Introduction
The prevalence and consequences of substance use disorders (SUDs) among adolescents constitute a major public health concern. This has recently led to prominent shifts in national funding priorities and an expansion of research efforts cutting across diverse disciplines (Casey et al., Reference Casey, Cannonier, Conley, Cohen, Barch, Heitzeg, Soules, Teslovich, Dellarco, Garavan, Orr, Wager, Banich, Speer, Sutherland, Riedel, Dick, Bjork, Thomas, Chaarani, Mejia, Hagler, Daniela Cornejo, Sicat, Harms, Dosenbach, Rosenberg, Earl, Bartsch, Watts, Polimeni, Kuperman, Fair and Dale2018; Volkow et al., Reference Volkow, Koob, Croyle, Bianchi, Gordon, Koroshetz, Pérez-Stable, Riley, Bloch, Conway, Deeds, Dowling, Grant, Howlett, Matochik, Morgan, Murray, Noronha, Spong, Wargo, Warren and Weiss2018). Among the many risks associated with SUDs in youth, antisocial behavior is a conspicuous concern. Large-scale epidemiological studies have consistently reported more than half of those in juvenile corrections meet diagnostic criteria for SUDs (CASA, 2010; Teplin et al., Reference Teplin, Abram, McClelland, Dulcan and Mericle2002), 10 times greater than the roughly 5% prevalence recognized among adolescents in the general population (CBHSQ, 2015). Furthermore, most youth involved with the criminal justice system exhibit a history of misusing multiple substances (McClelland et al., Reference McClelland, Elkington, Teplin and Abram2004). Longitudinal studies have indicated more than 80% of those in juvenile corrections endure some trajectory of persisting SUDs for more than 10 years following their initial incarceration (Welty et al., Reference Welty, Hershfield, Abram, Han, Byck and Teplin2017). Further, the presence of SUDs remains a prominent predictor of future recidivism among those released from juvenile corrections (Mason & Windle, Reference Mason and Windle2002; Welty et al., Reference Welty, Hershfield, Abram, Han, Byck and Teplin2017). It remains clear that efforts to address mental health and behavioral issues among high-risk youth must consider the powerful role of substance use and addiction.
The neural correlates of SUDs have been well described in various biological models of addiction. Neuronal changes accompanying SUDs prominently affect the structure and function of fronto-striatal reward circuits, but also extend to other parts of the brain involved in executive control and socio-affective systems (Volkow et al., Reference Volkow, Wang, Fowler, Tomasi, Telang and Baler2010, Reference Volkow, Wang, Fowler, Tomasi and Telang2011). Individual differences in susceptibility to SUDs are influenced by a combination of genetic and environmental factors that converge on the physiology of the brain (Conrod & Nikolaou, Reference Conrod and Nikolaou2016). Significant heritability in problematic use has been consistently reported in conjunction with shared environmental factors (Kendler et al., Reference Kendler, Ohlsson, Maes, Sundquist, Lichtenstein and Sundquist2015; Rhee et al., Reference Rhee, Hewitt, Young, Corley, Crowley and Stallings2003). One of the most conspicuous physical representations of these effects is in gray matter volume (GMV). In a recent “mega-analysis” of over 3000 individuals, patterns of reduced GMV associated with alcohol and other substance dependence were apparent in the hippocampus, amygdala, nucleus accumbens, putamen, globus pallidus, insula, and orbitofrontal cortex – among other regions (Mackey et al., Reference Mackey, Allgaier, Chaarani, Spechler, Orr, Bunn, Allen, Alia-Klein, Batalla, Blaine, Brooks, Caparelli, Chye, Cousijn, Dagher, Desrivieres, Feldstein-Ewing, Foxe, Goldstein, Goudriaan, Heitzeg, Hester, Hutchison, Korucuoglu, Li, London, Lorenzetti, Luijten, Martin-Santos, May, Momenan, Morales, Paulus, Pearlson, Rousseau, Salmeron, Schluter, Schmaal, Schumann, Sjoerds, Stein, Stein, Sinha, Solowij, Tapert, Uhlmann, Veltman, van Holst, Whittle, Wiers, Wright, Yücel, Zhang, Yurgelun-Todd, Hibar, Jahanshad, Evans, Thompson, Glahn, Conrod and Garavan2019). This large-scale study reflects many consistent findings in extant literature reporting widespread gray matter decrements associated with SUDs in adults, across many different substances of abuse (Barrós-Loscertales et al., Reference Barrós-Loscertales, Garavan, Bustamante, Ventura-Campos, Llopis, Belloch, Parcet and Ávila2011; Ersche et al., Reference Ersche, Williams, Robbins and Bullmore2013; Franklin et al., Reference Franklin, Acton, Maldjian, Gray, Croft, Dackis, O’Brien and Childress2002; Lorenzetti et al., Reference Lorenzetti, Lubman, Whittle, Solowij and Yücel2010; Makris et al., Reference Makris, Oscar-Berman, Jaffin, Hodge, Kennedy, Caviness, Marinkovic, Breiter, Gasic and Harris2008). Further, patterns linking more severe, protracted substance use with greater decrements in gray matter suggest these effects are at least partially caused by the deleterious effects of prolonged substance misuse on neural structures over many years (Infante et al., Reference Infante, Eberson, Zhang, Brumback, Brown, Colrain, Baker, Clark, De Bellis, Goldston, Nagel, Nooner, Zhao, Pohl, Sullivan, Pfefferbaum, Tapert and Thompson2022; Kaag et al., Reference Kaag, Schulte, Jansen, Van Wingen, Homberg, Van Den Brink, Wiers, Schmaal, Goudriaan and Reneman2018). A recent large-scale review of neuroimaging findings in SUDs emphasizes apparent abnormalities in cognitive control networks and their interaction with subcortical salience-processing networks; however, this review also highlights lingering gaps in our understanding of premorbid vulnerabilities and developmental representations of abnormal brain structure and function (Paulus, Reference Paulus2022).
