Challenge by pathogenic Escherichia coli is responsible for a large variety of disorders in production, resulting in intestinal disruption and subsequent compromise in growth performance of chickens( Reference He, Fu and Shen 1 – Reference Gao, Han and Huang 3 ). In the past few decades, antibiotics were widely used to prevent or control E. coli infection in animals. However, antibiotic treatment led to increasing drug residues and drug-resistant bacteria and affected the health of animals. As a consequence, alternatives to antibiotics that could be applied to control or attenuate colibacillosis are necessary to protect the health status and growth performance of chickens. Recent studies have focused on the importance of probiotics and prebiotics as potential substitutes for antibiotics to alleviate bacteria-related immune dysfunction and intestinal damage, as well as impaired performance in chickens( Reference Cao, Zeng and Chen 2 , Reference Ribeiro, Vogt and Canal 4 – Reference Penha-Filho, Díazb and Fernando 7 ).
Mannan-oligosaccharide (MOS) holds an important position among multifarious prebiotics, which has been indicated to suppress the adhesion of some bacteria to the gut( Reference Spring, Wenk and Dawson 8 ) and exert beneficial effects on growth performance, intestinal immunity and structure as well as gut microflora in broilers( Reference Yang, Iji and Kocher 5 , Reference Baurhoo, Letellier and Zhao 9 , Reference Abudabos and Yehia 10 ). However, a few studies have been carried out on whether MOS addition could alleviate intestinal inflammation and protect the intestinal barrier against bacteria in broilers.
An important constituent of probiotics is live yeast (LY, Saccharomyces cerevisiae) that has been reported to modulate intestinal microbial balance( Reference Heugten, Funderburke and Dorton 11 , Reference Haldar, Ghosha and Toshiwatia 12 ), improve humoral immune responses and intestinal structure and function of animals( Reference Haldar, Ghosha and Toshiwatia 12 – Reference Jang, Kang and Piao 14 ). Furthermore, LY addition was indicated to mitigate bacteria-associated immunological derangement and intestinal disorders in pigs( Reference Badia, Lizardo and Martinez 15 – Reference Trevisi, Colombo and Priori 17 ) and to attenuate pathogen-induced intestinal inflammation in mice( Reference Jawhara, Habib and Maggiotto 18 ). Similar results were also found in in vitro studies, where LY treatment reduced the expressions of pro-inflammatory cytokines and increased the expressions of anti-inflammatory cytokines of porcine intestinal epithelial cells following bacterial invasion( Reference Zanello, Berri and Dupont 19 , Reference Zanello, Meurens and Berri 20 ). Nevertheless, an understanding of the effects of LY addition on intestinal inflammation and barrier function in infected chickens is lacking. Therefore, the present study was conducted to determine the effects of supplemental LY and MOS on intestinal inflammatory responses and barrier function in broilers challenged with E. coli.
Methods
Birds and experimental design
The experimental animal protocol for this study was approved by the Animal Care and Use Committee of China Agricultural University. The experimental design was a 3×2 factorial arrangement. A total of 540 1-d-old male Arbor Acre broilers were randomly divided into three treatment groups with two subgroups each (nine replicates). Initial body weights were similar across all the groups. Birds received mashed basal diets with or without 0·5 g/kg MOS (SAF-mannan; Phileo Lesaffre Animal Care) or 0·5 g/kg LY (S. cerevisiae Actisaf Sc 47, 1·0×1010 colony-forming units (CFU)/g; Phileo Lesaffre Animal Care) throughout the trial period. The composition of the basal diets is shown in Table 1. All birds were maintained in wire-floored cages in a three-level battery on their respective diets, and were vaccinated using combined Newcastle disease virus and infectious bronchitis virus at 9 d of age and infectious bursal disease virus at 21 d of age via intranasal and intraocular administration. Feed and fresh water were available ad libitum. Birds were maintained at a 20 h light–4 h dark cycle throughout the trial period. Temperature was controlled with heaters and gradually reduced from 35°C on d 1 to 24°C at 21 d of age and then kept roughly constant.
Table 1 Composition of the experimental diets (g/kg)
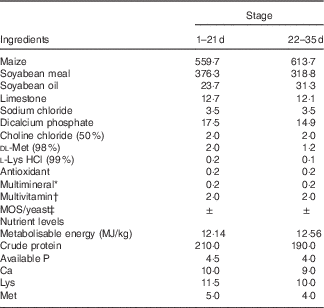
MOS, mannan-oligosaccharide.
* Supplied per kg of diet: Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0·15 mg; I, 0·35 mg.
† Supplied per kg of diet: retinyl acetate, 24 mg; cholecalciferol, 6 mg; menadione, 2·65 mg; thiamin, 2 mg; riboflavin, 6 mg; cyanocobalamin, 0·025 mg; α-tocopherol acetate, 20 mg; biotin, 0·0325 mg; folic acid, 1·25 mg; pantothenic acid, 12 mg; niacin, 50 mg.
‡ MOS or yeast was substituted for the same amount of maize.
