serum insulin levels in diabetes reflect abnormalities in β-cell function or structure. The decrease in the β-cell function and size of the islets (Figs. 4 and 5) is a clear indication that the n-STZ type 2 diabetes in these rats was not due to insulin resistance but was because of β-cell dysfunction, which is the major pathophysiology of non-obese type 2 diabetic subjects in the Indo-Pak region(Reference Roy, Biswas and Siddiqua35, Reference Karim, Zinnat and Akter36).
The untreated diabetic rats had higher blood glucose levels and lower insulin levels than the non-diabetic controls (Figs. 1 and 2, respectively). Interestingly, the A. officinalis extract treatment significantly improved serum insulin levels as well as the insulin:glucose ratio in the diabetic rats (Figs. 2 and 3, respectively). The increased serum insulin levels may be related to the up-regulation of insulin synthesis by the β-cells, enhanced insulin secretion or insulin release from the pancreatic β-cells. Previously, it has been reported that the ethanolic extract of A. racemosus roots increased insulin secretion in the isolated islets of rats in vitro (Reference Hannan, Marenah and Ali37). Thus, the control of blood glucose levels is most probably due to the enhanced insulin secretion from the pancreatic β-cells in the A. officinalis-treated diabetic rats.
The A. officinalis treatment improved the size of the islets in the diabetic rats (Fig. 4). The β-cell is the most abundant cell type in the endocrine pancreas, and β-cell number is the most important factor that determines the islet area. It has been reported elsewhere that α-cells increased in diabetic rats and the distribution pattern of the α- and β-cells changed in STZ-induced diabetic rats(Reference Papaccio and Mezzogiorno38, Reference Pons and Aoki39). In the present study, it was not clear from H&E staining whether the increased size of the islets was due to the β- or α-cells (Fig. 4). To determine this, we stained the same pancreatic sections with insulin and glucagon antibodies to observe the insulin-positive β-cells and glucagon-positive α-cells simultaneously. The immunohistochemical data revealed that it was the β-cell that played the major role for the increased size of the islets (Fig. 5).
A. officinalis improved β-cell function in the diabetic rats. However, it was not clear whether this improvement of β-cell function was due to the hyperplasia or hypertrophy of the β-cells. The immunohistochemical and morphometric studies suggest that hyperplasia rather than hypertrophy in the A. officinalis-treated rats (Fig. 5 and Table 1) was responsible for the improvement in β-cell function. Therefore, the increased number of β-cells may have a direct role in controlling the blood glucose levels in this diabetic rat model. Furthermore, the high-resolution insulin immunohistochemical data revealed that most of the β-cells were degranulated in the treatment group, suggesting that A. officinalis stimulated the release of insulin from the pancreatic β-cells (data not shown). Therefore, the present biochemical results along with the immunohistochemical findings further support that the anti-diabetic activity of A. officinalis may be due to the enhanced insulin secretion and/or insulin release.
The β-cell number in the pancreatic islets of the A. officinalis-treated rats was significantly higher than that of the untreated diabetic rats (Table 1). This suggests that A. officinalis may have a role in the regeneration or revitalisation of the β-cells or in the recovery of the partially damaged β-cells. A number of other plants have also been observed to exert anti-diabetic activity through these mechanisms(Reference Singh and Gupta40–Reference Hafizur, Kabir and Chishti42).
Although the A. officinalis extract showed potent antioxidant activity in vitro, we asked whether the antioxidant had any role in the mechanism of the anti-diabetic property of the A. officinalis extract. In support to the antioxidant activity of the extract, we found that TAS was increased significantly in the A. officinalis-treated rats (Fig. 6). The mechanisms responsible for the increased antioxidant status of A. officinalis are not clear. However, a strong correlation between the antioxidant activity and the total phenol content of asparagus has been reported, suggesting that phenols could be mainly responsible for the antioxidant activity, as observed for other vegetables(Reference Gardner, White and McPhail43, Reference Martinez-Valverde, Periago and Provan44). The antioxidant activity of A. officinalis extract may prevent STZ-induced oxidant damage to the β-cells. Thus, it may also suggest that the anti-diabetic activity may partially be due to the antioxidant property of the extract. Studies are ongoing in our laboratory to elucidate this hypothesis.
In conclusion, we have shown that long-term administration of A. officinalis has anti-diabetic effects on non-obese type 2 diabetic rats. The anti-diabetic activity appears mainly due to the enhanced insulin secretion and the modulation of pancreatic β-cell function. The antioxidant may also play an indirect role in the anti-diabetic activity of A. officinalis. Altogether, these findings indicate that asparagus may be an effective therapeutic strategy for the management of non-obese type 2 diabetes. Further experiment and clinical studies are required to explore the additional mechanisms and establish its clinical utility.
Acknowledgements
This study was supported by a grant (5-9/3394/PAS/) from the Pakistan Academy of Sciences (PAS), Islamabad, Pakistan. We would like to appreciate Professor Muhammad Iqbal Choudhary for his encouragement. We are thankful to Dr Suad Naheed and Dr Sajjad Ali for the in vitro assays, and Maryam Bano for the GB experiments. R. M. H. and N. K. designed the study. R. M. H. conducted the research, analysed the data and wrote the manuscript. R. M. H. and N. K. analysed the histological and immunohistochemical changes. S. C. was involved in animal handling, feeding the extract to the animals and blood glucose estimation. All the authors read and approved the final manuscript. None of the authors has conflicts of interest.