The effects of SUDs on gray matter commonly reported in adult populations are not as clear or consistent in juveniles. From a developmental perspective, adolescence represents a critical period of change with both social and biological influences that promote more risk-taking and reward-oriented decision-making (Blakemore & Robbins, Reference Blakemore and Robbins2012; Casey et al., Reference Casey, Getz and Galvan2008). Natural developmental trajectories during these years are accompanied by nonlinear and nonuniform patterns of gray matter change across the brain. In typical development, total gray matter volume (across the whole brain) reaches its global maximum around 12 years old, with regional variation in temporal and occipital regions peaking a few years later, up to about 20 years (Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans and Rapoport1999; Giedd, Reference Giedd2008; Sowell et al., Reference Sowell, Peterson, Thompson, Welcome, Henkenius and Toga2003). Significant neural pruning results in progressively lower density and volume of gray matter, beginning earlier in posterior brain areas and later in frontal regions (Giedd, Reference Giedd2008). Regionally specific pruning, arborization, and myelination promote changes in impulsivity, novelty-seeking, reward-oriented decision-making, and changes in self-monitoring and behavioral inhibition reflected during these important developmental years (Blakemore & Robbins, Reference Blakemore and Robbins2012; Christakou et al., Reference Christakou, Brammer and Rubia2011; Luna & Sweeney, Reference Luna and Sweeney2004).
These complex developmental patterns may obscure the measurement of neural abnormalities specifically related to substance use in this age group and make interpretation of empirical findings difficult. Certain deleterious effects of substance use recognized in adult populations might be collinear with local developmental changes in several brain regions during adolescence. Concurrently, young substance users may simply not have accumulated as much neural damage from substance use as adults. It is also possible that early-stage vulnerability to substance misuse among juveniles may be represented by neural markers that are distinct from those concomitant with the deleterious effects of long-term substance abuse commonly reported in adults. These challenges undoubtedly contribute to more variability apparent in reported findings among youth.
Some reports have suggested causal relationships between gray matter loss and substance use frequency/severity, such as in binge drinking among adolescents (Infante et al., Reference Infante, Eberson, Zhang, Brumback, Brown, Colrain, Baker, Clark, De Bellis, Goldston, Nagel, Nooner, Zhao, Pohl, Sullivan, Pfefferbaum, Tapert and Thompson2022), while others suggest that gray matter reductions associated with natural maturation processes (i.e., pruning) may drive higher frequency of alcohol use (Robert et al., Reference Robert, Luo, Yu, Chu, Ing, Jia, Papadopoulos Orfanos, Burke-Quinlan, Desrivières, Ruggeri, Spechler, Chaarani, Tay, Banaschewski, Bokde, Bromberg, Flor, Frouin, Gowland, Heinz, Ittermann, Martinot, Paillère Martinot, Nees, Poustka, Smolka, Vetter, Walter, Whelan, Conrod, Barker, Garavan and Schumann2020). Again, individual variation in gray matter may also be interpreted as a premorbid marker for vulnerability to substance misuse. Weiland and colleagues, for example, have reported reductions in frontal cortical gray matter as a marker for early risk of both substance abuse and higher externalizing behaviors, controlling for both family history and current substance use (Weiland et al., Reference Weiland, Korycinski, Soules, Zubieta, Zucker and Heitzeg2014).
Beyond the frontal cortex, subcortical and salience-processing networks in the brain are also conspicuous candidates for early vulnerability markers. Striatal dopamine modulation in adolescence naturally increases the risk for initiating substance use/abuse, and individual differences in novelty-seeking and reward sensitivity may play a role in differential risk for substance abuse and maintenance of maladaptive behaviors (Casey & Jones, Reference Casey and Jones2010; Kelley et al., Reference Kelley, Schochet and Landry2004). Correspondingly, increased excitatory activity in striatal dopaminergic pathways accompanying substance use during this critical developmental period may also modify structure and function while the striatum is undergoing development. It has been consistently noted that impulsivity and hyperactivation in networks involved in reward-related evaluations are reliable predictors of substance use outcomes and vulnerability. These effects are especially apparent in medial orbitofrontal and striatal system hyperexcitability of dopamine receptors in this network (Verdejo-Garcia & Albein-Urios, Reference Verdejo-Garcia and Albein-Urios2020). Dopaminergic function (e.g., fronto-striatal D2 receptors) has been found to be altered among young adults at risk for SUDs based on family history (Jaworska et al., Reference Jaworska, Cox, Tippler, Castellanos-Ryan, Benkelfat, Parent, Dagher, Vitaro, Boivin, Pihl, Côté, Tremblay, Séguin and Leyton2020; Weiland et al., Reference Weiland, Zucker, Zubieta and Heitzeg2017). Lees and colleagues reported markers for substance use vulnerability including hyperactivation of ventral striatal regions during reward feedback and risk evaluation accompanied by distinct patterns of frontal/fronto-parietal hypoactivation (during failures of inhibition) and hyperactivation (during successful trials) (Lees et al., Reference Lees, Garcia, Debenham, Kirkland, Bryant, Mewton and Squeglia2021). Also, in children (aged 8–12) at risk for SUDs, early exposure to drug use is associated with hyperactivation of the nucleus accumbens during an incentive delay task (Cope et al., Reference Cope, Martz, Hardee, Zucker and Heitzeg2019).