Oral challenge and sampling
The E. coli O78 strain (CVCC1490; China Veterinary Culture Collection Center) was cultured in lactose broth at 37°C for 16 h. To enumerate bacteria, inoculum was diluted and plated on MacConkey agar at 37°C for 24 h. From 7 to 11 d of age, half of the birds from each treatment group were orally gavaged with 1 ml of E. coli O78 culture (1·0×109 CFU/ml) or the same amount of lactose broth. One bird per replicate was randomly selected for sample collection at 14 d of age. Individual blood samples were collected aseptically from the wing vein; serum samples were separated by centrifugation of blood at 3000 rpm for 10 min at 4°C and stored at −30°C until analysed. After blood collection, these birds were slaughtered rapidly, and the midpoints of the jejunum and ileum of each bird were harvested and cut into two segments. One sample was fixed in 4 % paraformaldehyde solution, and the other was frozen in liquid N2 and kept at −80°C for quantification of gene expression. Besides, chyme and mucosa samples were collected from jejunal and ileal segments and quick-freezed using liquid N2, followed by the preservation at −80°C until further analysis.
Performance determination
Body weight and feed intake of broilers were recorded for each replicate at 21 and 35 d of age. Average body weight (ABW), average daily gain (ADG), average daily feed intake (ADFI), feed conversion ratio (FCR), mortality during the grower period (1–21 d) and the overall period (1–35 d) were calculated.
Biochemical assay of serum and intestinal mucosa
Serum endotoxin was quantified using a quantitative chromogenic substrate assay kit (Xiamen TAL Experimental Plant Co., Ltd). Serum diamine oxidase (DAO) activity was determined colorimetrically using a commercial kit according to the manufacturer’s protocols (Huaying Biotechnology Research Institute). Intestinal lysozyme and myeloperoxidase (MPO) activities were also measured using colorimetric kits according to the manufacturer’s protocols (Jiancheng Bioengineering Institute). Disaccharidase activity was determined as described previously( Reference Lamb-Rosteski, Kalischuk and Inglis 21 ). The results of the above-mentioned intestinal parameters were normalised by total protein content, which was measured using BCA protein quantitation kits (Jiancheng Bioengineering Institute).
Intestinal morphological analyses
We obtained 4-μm cross-sections of jejunal and ileal tissues after staining with haematoxylin–eosin using standard paraffin-embedding procedures. From each section, ten longest and intact villi were selected for morphology measurement using Leica DMI6000B light microscope equipped with an image-processing software (Leica application suite V4.2). Villus height (VH) was measured from the tip of villus to the junction of villus and crypt. Crypt depth was defined as the depth of emboli between adjacent villi. Villus width (VW) was defined as the width of the widest point of it. Villus height:crypt depth ratio (VCR) and villus surface area (π×VH×VW) were calculated. The mean value of these ten values represents the final value of each sample.
RNA extraction and real-time quantitative PCR
Total RNA was extracted from ileal samples using Trizol reagent (Invitrogen Biotechnology Inc.) and dissolved in RNase-free water. The concentration of extracted RNA was determined using NanoDrop spectrophotometer (ND-2000 UV-Vis; Thermo Scientific Inc.). RNA purity was verified by measuring the absorbance ratio at 260:280 nm. RNA integrity was evaluated on the basis of the spectral curve(
Reference Fleige and Pfaffl
22
). Total RNA was then reverse transcribed using PrimeScriptTM RT reagent kit with gDNA Eraser (Takara Biotechnology Inc.), and complementary DNA (cDNA) was stored at −20°C until analysed. Real-time PCR for measuring intestinal gene expression was carried out using SYBR® Premix Ex TaqTM (Tli RNaseH Plus) (Takara Biotechnology Inc.) in ABI 7500 Real Time PCR Systems (Applied Biosystems). The expression of β-actin was used as an internal control to normalise the amount of initial RNA for each sample. The reaction volume of 20 μl mixture contained 10 μl SYBR® Premix Ex Taq (Tli RNaseH Plus), 0·4-μl ROX reference dye, 0·4 μl of each forward and reverse primer, 6·8-μl easy dilution and 2-μl cDNA template. The primer sequences for the target and reference genes are shown in Table 2. The optimised protocol for all the genes was 95°C for 30 s followed by forty cycles of 95°C for 5 s and 60°C for 34 s. All measurements were carried out in triplicate, and the average values were obtained. Real-time PCR efficiency for each gene was calculated on the basis of the slope of cDNA relative standard curve that was generated using pooled samples. The efficiency values between the reference gene and target genes were consistent. The abundance of β
-actin mRNA was not affected by dietary treatment or E. coli challenge. Specificity of PCR products was evaluated by the analysis of melting curve. The results of relative mRNA expression of intestinal genes were calculated using the
![]() $2^{{{\minus}\Delta \Delta C_{t} }} $
method(
Reference Livak and Schmittgen
23
).
$2^{{{\minus}\Delta \Delta C_{t} }} $
method(
Reference Livak and Schmittgen
23
).
Table 2 Sequences for real-time PCR primers
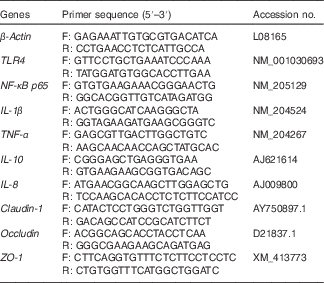
F, forward; R, reverse; TLR4, Toll-like receptor 4; ZO, zonula occludens.
