Chronic affective disorders are a major cause of psychiatric morbidity (Reference Scott, Barker and EcclestonScott et al, 1988), affecting up to 1% of the population. Studies of bipolar patients have identified a group whose illnesses respond poorly to treatment and have a poor outcome, with 20% remaining unwell for up to a year (Reference Cole, Scott and FerrierCole et al, 1993). Treatment resistance might be anticipated in individuals whose illness had specific neurobiological underpinnings. In bipolar subjects, T2-weighted magnetic resonance imaging (MRI) has revealed deep subcortical white matter lesions (DWML) and periventricular white matter lesions (PVWML), which have differing neuropathologies (Reference Fazekas, Kleinert and OffenbacherFazekas et al, 1993). Although not all MRI studies find an excess of white matter lesions (Reference Brown, Lewine and HudginsBrown et al, 1992; Reference Strakowski, Woods and TohenStrakowski et al, 1993), the majority do (Dupont et al, Reference Dupont, Jernigan and Gillin1987, Reference Dupont, Jernigan and Butters1990, Reference Dupont, Butters and Schafter1995a ,Reference Dupont, Jernigan and Heindel b ; Reference Swayze, Andreasen and AlligerSwayze et al, 1990; Reference Figiel, Krishnan and RaoFigiel et al, 1991; Reference McDonald, Krishnan and DoraiswamyMcDonald et al, 1991; Reference Puzynski, Beresewicz and KoszewskaPuzynski et al, 1995; Reference Woods, Yurgelun-Todd and MikulisWoods et al, 1995), and meta-analysis indicates that more bipolar patients than controls exhibit WMLs (Reference Altshuler, Curran and HauserAltshuler et al, 1995). Specific, but as yet unidentified subgroups, may exhibit WMLs. Dupont et al (Reference Dupont, Jernigan and Butters1990) reported that patients showing WMLs were more seriously unwell and performed poorly on neuropsychological testing.
Hypothesis
These observations lead us to predict that poor outcome bipolar patients have underlying structural brain abnormalities. It is our hypothesis that poor outcome bipolar subjects have more white matter abnormalities on MRI than either good outcome patients or controls.
METHOD
To explore our hypothesis we have compared T2-weighted MRI scans of poor and good outcome bipolar patients with healthy controls. Patients who did not meet the criteria for inclusion in either the good or the poor outcome groups were eliminated from the study and did not undergo MRI.
Subjects
Patients with a DSM—IV (American Psychiatric Association, 1994) diagnosis of bipolar affective disorder aged 20-65 years were identified from the in-patient and out-patient services of the Newcastle Mental Health Trust. To be included, all patients had been diagnosed as suffering from bipolar disorder for at least 3 years and had had a minimum of two episodes of illness. After identification, their case notes were reviewed. Those meeting the criteria for bipolar disorder (DSM—IV) were subjected to a full clinical evaluation by an experienced psychiatrist (I.C.M. or P.B.M.), who ensured that they met the criteria for inclusion but not the exclusion criteria for the study. A full history, including family history of mental illness, smoking history and obstetric history, was compiled and old case notes were reviewed. To screen out subjects with considerable cognitive decline (early dementia or pseudodementia), a Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) was completed. This instrument is insufficiently sensitive to detect subtle cognitive changes, for example those reported in patients with depression, thereby preventing inappropriate exclusion of patients. After a complete description of the study of the patients, written informed consent was obtained. Thereafter, subjects were allocated to either a good or poor outcome group (see below) or eliminated because of an intermediate outcome. Immediately prior to MRI scanning, the patient was interviewed to assess current mental state and a Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961) was completed by the subject.
Patient selection criteria
These are set out in the Appendix.
Poor outcome group (B)
At the time of study, patients in this group had been unwell for 2 years or more, despite adequate therapy. Any periods of remission lasted 8 weeks or less, during which time they still had significant functional impairment. All patients had made a poor response to lithium. Almost all were patients of a regional unit specialising in the treatment of affective disorder.
Good outcome group (A)
At the time of study, these patients had been clinically euthymic for at least 8 eight weeks. After any episode of illness, they had shown full symptomatic recovery and had returned to normal premorbid functioning. The good and poor outcome groups were age and gender matched.
Controls
Controls were recruited from the families of patients and the staff of Newcastle University and its associated hospitals. Controls were selected to match, as closely as possible, the ages and genders of subjects in the poor outcome group. No controls met the exclusion criteria applied to the patient groups.
Patient characteristics
In total, 17 patients were recruited into the poor outcome group and 16 into the good outcome group. One patient was excluded because he experienced a panic attack during MRI scanning and the scanning sequence could not be completed. Three patients were eliminated after the scans were examined because of evidence of demyelination (n=1), an Arnold—Chiari malformation with hydrocephalus (n=1) and cerebrovascular infarcts (n=1). A total of 29 patients completed the study, 15 in the poor outcome and 14 in the good outcome group.