Considering corresponding effects in gray matter morphology, several studies have reported increased gray matter in fronto-striatal regions among young substance users and in prospective substance abusers. In a study of adolescents using methamphetamine and cannabis, drug use and novelty-seeking traits were both positively associated with striatal GMV (Churchwell et al., Reference Churchwell, Carey, Ferrett, Stein and Yurgelun-Todd2012). Young cigarette smokers have shown increased GMV in the left putamen (accompanied by decreased volume in the anterior cingulate) compared with nonsmoking controls (Bu et al., Reference Bu, Yu, Su, Ma, Von Deneen, Luo, Zhai, Liu, Cheng, Guan, Li, Bi, Xue, Lu and Yuan2016). Girls with alcohol use disorder have exhibited larger putamen and thalamic volumes than non-drinking girls (although the effect was reversed in boys) (Fein et al., Reference Fein, Greenstein, Cardenas, Cuzen, Fouche, Ferrett, Thomas and Stein2013). In young recreational cannabis users, gray matter was relatively increased in the left nucleus accumbens and extended subcallosal cortex, hypothalamus, and amygdala, even after controlling for age, sex, alcohol use, and cigarette smoking (Gilman et al., Reference Gilman, Kuster, Lee, Lee, Kim, Makris, van der Kouwe, Blood and Breiter2014). Early cannabis use (younger than age 16) has been associated with larger left nucleus accumbens and increased thickness in right frontal cortex gray matter (Lisdahl et al., Reference Lisdahl, Tamm, Epstein, Jernigan, Molina, Hinshaw, Swanson, Newman, Kelly and Bjork2016). Further, in a longitudinal study, high novelty-seeking 14-year-olds who eventually developed problematic drug use (by age 16) had relatively increased gray matter density in the left ventral striatum, midbrain, and bilateral dorsolateral prefrontal cortex (Büchel et al., Reference Büchel, Peters, Banaschewski, Bokde, Bromberg, Conrod, Flor, Papadopoulos, Garavan, Gowland, Heinz, Walter, Ittermann, Mann, Martinot, Paillère-Martinot, Nees, Paus, Pausova, Poustka, Rietschel, Robbins, Smolka, Gallinat, Schumann and Knutson2017) compared with those who did not develop problematic drug use. In support of these morphometric differences reflecting a possible neurocognitive vulnerability predicating substance abuse, increased GMV in the medial temporal lobe and basal ganglia have been reported in both drug-dependent individuals and their non-drug-abusing siblings relative to healthy controls, while non-affected siblings also demonstrated concomitant abnormalities in a stop-signal task, reflecting impairments in impulse control (Ersche et al., Reference Ersche, Jones, Williams, Turton, Robbins and Bullmore2012). Lees and colleagues reported larger ventral striatal volume (but smaller fronto-parietal and amygdala volume) were associated prospectively with future substance misuse (Lees et al., Reference Lees, Garcia, Debenham, Kirkland, Bryant, Mewton and Squeglia2021).
In sum, neural development supporting reward-seeking, exploratory, and elevated risk-taking behavior is a common feature of adolescence. These developmental patterns may promote risky behaviors that include experimentation with substances of abuse. Less is known about neurobiological features contributing to individual differences in substance use behaviors that distinguish enduring, maladaptive patterns of substance abuse. It may be true that exaggerated proportional differences in gray matter reflect a vulnerability for initiation of substance use, amplifying the rewarding effects of drugs and promoting continued use, and/or stronger perseverative motivation following initial experimentation. Relatively little research has focused on structural brain correlates of substance use severity among high-risk juveniles, particularly those exhibiting early antisocial behavior. In the current study, we set out to examine morphometric differences in gray matter accompanying histories of substance use in boys incarcerated in a secure correctional facility and treatment program for youth. From a design perspective, data from these individuals may be helpful in ascribing effects to substance use, per se, as the sample is relatively more homogeneous on several collateral risk factors related to their incarceration status (e.g., antisocial behavior, psychopathic personality traits, sociodemographic risk factors). These are often difficult factors to eliminate or control for when comparing substance abusing youth with typical healthy controls. We present two complementary data analysis approaches, utilizing both traditional, univariate, voxel-based morphometry (VBM) alongside multivariate source-based morphometry (SBM) using independent component analysis (ICA) of structural MRI data. In the univariate approach, we test differences in a priori regions of interest (ROIs) selected for their roles in reward-motivated decision-making and highlighted in prior studies examining SUDs in juveniles. The SBM analysis is a data-driven approach, organizing whole-brain data into naturally occurring (intrinsic) networks of gray matter with closely covarying volume. As such, this method is ideal for validating the univariate findings and demonstrating the natural organizational structure of affected networks, without constraining analyses to artificially defined ROIs. We hypothesized that more severe substance abuse in juveniles would be associated with hypertrophy of gray matter in fronto-striatal reward circuits, reflected in both univariate a priori regions of interest and corresponding intrinsic structural networks derived from SBM analysis.
Methods
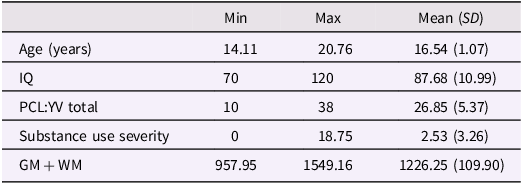
Participants were n = 175 adolescent males incarcerated at the Mendota Juvenile Treatment Center in Madison, Wisconsin. The facility houses and treats youth who have been transferred from other state juvenile facilities, largely due to unmanageable behavior in traditional corrections programs. This cohort represents a particularly high-risk group, even as compared with more typical juvenile corrections samples. All participants provided their own assent accompanied by their guardian’s/representative’s consent. Participants were aware that their cooperation with research was completely voluntary and would have no impact on their sentence or status at the facility. Research protocols were conducted in accordance with principles established by the Declaration of Helsinki, approved by local IRB, Ethical and Independent Review Services, and by the National Institutes of Health Office of Human Research Protections with respect to the vulnerable status of incarcerated subjects. Participants were admitted into the study if they had at least a fourth-grade reading level and were able to understand informed assent procedures. Participants were excluded from analysis if their IQ was below 70 (n = 18) and for history of head injuries/traumatic brain injury (TBI) resulting in prolonged loss of consciousness (60 min, cumulative) or abnormal radiological report following MRI (n = 5). A total of n = 152 boys were included in the final analysis. Based on NIH race classifications, participants self-identified as American Indian or Alaskan Native (n = 5), Black or African American (n = 81), White (n = 46), and more than one race (n = 19). One participant chose not to self-disclose their race. Additionally, n = 19 participants identified as Hispanic/Latin@, and n = 133 participants identified as non-Hispanic/Latin@. Descriptive features of this sample are provided in Table 1.