Pyrosequencing of ileal microbiota
DNA samples were extracted from ileal digesta using QIAamp DNA Stool Mini Kits (Qiagen Inc.) according to the manufacturer’s protocol. The concentration and quality of extracted DNA were checked with gel electrophoresis and NanoDrop 2000 spectrophotometer. Bacterial 16S rRNA sequences spanning the variable regions V3–V4 were amplified using primer 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). A 10-ng aliquot of each sample DNA was used for PCR reaction using TransStart FastPfu DNA Polymerase in a 20-µl reaction volume. Amplification by PCR consisted of the following steps: denaturation at 95°C for 3 min, twenty-eight cycles of 30 s at 95°C, 55°C for 30 s, and 72°C for 45 s, and extension at 72°C for 10 min. The PCR products were detected by 2 % agarose gel electrophoresis and purified with QIAquick Gel Extraction Kit (Qiagen Inc.). A DNA Library was constructed using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina) and detected by Qubit and q-PCR quantification. Pyrosequencing was carried out on the Illumina HiSeq2500 PE250 platform (Illumina). All the procedures were conducted by Novogene Bioinformatics Technology Co., Ltd. Sample reads were assembled using mothur software. Clustering of filtered sequences into operational taxonomic units (OTU) was achieved using Uparse at 97 % sequence identity. Taxonomic classification at different taxonomic levels of these OTU sequences was performed by comparing sequences with the GreenGene database. Qiime software and Python scripts were used for the analysis of microbial diversity. α-Diversity metrics including Shannon, Simpson, Chao1 and abundance-based coverage estimator (ACE) indices were calculated. β-Diversity (UniFrac distances) was visualised by principal component analysis (PCA) and principal coordinates analysis (PCoA).
Statistical analysis
Data are presented as mean values with their standard errors and analysed by two-way ANOVA to measure the main effects of dietary treatment and E. coli challenge using the general linear model procedure of SPSS 18.0 software. Differences between different groups were analysed by Duncan’s multiple comparisons. Significance was set at P<0·05, and 0·05<P<0·10 was viewed as a trend towards significance. One-way ANOVA was used to analyse the results if interaction was significant.
Results
Growth performance
E. coli challenge reduced (P<0·05) ABW of birds on d 21 and 35, as well as ADG and ADFI during the grower period coupled with ADG during the overall period (Table 3). Besides, there was an increase in (P<0·05) FCR during the grower period, along with a decreasing trend for ADFI (P<0·10) during the overall period in response to the challenge. Dietary treatment with LY or MOS did not modify (P>0·05) ABW, ADG and ADFI of broilers, but the addition of LY reduced (P<0·05) FCR of birds during the grower period. The increased mortality of birds due to E. coli challenge was not influenced (P>0·05) by dietary treatment (data not shown).
Table 3 Effects of dietary treatment on growth performance of broilers challenged with Escherichia coli (Mean values with their standard errors; n 9)
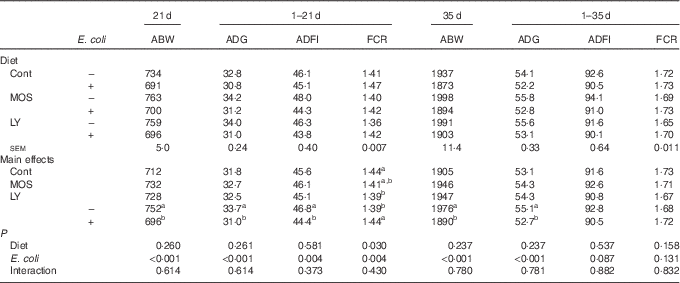
ABW, average body weight (g); ADG, average daily gain (g); ADFI, average daily feed intake (g); FCR, feed conversion ratio; Cont, control; MOS, mannan-oligosaccharide; LY, live yeast.
a,b Mean values within a column with unlike superscript letters are significantly different (P<0·05).
Serum and intestinal biochemical parameters
Challenged groups had higher (P<0·05) endotoxin and DAO levels in serum as well as MPO and lysozyme activities in the ileum compared with unchallenged groups (Table 4). A decreasing trend (P<0·10) of maltase activity in the jejunum and a reduction (P<0·05) of it in the ileum were recorded after E. coli challenge. Dietary treatment with both LY and MOS reduced (P<0·05) serum DAO activity and ileal MPO activity, and tended to alleviate (P<0·10) E. coli-induced increase in serum endotoxin content.
Table 4 Effects of dietary treatment on serum and intestinal biochemical parameters of broilers challenged with Escherichia coli (Mean values with their standard errors; n 9)
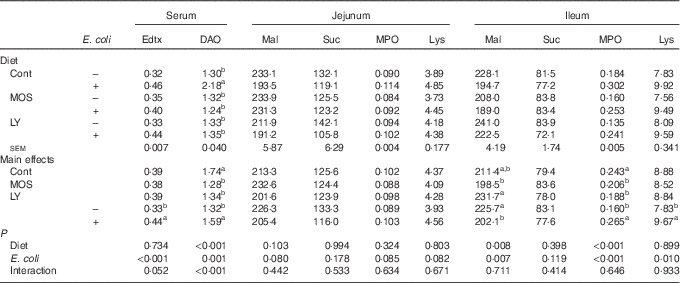
Edtx, endotoxin (EU/ml); DAO, diamine oxidase (U/ml); Mal, maltase (U/g protein); Suc, sucrase (U/g protein); MPO, myeloperoxidase (U/g tissue); Lys, lysozyme (U/mg protein); Cont, control; MOS, mannan-oligosaccharide; LY, live yeast.
a,b Mean values within a column with unlike superscript letters are significantly different (P<0·05).