The patient characteristics of the good (A) and poor (B) outcome groups and controls are compared in Table 1. Good outcome patients tend to be older, better educated and less likely to be left handed, but the differences do not reach significance. The illness duration, gender ratios, ages and prevalence of a family history of bipolar disorder are similar in both groups. The major difference between the groups arises in the severity of illness at the time of investigation. All good outcome patients were euthymic on the day of scanning, reflected in mean BDI scores of 9.0, s.d.=6.7. In contrast, the poor outcome group had significantly higher (P<0.05, unpaired Student's t-test) BDI scores of 16.0, s.d.=7.0, and several of this group showed clinical mood disturbances.
Table 1 Characteristics of patient groups and controls
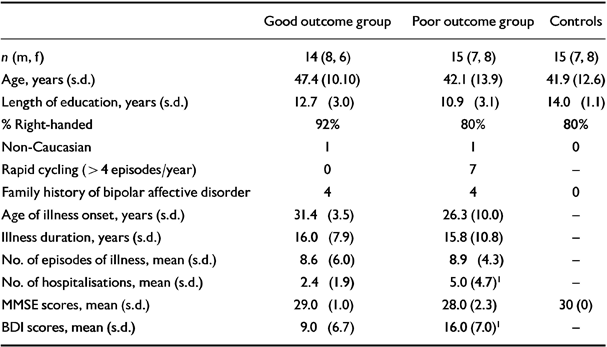
| Good outcome group | Poor outcome group | Controls | |
|---|---|---|---|
| n (m, f) | 14 (8, 6) | 15 (7, 8) | 15 (7, 8) |
| Age, years (s.d.) | 47.4 (10.10) | 42.1 (13.9) | 41.9 (12.6) |
| Length of education, years (s.d.) | 12.7 (3.0) | 10.9 (3.1) | 14.0 (1.1) |
| % Right-handed | 92% | 80% | 80% |
| Non-Caucasian | 1 | 1 | 0 |
| Rapid cycling (>4 episodes/year) | 0 | 7 | - |
| Family history of bipolar affective disorder | 4 | 4 | 0 |
| Age of illness onset, years (s.d.) | 31.4 (3.5) | 26.3 (10.0) | - |
| Illness duration, years (s.d.) | 16.0 (7.9) | 15.8 (10.8) | - |
| No. of episodes of illness, mean (s.d.) | 8.6 (6.0) | 8.9 (4.3) | - |
| No. of hospitalisations, mean (s.d.) | 2.4 (1.9) | 5.0 (4.7)1 | - |
| MMSE scores, mean (s.d.) | 29.0 (1.0) | 28.0 (2.3) | 30 (0) |
| BDI scores, mean (s.d.) | 9.0 (6.7) | 16.0 (7.0)1 | - |
Magnetic resonance imaging
All subjects were examined using a General Electrics (Slough, UK) MR max plus 0.5 tesla scanner. Sagittal, coronal and axial T1-weighted scan sequences, axial T2-weighted scan sequences and coronal inversion recovery scan sequences were recorded on all subjects. The T1-weighted and inversion recovery scans were examined by a consultant neuroradiologist (V.L.M.) for the presence of structural abnormalities. Up to 15 axially oriented 7-mm slices separated from each other by 1 mm were recorded during the T2-weighted sequence.
Axial T2-weighted scan sequences with a relaxation time of 2300 ms and echo delays of 25 and 100 ms were examined for the presence of white matter hyper-intensities. To be counted, WMLs had to be present on both the proton density and T2 scans. Two investigators (D.J.S. and V.L.M.) examined scans independently and blindly and their results were in complete agreement. White matter lesions were classified as either deep subcortical or periventricular and images were graded 0-3 using the scheme proposed by Fazekas (Fazekas et al, Reference Fazekas, Chawluk and Alavi1987, Reference Fazekas, Kleinert and Offenbacher1993).
Statistical analysis
Prior to analysis, all data were checked for deviation from normality using the Anderson—Darling method (Reference Stephens, D'Agostino and StephensStephens, 1980). The MMSE scores, age of onset of illness and duration of education differed significantly from normality. All data were analysed using the appropriate parametric or non-parametric methods: for parametric data, ANOVA, ANCOVA, Student's t-test and Pearson product moment coefficient were calculated; for non-parametric data, including white matter hyperintensity gradings, χ2, Mann-Whitney and Spearman rank correlations were calculated using the computerised statistical package Minitab 10.2 for Windows (Minitab Inc., PA).
RESULTS
The principal findings of the study are shown in Table 2. Comparing the numbers of subjects with DWMLs, significantly more (odds ratio=11.4) patients (7/15, 47%) in the poor outcome group had DWMLs than in the good group (1/14, 7%, Fisher's exact test, P=0.035) or controls (0/15, 0%, P=0.003). The grades (severity) of DWMLs in the poor outcome group significantly exceeded (Mann—Whitney U-test) the good outcome patients (P=0.030) or controls (P<0.014). In contrast, the grade of DWMLs in the good patient group and controls did not differ significantly (P=0.96).