Table 1. Descriptive features n = 152 incarcerated male youth
Assessments
Substance use severity: The history of substance use was measured using a modified version of the Addiction Severity Index (McLellan et al., Reference McLellan, Kushner, Metzger, Peters, Smith, Grissom, Pettinati and Argeriou1992). Participants reported duration of regular substance use in their lifetime based on a structured interview tabulating several categories including alcohol, cannabis, heroin, other opiates (e.g., painkillers, methadone, levacetylmethadol), cocaine/crack, methamphetamine, other amphetamines (e.g., Adderall), anxiolytics (e.g., benzodiazepines, Xanax), hallucinogens, inhalants, and others (e.g., DXM, spice). Regular use was defined as using a non-prescribed substance at least three or more times per week. Cumulative years of regular use were summed and summarized for each category. For neuroimaging analyses, years of regular substance use were divided by the participant’s age to account for opportunity for use, and a square root transformation was applied to account for non-normality, similar to a method used in prior publications (Ermer et al., Reference Ermer, Cope, Nyalakanti, Calhoun and Kiehl2012; Sajous-Turner et al., Reference Sajous-Turner, Anderson, Widdows, Nyalakanti, Harenski, Harenski, Koenigs, Decety and Kiehl2020). This approach captures variance attributable to the relative level of severity of multiple periods of use across the lifespan.
Psychopathic traits
The Hare Psychopathy Checklist: Youth Version (PCL:YV) (Forth et al., Reference Forth, Kosson and Hare2003), an expert-administered rating scale consisting of a semi-structured interview and review of collateral information, including institutional and medical records, was used to assess youth psychopathic traits. The PCL:YV is composed of 20 different items assessing personality traits, emotional factors, interpersonal style, developmental characteristics, and behaviors concomitant with psychopathy. PCL:YV total scores can range from 0 to 40. Four facets are also available as separable features of the total score. In the current analysis, the total score is utilized as a covariate to account for individual variation in antisocial traits and delinquent behavior in this high-risk sample.
Intelligence: Full-scale IQ was estimated using the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale – 3rd Edition (Wechsler, Reference Wechsler1997) for participants 16 years of age or older and from the Wechsler Intelligence Scale for Children – 4th Edition (Wechsler, Reference Wechsler2003) for participants younger than 16 years of age. IQ < 70 was an exclusionary criterion for this study. IQ is also entered as a covariate in analyses.
Head Injury: A history of prior head injuries was taken using a modified post-head injury symptoms questionnaire (King et al., Reference King, Crawford, Wenden, Moss and Wade1995). A total of n = 81 (46%) self-reported some prior history of head injury ranging from minor sub-concussive events (e.g., fell out of bed) to severe incidents resulting in loss of consciousness and hospitalization. Prolonged loss of consciousness (1 hr or more, cumulative) or abnormal radiological report was an exclusionary criterion for this study.
MRI collection and processing
Structural MRI scans were acquired on-site at the correctional facility, using the Mind Research Network Siemens 1.5 T Avanto mobile scanner. High-resolution T1-weighted scans were collected using a multi-echo MPRAGE pulse sequence provided by Massachusetts General Hospital Radiology Department (repetition time = 2530 ms, echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms, inversion time = 1100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256). This yielded 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm. Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM12; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). T1 images were manually inspected by an operator blind to subject identity and realigned using the anterior and posterior commissures to ensure proper spatial normalization. Images were then spatially normalized to the SPM12 T1 Montreal Neurological Institute template using nonlinear registration, segmented into gray matter, white matter, and cerebrospinal fluid, and modulated with the Jacobian determinants to preserve total volume after normalization (Ashburner & Friston, Reference Ashburner and Friston2000, Reference Ashburner and Friston2005); that is, voxelwise parameters are interpreted as gray matter volume. Finally, the images were resampled to 1.5 × 1.5 × 1.5 mm and smoothed with a 10 mm full-width at half-maximum Gaussian kernel. Voxels with a gray matter value of < .15 were excluded to remove possible edge effects between gray matter and white matter. Additional quality assurance on MRI data was verified using the MRIQC pipeline (https://mriqc.readthedocs.io/en/latest/). No additional exclusions or modifications were necessary following QA assessment.
Voxelwise multiple regression analysis was performed within a priori ROIs, examining the effects of substance use severity on local gray matter while accounting for covariates of age, race, IQ, PCL:YV total scores, and total brain volume (gray matter + white matter). A robust regression method was applied, which reduces bias from outliers and distribution non-normality using an iteratively reweighted least squares approach (Wager et al., Reference Wager, Keller, Lacey and Jonides2005; see also https://github.com/canlab/RobustToolbox). Bilateral anatomical masks were calculated for the amygdala, nucleus accumbens, caudate, putamen, pallidum, anterior insula, and orbitofrontal cortex (frontal inferior orbital) using automated anatomical labels (AAL3) atlas and extracted with MATLAB. For each region, the familywise error (FWE) rate was maintained at p < .05 by correcting for the volumes of each mask; we report the FWE corrected p-values.
As a complementary approach, a multivariate data-driven analysis of brain structure (SBM) was used to define intrinsic networks of gray matter using ICA. Detailed methods for this approach have been described elsewhere (Xu et al., Reference Xu, Groth, Pearlson, Schretlen and Calhoun2009). The complementary value of this analysis strikes a balance between confining analysis to a few circumscribed regions of interest and the problematic multiple comparisons issue introduced by whole-brain, voxelwise (univariate) GLM solutions. The algorithm produces a set of maximally independent components (networks) of covarying voxels and parameter values (loading coefficients) for each subject on each component. Stronger/weaker parameter values scale proportionally with individual gray matter values in each represented network. These parameter values are then carried forward in subsequent analyses and statistical estimates can be made for each component network, rather than on a voxel-by-voxel basis (i.e., VBM). In this way, statistical estimates represent individual characteristics of a cohesive network as opposed to properties of a single voxel. Following preprocessing, the Group ICA fMRI Toolbox (GIFT) software (http://mialab.mrn.org/software/gift) was used to extract 30 maximally independent components. The resulting components (networks) were then evaluated for their voxelwise overlap with selected ROIs, using a selection criterion of at least 10% overlap with a single mask defining voxels corresponding to the combined a priori ROIs. Subsequent analyses were carried out on properties of naturally derived networks corresponding closely with a priori ROIs. Bivariate and partial correlations were then calculated between component parameters (loading coefficients) and individual factors representing substance use severity, with partial correlations controlling for the same nuisance covariates as in the previous univariate ROI regression models.