Intestinal morphology structure
As shown in Table 5, E. coli challenge resulted in a reduction (P<0·05) in jejunal VCR and a decreasing trend (P<0·10) in ileal VCR. There was an increase in (P<0·05) ileal VCR and a tendency towards an increased (P<0·10) VH in the ileum after dietary treatment with both LY and MOS.
Table 5 Effects of dietary treatment on the intestinal morphology structure of broilers challenged with Escherichia coli (Mean values with their standard errors; n 9)
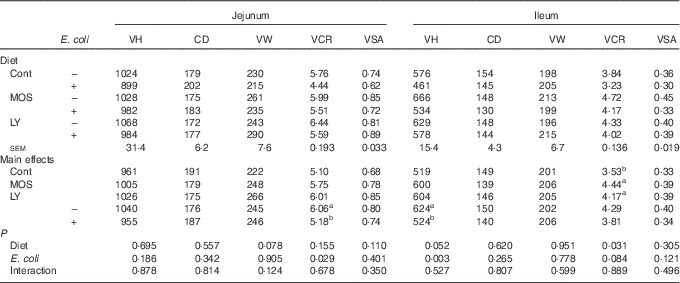
VH, villus height (µm); CD, crypt depth (µm); VW, villus width (µm); VCR, the ratio of VH:CD; VSA, villus surface area (mm2); Cont, control; MOS, mannan-oligosaccharide; LY, live yeast.
a,b Mean values within a column with unlike superscript letters are significantly different (P<0·05).
Relative mRNA expression of ileal genes
E. coli challenge increased (P<0·05) the mRNA expressions of ileal Toll-like receptor 4 (TLR4), NF- κ B, IL-1 β and IL-8, but tended to down-regulate (P<0·10) ileal IL-10 expression (Fig. 1). There were interactions (P<0·05) between dietary treatment and E. coli challenge on ileal TLR4, NF-κ B and IL-1 β expressions, as exhibited by the mitigatory effects (P<0·05) of dietary treatment with both LY and MOS on the elevated (P<0·05) expressions of ileal TLR4, NF- κ B and IL-1 β in challenged birds. In addition, LY-treated birds showed higher (P<0·05) ileal TNF- α expression compared with unchallenged birds. Administration of MOS into challenged birds prevented (P<0·05) the reduction in IL-10 expression. A reduced (P<0·05) expression of ileal occludinand a decreasing trend (P<0·10) of ileal zonula occludens (ZO)-1 expression were observed because of E. coli challenge (Fig. 2). The effect of dietary treatment (P>0·05) with MOS or LY was not noted for ileal claudin-1 and ZO-1 expressions. However, there was an interaction (P<0·05) between E. coli challenge and dietary treatment on ileal occludin expression, as evidenced by the counteraction (P<0·05) of the reduced occludin expression in challenged birds due to MOS addition.
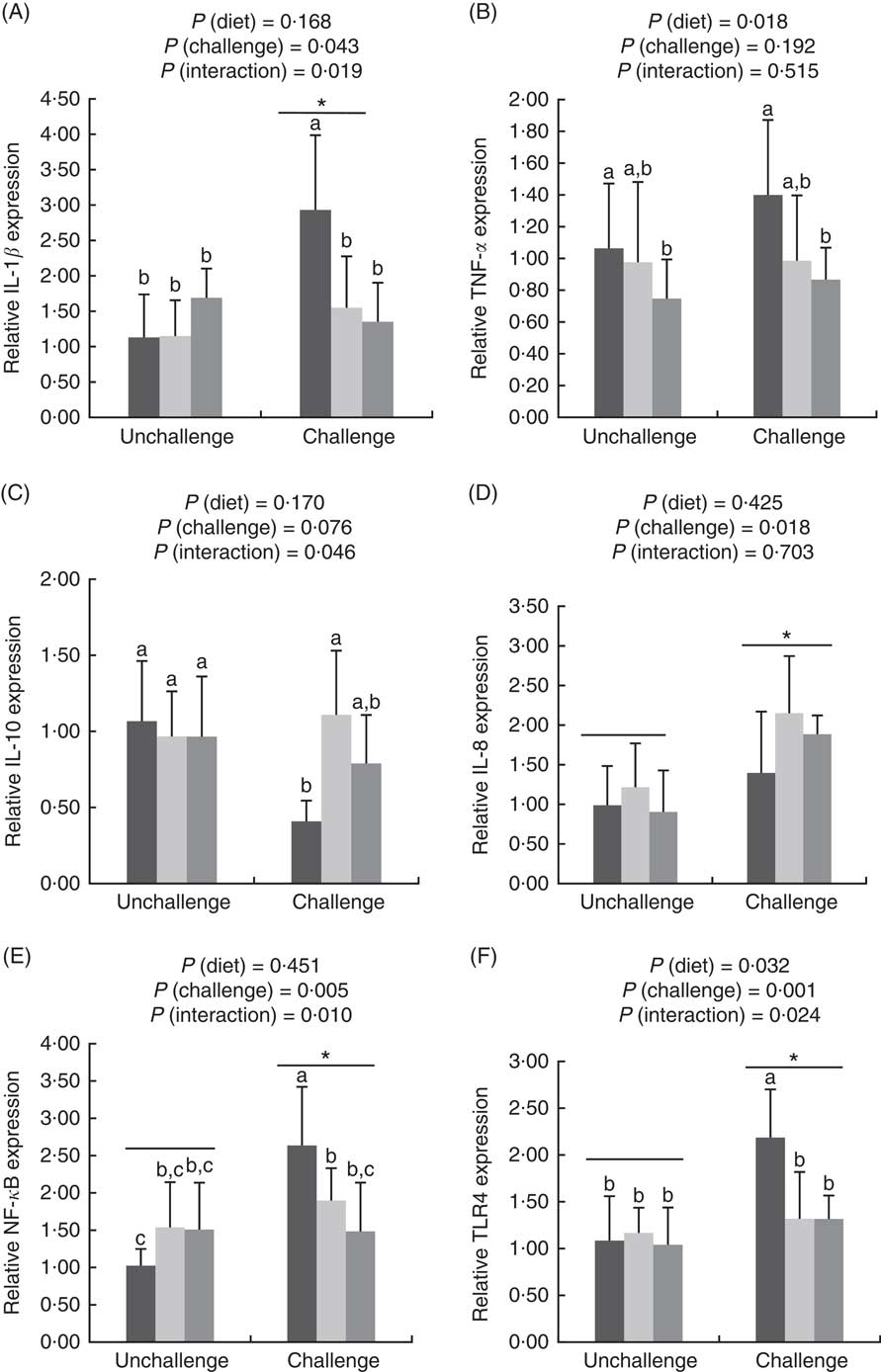
Fig. 1 Effects of dietary treatments (control, mannan-oligosaccharide (MOS) and live yeast (LY)) on the expressions of inflammation-related genes (IL-1β (A), TNF-α (B), IL-10 (C), IL-8 (D), NF-κ
B (E), Toll-like receptor 4 (TLR4) (F)) in the ileum of broilers challenged with Escherichia coli. Values are means (n 9) and standard deviations represented by vertical bars. * Suggests significant main effect (P<0·05) of E. coli challenge. a,b,c Treatments with unlike letters are significantly different (P<0·05). ![]() , Control;
, Control; ![]() , MOS;
, MOS;
![]() , LY.
, LY.
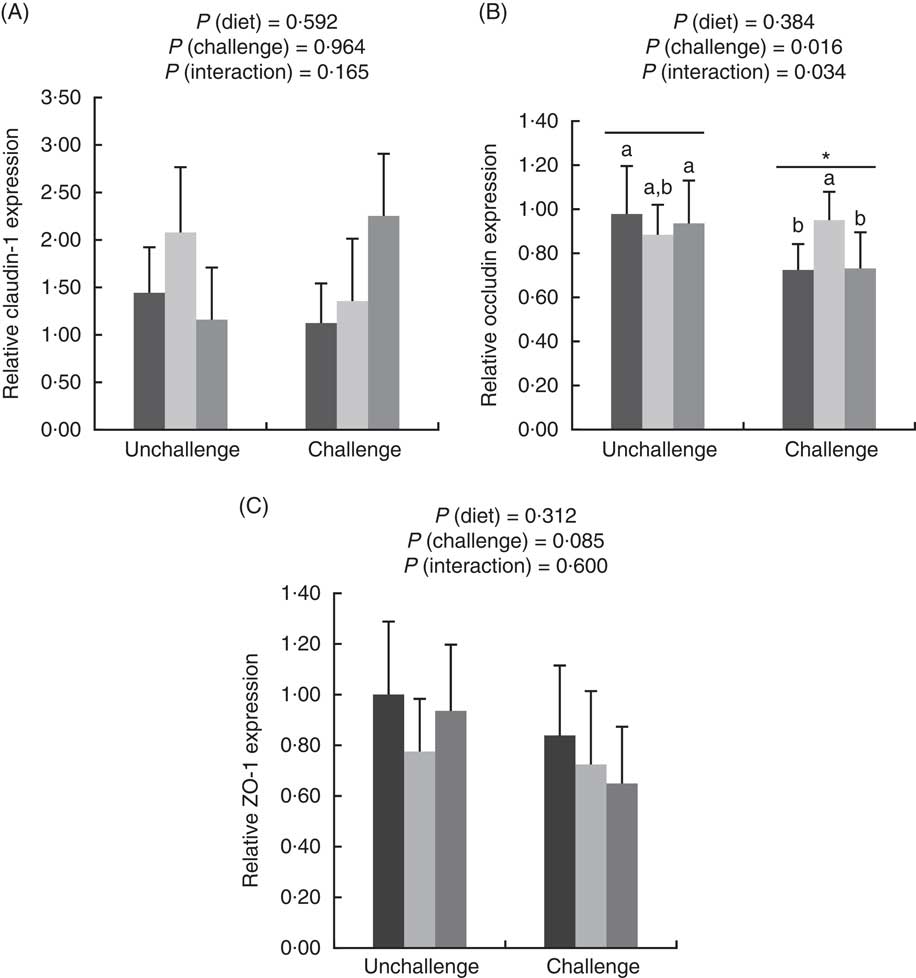
Fig. 2 Effects of dietary treatments (control, mannan-oligosaccharide (MOS) and live yeast (LY)) on the relative expressions of tight junction proteins (claudin-1 (A), occludin (B) and zonula occludens (ZO)-1 (C)) in the ileum of broilers challenged with Escherichia coli. Values are means (n 9) and standard deviations represented by vertical bars. * Suggests significant main effect (P<0·05) of E. coli challenge. a,b Treatments with unlike letters were significantly different (P<0·05). ![]() , Control;
, Control; ![]() , MOS;
, MOS; ![]() , LY.