Results
The final analysis included n = 152 boys, excluding n = 18 for low IQ and n = 5 for TBI. Descriptive features of this sample are provided in Table 1. Substance use severity in this sample is described in Table 2, which accounts for those in the sample meeting criteria for problematic use of each substance, defined as three or more times per week for a minimum period of 1 month.
Table 2. Substance use in n = 152 incarcerated male youth

For visualization purposes, whole-brain correlations between GMV and substance use severity, controlling for age, race, IQ, PCL:YV total scores, and brain volume (gray matter + white matter), are represented in axial slices in Figure 1. These effects were thresholded to p < .005, using a cluster-based threshold of 1387 voxels (3DClusSim, Nearest Neighbor 3, allowing faces touching, edges touching, corners touching). This figure is intended to highlight the whole-brain orientation of significant effects in a priori ROIs, presented in data tables.

Figure 1. Elevated gray matter associated with substance use severity. VBM whole-brain (positive) correlations with substance use severity, p < .005, K(NN3) = 1387, and N = 152, are highlighted in orange/yellow. More severe substance use is associated with increased gray matter in highlighted regions. Covariates of age, race, IQ, PCL:YV, and total brain volume (GM + WM) are included in this analysis.
ROI results
Analyses in a priori regions of interest (amygdala, nucleus accumbens, caudate, putamen, pallidum, anterior insula, and orbitofrontal cortex) revealed significant effects in bilateral amygdala, right putamen, right pallidum, right insula, and right orbitofrontal cortex. Effects in other ROIs did not survive FWE corrections across regional volumes. Coordinates and statistics for significant effects are presented in Table 3.
Table 3. VBM analysis in ROIs: significant effects surviving FWE correction in volume
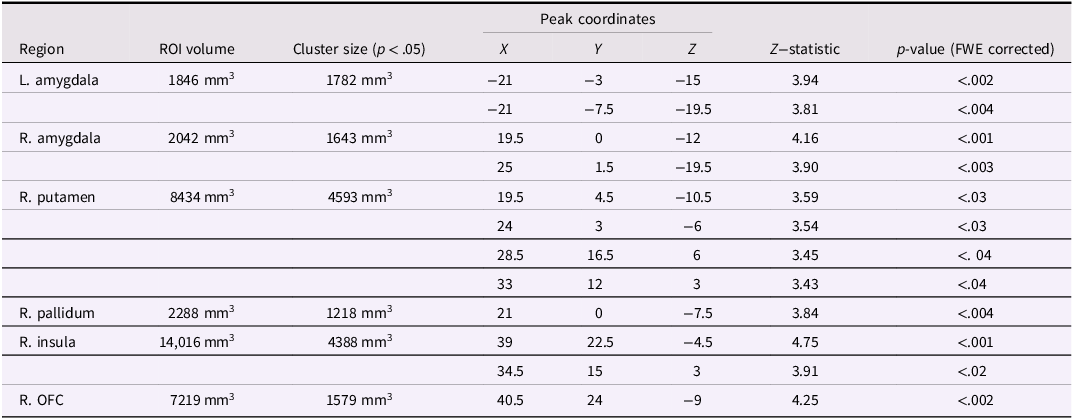
Note. Significant effects represent increased gray matter associated with more severe substance use in juvenile offenders, supporting our hypotheses. FWE = familywise error correction for multiple comparisons; R. OFC = right orbitofrontal cortex.
SBM results
Two structural networks (derived by ICA) exhibited more than 10% overlap with a priori ROIs, together accounting for 80% of voxels examined in the GLM analysis. A network including bilateral striatum was positively correlated with substance use severity using bivariate correlations, r = .213, p = .008 (see Fig. 2). A second structural network including bilateral insula was also positively correlated with substance use severity, r = .166, p = .04 (see Fig. 3). After controlling for other covariates (age, race, IQ, PCL:YV, GM + WM), the striatal component remained significant (partial r = .194, p = .018), but the insular component only trended in the same direction (partial r = .137, p = .096). Three additional components (out of 28 remaining), which did not include a priori regions of interest, also exhibited potentially meaningful relationships with substance use severity. These components included structural networks comprising the posterior cingulate and precuneus (both positively correlated with substance use), and a network including the posterior cerebellum was significantly negatively correlated with substance use severity. These incidental findings are presented for completeness in the supplementary materials; however, these effects do not survive corrections for multiple comparisons if evaluated from an agnostic, whole-brain approach. Nevertheless, they may be of interest in the context of developing new hypotheses for future investigations.