, LY.
Composition and community diversity of ileal microbiota
E. coli challenge decreased (P<0·05) the relative abundance of ileal Enterococcus, and showed a trend towards an increase (P<0·10) in the relative abundance of Ruminococcus in the ileum of broilers (Table 6). LY addition showed a trend towards an increase (P<0·10) in the relative abundance of ileal Enterococcus and Brevibacillus of broilers. As shown in Table 7, LY addition increased (P<0·05) Shannon and Simpson indices of ileal microbiota and tended to elevate (P<0·10) Chao1 and ACE indices of ileal microbiota in challenged birds. PCA and PCoA indicated that there was no obvious difference in β diversity of ileal microbiota between each group (Fig. 3).
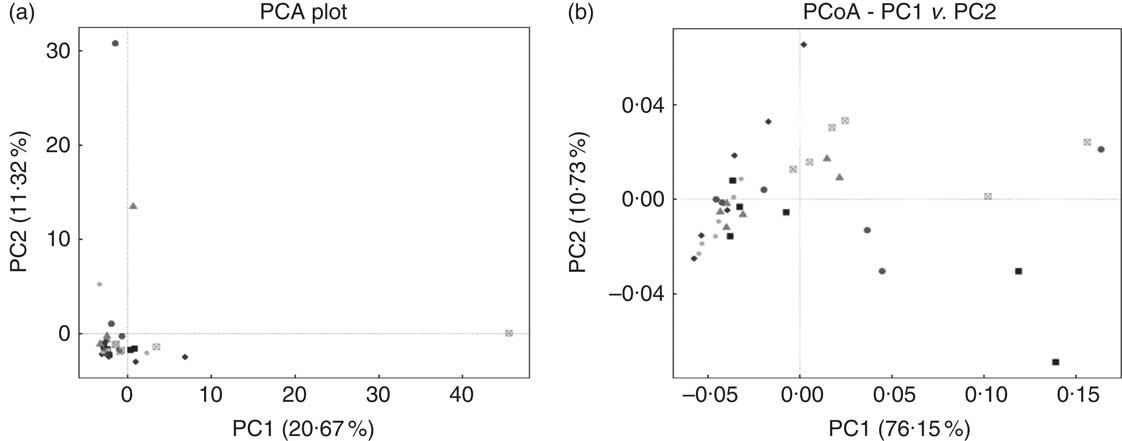
Fig. 3 Effects of dietary treatment on community similarity of ileal microbiota of broilers challenged with Escherichia coli (n 6). (a) Principal component analysis (PCA) and (b) principal coordinates analysis (PCoA) of ileal microbiota. ![]() , Control group and received challenge;
, Control group and received challenge; ![]() , mannan-oligosaccharide (MOS)-treated group and received challenge;
, mannan-oligosaccharide (MOS)-treated group and received challenge; ![]() , live yeast (LY)-treated group and received challenge;
, live yeast (LY)-treated group and received challenge; ![]() , control group and free from challenge;
, control group and free from challenge; ![]() , MOS-treated group and free from challenge;
, MOS-treated group and free from challenge; ![]() , LY-treated group and free from challenge.
, LY-treated group and free from challenge.
Table 6 Effects of dietary treatment on relative abundance (%) of ileal bacterial taxa at the genus level (top ten) of broilers challenged with Escherichia coli (Mean values with their standard errors; n 6)
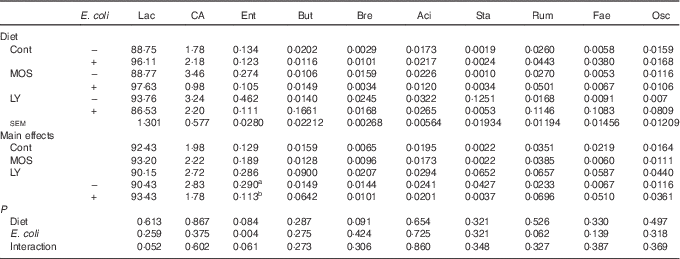
Lac, Lactobacillus; CA, Candidatus arthromitus; Ent, Enterococcus; But, Butyricicoccus; Bre, Brevibacillus; Aci, Acinetobacter; Sta, Staphylococcus; Rum, Ruminococcus; Fae, Faecalibacterium; Osc, Oscillospira; Cont, control; MOS, mannan-oligosaccharide; LY, live yeast.
a,b Mean values within a column with unlike superscript letters are significantly different (P<0·05).
Table 7 Effects of dietary treatment on the α diversity metric of ileal microbiota of broilers challenged with Escherichia coli (Mean values with their standard errors; n 6)
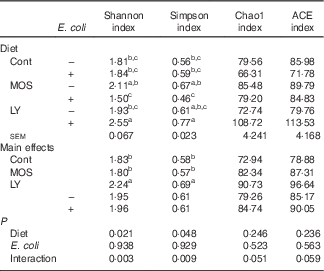
Cont, control; MOS, mannan-oligosaccharide; LY, live yeast; ACE, abundance-based coverage estimator.
a,b,c Mean values within a column with unlike superscripts letters are significantly different (P<0·05).