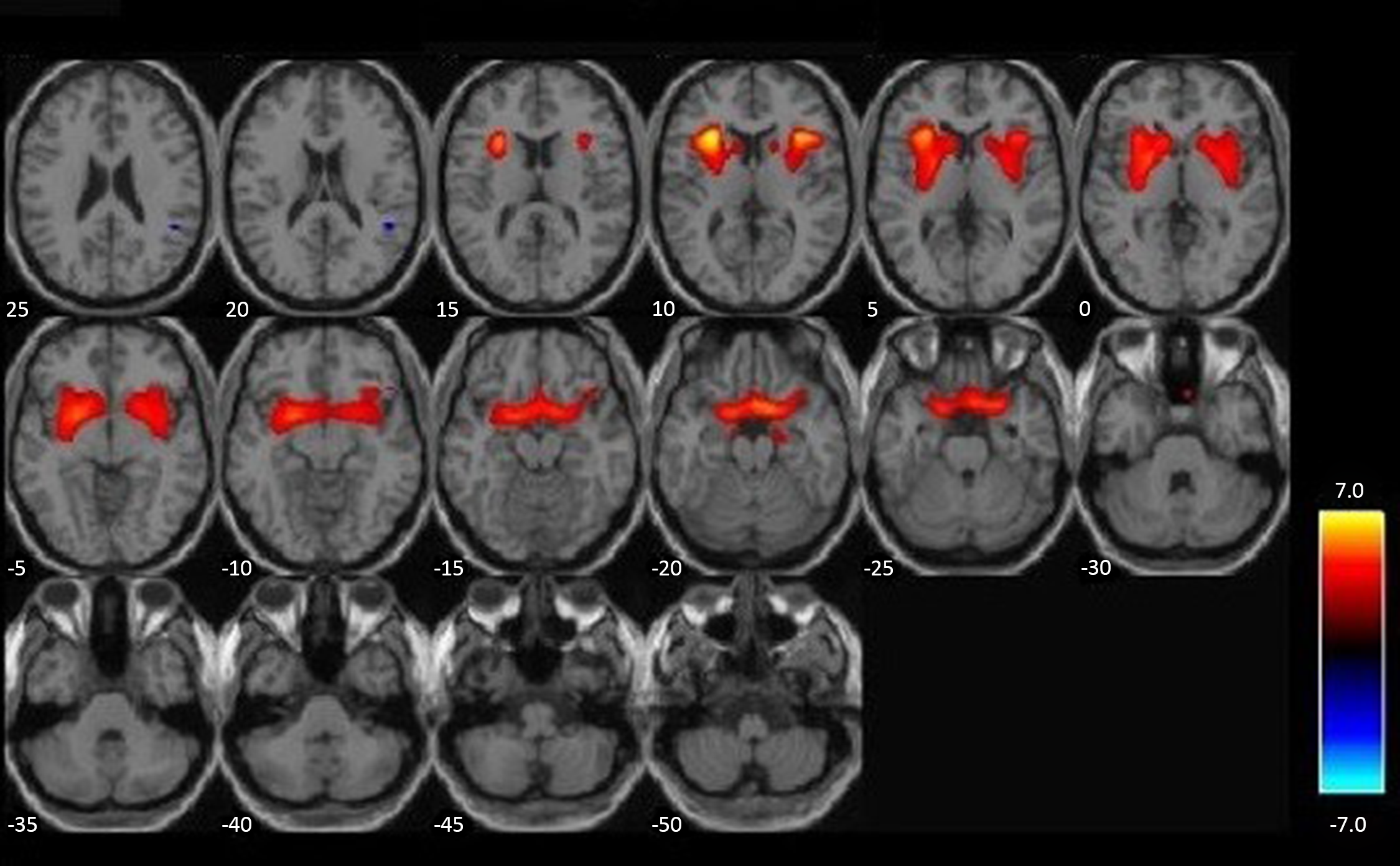
Figure 2. An intrinsic network with elevated gray matter in those with more severe substance use. A structural network, derived from ICA, n = 152, positively correlated with substance use severity is highlighted in red/orange. More severe substance use is associated with proportionally more gray matter throughout this intrinsic network, which includes caudate, putamen, nucleus accumbens, ventromedial PFC, and parts of the anterior insula (a priori ROIs). ICA = independent component analysis.
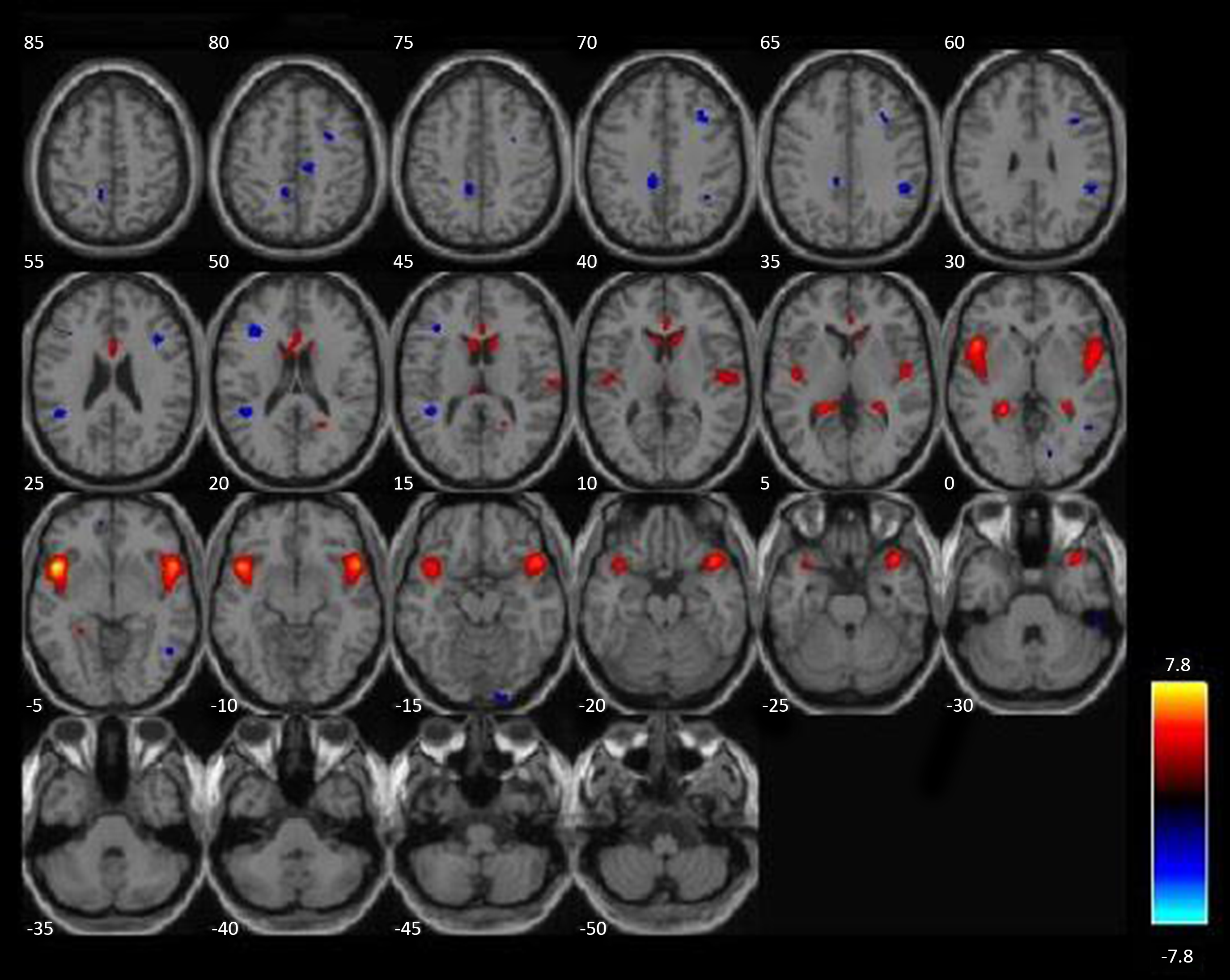
Figure 3. A second intrinsic network with increased gray matter associated with more severe substance use. A second structural network, derived from ICA, n = 152, positively correlated with substance use severity, highlighted in red/orange. More severe substance use is associated with proportionally more gray matter throughout this intrinsic network, which includes bilateral insula (a priori ROIs). ICA = independent component analysis.