Discussion
The suppressed growth performance of animals bred in an unclean environment could be a result of intestinal dysfunction induced by bacterial challenge( Reference He, Fu and Shen 1 – Reference Gao, Han and Huang 3 ). In this study, we confirmed the negative effects of E. coli challenge on growth performance of broilers, exhibited as reduced ABW, ADG and ADFI accompanied by an increased FCR. Improved growth performance of chickens had been reported following the administration of MOS( Reference Abudabos and Yehia 10 ); however, the different findings are described elsewhere( Reference Santos, Costa and Silva 24 ). On the other hand, the effects of LY addition on growth performance of animals were also variable( Reference Haldar, Ghosha and Toshiwatia 12 , Reference Rezaeipour, Fononi and Irani 25 ). In this study, dietary treatment with MOS or LY did not affect ABW, ADG and ADFI of broilers independent of the immunological status. However, broilers fed LY had a reduced FCR during the grower period compared with control birds. It could be deduced that application of LY at an early stage appears to provide the optimal effect for broilers because of immature intestinal function( Reference Uni 26 ).
Endotoxin is a part of the cell wall of gram-negative bacteria, whereas DAO is a highly active intracellular enzyme in intestinal villi( Reference Luk, Bayless and Baylin 27 ). They are released into circulation when the intestinal barrier is destroyed, and their levels in blood can be used as indicators for monitoring the extent of intestinal injury( Reference Luk, Bayless and Baylin 27 , Reference Mani, Weber and Baumgard 28 ). In the present study, we observed increases in serum endotoxin and DAO levels in challenged birds, suggesting the idea of an increase in intestinal permeability and an impairment of barrier function after E. coli challenge. In support of this view, we found that E. coli challenge also exerted negative effects on intestinal maltase activity (brush-border enzyme) and morphological structure, a marker of intestinal mucosal integrity( Reference Lamb-Rosteski, Kalischuk and Inglis 21 , Reference Tan, Applegate and Liu 29 ), confirming a disruption in the intestinal barrier by E. coli. It was reported that dietary MOS or LY addition was positive for brush-border enzymes production and intestinal morphology in animals( Reference Haldar, Ghosha and Toshiwatia 12 , Reference Xiong, Yang and Li 13 , Reference Cheled-Shoval, Amit-Romach and Barbakov 30 , Reference Jiang, Wei and Wang 31 ). However, a few studies have been carried out to study their effects on intestinal structure of broilers under challenged conditions. In the present study, there was an increasing trend for ileal VH and an enhanced VCR in response to dietary treatment with both LY and MOS, revealing an improvement in ileal villi structure of LY- and MOS-treated birds, which could be helpful for the intestinal barrier of broilers against E. coli challenge. In addition, this result coincided with the alleviation of the increased serum DAO and endotoxin levels in challenged birds treated with both MOS and LY.
Tight junctions (TJ) are composed of several unique proteins, which create a paracellular permeability barrier and act as a fence preventing macromolecular translocation( Reference Ballard, Hunter and Taylor 32 ). Following E. coli challenge, we found destruction in intestinal TJ, characterised by the reduced occludin and ZO-1 expressions in the ileum. This was similar to some previous studies( Reference Gao, Han and Huang 3 , Reference Ewaschuk, Murdoch and Johnson 33 ). Decreased expression of TJ proteins was correlated with an increased intestinal permeability and a disruption of the intestinal barrier, thereby allowing the transmission of macromolecules from the intestinal lumen into blood circulation( Reference Zhang and Guo 34 ). This could conduce to elevated serum endotoxin and DAO levels of challenged birds. Recent studies have evidenced that treatment with some prebiotics and probiotics could up-regulate the expressions of intestinal TJ proteins in animals( Reference Zhou, Kong and Lian 35 – Reference Li, Zhang and Chen 39 ). However, it was unknown whether MOS or LY could impact TJ profile in broilers. In the present study, MOS inclusion restored the reduced expression of ileal occludin in challenged birds. These results support the idea that dietary MOS addition could be, to a degree, beneficial for the TJ structure of broilers after E. coli challenge, by which it might be implicated in tightening intestinal permeability.
Lipopolysaccharides of gram-negative bacteria such as E. coli can be detected by TLR4, the stimulation of which activates downstream signalling such as NF- κ B leading to the sharp increase in synthesis and release of pro-inflammatory cytokines, thereby resulting in tissue damage along with high consumption of nutrients( Reference Dobrovolskaia and Vogel 40 , Reference Dinarello 41 ). Similar to the previous studies( Reference Li, Zhang and Chen 39 , Reference MacKinnon, He and Nerren 42 ), we found increases in ileal TLR-4, NF- κ B, IL-1 β and IL-8 expression in challenged birds, demonstrating that E. coli-induced intestinal inflammatory responses probably through the TLR4/NF- κ B signalling pathway. As inflammatory responses are involved in the recruitment of phagocytes to the infection site( Reference Gong, Hart and Shchurin 43 ), it could be deduced that products of phagocytes in the infection site would be increased. Indeed, we found that E. coli challenge elevated the activities of ileal lysozyme and MPO, which are produced by activated macrophages and neutrophils and serve as a marker for evaluating the degree of inflammation( Reference Persson, Carlsson and Hambleton 44 , Reference Lavi, Levinson and Peri 45 ), providing another clue for intestinal inflammation in challenged birds. It has been shown that dietary MOS addition exerted an anti-inflammatory role in pigs confronted with immunological stress, as revealed by the reduced expression of intestinal pro-inflammatory cytokines and increased expression of anti-inflammatory cytokines( Reference Che, Johnson and Kelley 46 ). Similar effects were also observed for LY treatment, which prevented E. coli-induced increase in intestinal TLR4 expression in pigs( Reference Badia, Lizardo and Martinez 15 ), and inhibited the expression of intestinal pro-inflammatory cytokines in response to pathogen invasion in mice( Reference Jawhara, Habib and Maggiotto 18 ) and in vitro ( Reference Zanello, Berri and Dupont 19 , Reference Zanello, Meurens and Berri 20 ). In this study, we observed that dietary treatment with both MOS and LY reduced ileal MPO activity, and simultaneously alleviated the increased expressions of ileal IL-1 β, TLR4 and NF- κ B in challenged birds. These results suggest that both MOS and LY addition relieved the inflammatory infiltration of the ileum to some degree and exerted active roles in reducing massive production of ileal pro-inflammatory cytokines following E. coli challenge probably through the inhibition of TLR4/NF- κ B pathway. Attenuated local inflammation had been suggested to be associated with the alleviation of intestinal morphology impairment( Reference Liu, Huang and Hou 47 ). Therefore, the suppression of TLR4pathway in the present study might partially contribute to the mitigation of ileal villus damage. IL-10 is a pivotal anti-inflammatory cytokine secreted by activated macrophages that serves to maintain immune balance by suppressing the excessive production of pro-inflammatory cytokines( Reference Corwin 48 ). Accordingly, increase in ileal IL-10 expression of challenged birds might also be related to the alleviation of E. coli-induced intestinal inflammation of broilers fed MOS.