Discussion
This study reports GMV differences associated with substance use severity among incarcerated juveniles. Consistent with hypotheses, gray matter hypertrophy was apparent in several regions corresponding to salience network, fronto-striatal reward circuits, and basal ganglia. These effects were apparent across two complementary analytic strategies: one prioritizing circumscribed ROIs and another using a data-driven approach for defining naturalistic structural networks across the whole brain. These findings confirmed expectations for abnormal developmental markers associated with early-life substance abuse among high-risk, antisocial youth in brain areas governing reward/reinforcement learning. Addressing possible limitations of prior studies, these effects were not attributable to variation in antisocial behavior/psychopathic personality traits, nor were they attributable to age, race, IQ, or overall brain size, which were accounted for in statistical models. The direction of effects (increased gray matter associated with greater substance use severity) is consistent with notions that relative hypertrophy and/or neurodevelopmental delays (i.e., pruning) in fronto-striatal reinforcement networks may be a marker for vulnerability to more extreme substance use behaviors. The present findings add support to a growing body of work denoting the importance of altered neurodevelopmental trajectories in early-onset substance misuse. These findings also underscore the relevance of these factors in highly antisocial youth.
The evident patterns of gray matter differences in the present study may be interpreted in more than one way. For instance, these findings may reflect differential rates of development in reward-oriented brain structures. While age is used as a covariate in our analyses, differential developmental trajectories do not perfectly align with chronological age (Kiehl et al., Reference Kiehl, Anderson, Aharoni, Maurer, Harenski, Rao, Claus, Harenski, Koenigs, Decety, Kosson, Wager, Calhoun and Steele2018). We know the volume of gray matter in the striatum steadily decreases through adolescence (Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans and Rapoport1999; Giedd, Reference Giedd2008) even as youth experience exaggerated dopamine responses and feelings of reinforcement from rewarding stimuli, relative to adults (Galvan, Reference Galvan2010). In this way, if we attribute increased gray matter in high substance users to developmental processes, less mature brains are apparently more susceptible to problematic substance use behaviors in high-risk youth. Alternatively, it is possible that increased substance use (and more frequent, exaggerated stimulation of striatal reward networks) produces a hypertrophic effect on gray matter in these areas, apparent in these younger users, which would hypothetically give way to the well-established deleterious effects of chronic use often reported in adults (Kaag et al., Reference Kaag, Schulte, Jansen, Van Wingen, Homberg, Van Den Brink, Wiers, Schmaal, Goudriaan and Reneman2018; Mackey et al., Reference Mackey, Allgaier, Chaarani, Spechler, Orr, Bunn, Allen, Alia-Klein, Batalla, Blaine, Brooks, Caparelli, Chye, Cousijn, Dagher, Desrivieres, Feldstein-Ewing, Foxe, Goldstein, Goudriaan, Heitzeg, Hester, Hutchison, Korucuoglu, Li, London, Lorenzetti, Luijten, Martin-Santos, May, Momenan, Morales, Paulus, Pearlson, Rousseau, Salmeron, Schluter, Schmaal, Schumann, Sjoerds, Stein, Stein, Sinha, Solowij, Tapert, Uhlmann, Veltman, van Holst, Whittle, Wiers, Wright, Yücel, Zhang, Yurgelun-Todd, Hibar, Jahanshad, Evans, Thompson, Glahn, Conrod and Garavan2019). A third possibility is that patterns of increased gray matter reflect some other intrinsic marker for susceptibility to substance abuse, apart from typical maturational variations – fundamentally an endophenotype of vulnerability. This interpretation aligns with prior work recognizing similar patterns of enlarged gray matter in substance abusing youth as well as their non-substance-abusing siblings (Ersche et al., Reference Ersche, Jones, Williams, Turton, Robbins and Bullmore2012), suggesting some heritable influence independent of substance use. This interpretation also aligns with a prospective, longitudinal study that reported increased gray matter in the striatum, midbrain, and prefrontal cortex of high novelty-seeking youth (pre-exposure to substance use) who went on to develop problematic substance use behaviors years later (Büchel et al., Reference Büchel, Peters, Banaschewski, Bokde, Bromberg, Conrod, Flor, Papadopoulos, Garavan, Gowland, Heinz, Walter, Ittermann, Mann, Martinot, Paillère-Martinot, Nees, Paus, Pausova, Poustka, Rietschel, Robbins, Smolka, Gallinat, Schumann and Knutson2017).
As noted in the introduction, there is other prior evidence for positive associations between GMV in the striatum and substance misuse, especially in young populations (Büchel et al., Reference Büchel, Peters, Banaschewski, Bokde, Bromberg, Conrod, Flor, Papadopoulos, Garavan, Gowland, Heinz, Walter, Ittermann, Mann, Martinot, Paillère-Martinot, Nees, Paus, Pausova, Poustka, Rietschel, Robbins, Smolka, Gallinat, Schumann and Knutson2017; Bu et al., Reference Bu, Yu, Su, Ma, Von Deneen, Luo, Zhai, Liu, Cheng, Guan, Li, Bi, Xue, Lu and Yuan2016; Gilman et al., Reference Gilman, Kuster, Lee, Lee, Kim, Makris, van der Kouwe, Blood and Breiter2014; Lisdahl et al., Reference Lisdahl, Tamm, Epstein, Jernigan, Molina, Hinshaw, Swanson, Newman, Kelly and Bjork2016). Elevated striatal gray matter has also been reported in adult methamphetamine users (Chang et al., Reference Chang, Cloak, Patterson, Grob, Miller and Ernst2005), with more exaggerated effects in younger adult users (Jernigan et al., Reference Jernigan, Gamst, Archibald, Fennema-Notestine, Mindt, Marcotte, Heaton, Ellis and Grant2005). However, among adults, this has often been attributed to a kind of compensatory response to chronic neuropathological insults to maintain function (Chang et al., Reference Chang, Cloak, Patterson, Grob, Miller and Ernst2005). Prior reports also suggest that impulsive, short-term oriented decision-making (e.g., choosing immediate rewards over future larger rewards) is associated with hypertrophy of striatal gray matter in young adult men and women (Tschernegg et al., Reference Tschernegg, Pletzer, Schwartenbeck, Ludersdorfer, Hoffmann and Kronbichler2015). In an exploratory analysis from the multisite Adolescent Brain Cognitive Development study, in more than 11,000 children aged 9–10, elevated scores on behavioral activation (as opposed to inhibition) were positively correlated with GMV in the ventral striatum, and this effect was stronger in monozygotic twins, suggesting a strong genetic influence (Ide et al., Reference Ide, Li, Chen, Le, Li, Zhornitsky and Li2020). Such findings suggest that, even in the absence of severe substance use behaviors, increased gray matter in striatal areas aligns with personality and behavioral patterns that may promote more drug use/misuse among affected youth.
Several prior studies examining neural correlates of substance use have noted certain limitations in their interpretations related to sampling characteristics (Fein et al., Reference Fein, Greenstein, Cardenas, Cuzen, Fouche, Ferrett, Thomas and Stein2013; Schiffer et al., Reference Schiffer, Müller, Scherbaum, Hodgins, Forsting, Wiltfang, Gizewski and Leygraf2011). When comparing substance using individuals with healthy controls, there remain a number of convergent factors that are difficult to control for across cohorts, particularly factors like behavioral disinhibition and antisocial personality traits when comparing substance abusing youth with healthy controls. Also, acute or recent lingering effects of substance use on MRI data are a concern among volunteers from the general population. By studying these effects among highly antisocial youth with varied substance use histories, we have reduced the impact of factors covarying with antisocial behavior and general disinhibitory traits. The boys involved in the correctional program at this particular site are all highly antisocial and indeed matriculated into this program based on unmanageable behavior at other statewide juvenile correctional facilities. Further, as participants are housed in a maximum security/controlled environment, they are under strict enforcement of controlled abstinence, reducing concerns of acute substance use. This study was specifically intended to narrow some of these prior inconsistencies and limitations.
Limitations
The consequence of studying these effects in this group of high-risk offenders is that it is not yet clear whether these effects reliably generalize to other juvenile samples or whether they exhibit a pathological trend specific to those with prominent risk factors for antisocial behavior. Further, there is scant research examining these effects among girls, but there is some supportive research describing overlapping and gender-specific effects (Fein et al., Reference Fein, Greenstein, Cardenas, Cuzen, Fouche, Ferrett, Thomas and Stein2013). Additional research will be necessary to address this with more specificity. As noted above, the present findings align well with other recent research suggesting enlarged gray matter in key brain areas may represent features of an endophenotype for vulnerability to problematic substance use. This interpretation remains uncertain, however. Longitudinal research implementing within-subjects neuroimaging at multiple time points will be necessary to confirm these interpretations, both in high-risk and lower-risk samples.
Conclusion
Among the approximately seven million individuals under correctional supervision in the United States, more than half meet the criteria for alcohol or other SUDs (CASA, 2010; Glaze & Kaeble, Reference Glaze and Kaeble2014; Mumola & Karberg, Reference Mumola and Karberg2006), compared to approximately 12% in the general population (Merikangas & McClair, Reference Merikangas and McClair2012). SUDs also predict maladaptive behavior within the corrections system and remain a risk factor for recidivism following release into the community (Cochran et al., Reference Cochran, Mears, Bales and Stewart2014; Henry, Reference Henry2020). Among those with SUDs, about 60% of those left untreated during incarceration will relapse within 3 years of release, often resulting in parole violations and recidivism (Pearson & Lipton, Reference Pearson and Lipton1999). Among youth with early encounters in the criminal justice system, it remains a priority to address known risk factors and neurodevelopmental liabilities with better precision. Periods of incarceration provide us with a valuable opportunity for research and intervention alike, as youth in the correctional system are encountering enforced abstinence, many for the first time. These population characteristics help to reduce the impact of otherwise uncontrollable nuisance factors in general community samples. The present findings may represent a foothold for developing more targeted intervention strategies, which recognize specific neuropathological vulnerabilities influencing the initiation and maintenance of substance misuse at an early age. Continued research in this venue serves both public mental health priorities and addresses humanitarian and practical concerns serving our best interests as a society.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579424000804.
Funding statement
This study was supported by the National Institute of Mental Health and National Institute of Child Health and Human Development through grant numbers R01HD092331, R01HD082257, and F32MH098532. We are grateful to the Wisconsin Department of Corrections and Mendota Juvenile Treatment Center for their cooperative efforts.
Competing interests
None.