Gut microbiota conduce to maintain the physiological structure and function of the intestine( Reference Sekirov, Russell and Antunes 49 ), which are understood to be associated with intestinal inflammation and barrier function as well as growth performance of the host( Reference Santacruz, Collado and Garcia-Valdes 50 – Reference Singh, Shah and Deshpande 52 ). In this study, although E. coli challenge decreased the relative abundance of ileal Enterococcus and tended to increase the abundance of ileal Ruminococcus, it did not affect the majority of micro-organism, especially the predominant member (Lactobacillus) in the ileum. Therefore, it could be concluded that E. coli challenge altered the composition of ileal microbiota in broilers, but these changes were minor in nature. This is similar to a previous report by Videnska et al.( Reference Videnska, Sisak and Havlickova 53 ), who thought that bacteria-induced intestinal disruption of chickens was characterised more as an indirect consequence of the infection and inflammation instead of a positively selected evolutionary trait. It was indicated that intestinal microflora of animals could be modulated by the addition of MOS( Reference Kim, Seo and Kim 54 , Reference Corrigan, de-Leeuw and Penaud-Frézet 55 ) or LY( Reference Heugten, Funderburke and Dorton 11 , Reference Haldar, Ghosha and Toshiwatia 12 ). In the present study, MOS addition had little relation to the ileal microbial community; however, LY supplementation tended to increase the relative abundance of ileal Enterococcus and Brevibacillus, which are indicated to modulate intestinal microbiota and immune responses of the host, and thus considered as potential probiotics in chickens( Reference Alizadeh, Akbari and Difilippo 36 , Reference de Oliveira, van der Hoeven-Hangoor and van de Linde 56 – Reference Samli, Senkoylu and Koc 59 ). This might conduce to the alleviated intestinal inflammation of broilers fed LY. α Diversity of the microbial community could be reflected by Shannon and Simpson indices as well as Chao1 and ACE indices( Reference Li, Xu and Huang 60 ). Although PCA and PCoA revealed no obvious difference in β diversity of the ileal microbiota between each group, LY treatment enhanced the α diversity of ileal microbiota, which was characterised by the increased Shannon and Simpson indices and the tendency towards elevated Chao1 and ACE indices of the ileal microbial community in challenged birds. Ecological theory suggests that species-rich community results in enhanced stability of the intestinal micro-ecology, which could be related to the reduced susceptibility to bacterial invasion and intestinal inflammation coupled with an improvement in intestinal absorption and growth performance of the host( Reference Li, Xu and Huang 60 – Reference Semova, Carten and Stombaugh 63 ). Therefore, overall, it could be deduced that modulation of intestinal microbial community structure, including the increased microbial diversity and the abundance of Enterococcus and Brevibacillus, was associated with the alleviated intestinal inflammation and the reduced FCR during the grower period of broilers due to LY addition.
In conclusion, the present study suggests that dietary supplementation with both MOS and LY alleviated E. coli-induced intestinal disruption by attenuating intestinal inflammation and barrier dysfunction in broilers. It is possible that the ability of MOS and LY to alleviate intestinal inflammation is associated with the suppression of TLR4/NF- κ B signalling pathway. Moreover, LY addition improved intestinal microbial community structure, and this might also contribute to the alleviation of intestinal inflammation along with the improvement of feed efficiency in broilers.
Acknowledgements
The authors thank Youli Wang, Wenyi An, Ning Tian and Li Zhu (China Agricultural University, Beijing, China) for their assistance in the process of sample collection.
This study was financially supported by Phileo Lesaffre Animal Care (Marcq-en-Baroeul, Lille, France). The funder had no role in the design and analysis of the study and in the writing of this article.
W. W. conducted the animal trial, performed the sample analyses and wrote the manuscript; Z. L. and Q. H. assisted with animal feeding and data analysis; Y. G., B. Z. and R. D. contributed to the experimental design.
None of the authors had any conflicts of interest.