Table 2 White matter abnormalities on magnetic resonance imaging in bipolar subjects and controls
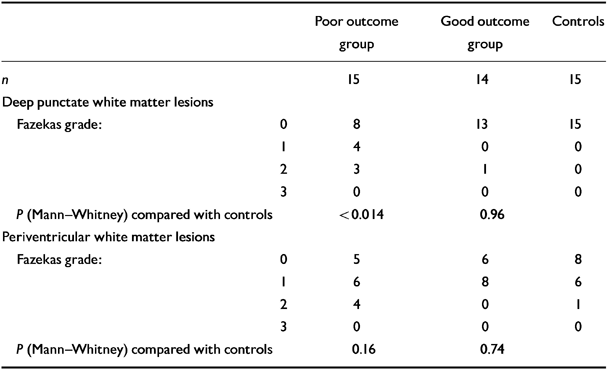
| Poor outcome group | Good outcome group | Controls | ||
|---|---|---|---|---|
| n | 15 | 14 | 15 | |
| Deep punctate white matter lesions | ||||
| Fazekas grade: | 0 | 8 | 13 | 15 |
| 1 | 4 | 0 | 0 | |
| 2 | 3 | 1 | 0 | |
| 3 | 0 | 0 | 0 | |
| P (Mann—Whitney) compared with controls | <0.014 | 0.96 | ||
| Periventricular white matter lesions | ||||
| Fazekas grade: | 0 | 5 | 6 | 8 |
| 1 | 6 | 8 | 6 | |
| 2 | 4 | 0 | 1 | |
| 3 | 0 | 0 | 0 | |
| P (Mann—Whitney) compared with controls | 0.16 | 0.74 |
In contrast, PVWMLs were not present in strikingly different numbers in the good (8/14, 57%), poor (10/15, 67%) or control (7/15, 47%) groups. Fisher's exact test failed to reveal any differences between the numbers in each group with PVWMLs. Although more patients in the poor outcome group had more severe abnormalities, Mann—Whitney tests failed to reveal any significant differences between the groups
(good v. poor, P=0.22; good v. controls, P=0.74; poor v. controls, P=0.16).
It is concluded that DWMLs but not PVWMLs are linked significantly to poor outcome in bipolar disorder.
Obstetric, smoking and family histories of mental disorder were collected. A detailed statistical analysis of the data is not warranted because of the small sample size. However, inspection of the data failed to suggest that, for an individual, there was a clear link between WMLs and these histories. A larger study would be required to explore correlations between these variables.
DISCUSSION
Deep white matter lesions
The principal finding of this study is that DWMLs are seen most frequently in bipolar patients with a poor outcome when compared with those who have a good outcome or with normal controls. In contrast, the frequencies of PVWML hyperintensities do not differ significantly between these groups. Although the pathology of WMLs in bipolar disorder is not known, they may be direct consequences of the disease process, they may result from independent processes that then predispose to treatment resistance or they may simply be coincidental findings. Clinicopathological studies in elderly subjects indicate that DWMLs and PVWMLs have different aetiologies. Discrete and confluent subcortical punctate WMLs probably arise from perivascular abnormalities and in severe cases may indicate the presence of areas of microcystic infarct. Hyperintense ventricular caps and smooth periventricular halos may result from myelin pallor and are associated with the loss of ependymal lining of the ventricles and local increase in tissue fluid. In contrast, extensive irregular PVWMLs have a similar aetiology to punctate lesions (Reference Fazekas, Kleinert and OffenbacherFazekas et al, 1993). Hence, analysis of white matter abnormalities in diseased states needs to differentiate clearly between these lesions. Although our study shows that there is an association between deep punctate and periventricular lesions in our patient group (Spearman: P=0.39, P=0.04), only the deep punctate lesions are associated with a poor outcome bipolar disorder. A similar finding has been reported in elderly patients with depression — in this group, DWMLs but not PVWMLs are associated with depression (Reference O'Brien, Desmond and AmesO'Brien et al, 1996). This difference probably reflects the differing neuropathological bases of these abnormalities. The present results suggest that a factor associated with poor outcome in bipolar disorder is the presence of microvascular abnormalities in the cerebral white matter.
A number of studies have reported WMLs in bipolar patients but many fail to differentiate periventricular from subcortical punctate lesions. White matter lesions have been reported more frequently in young bipolar patients (aged <60 years) than controls by Figiel et al (Reference Figiel, Krishnan and Rao1991), Swayze et al (Reference Swayze, Andreasen and Alliger1990) and Dupont et al (Reference Dupont, Jernigan and Gillin1987, Reference Dupont, Jernigan and Butters1990), whereas Strakowski et al (Reference Strakowski, Woods and Tohen1993) failed to find any difference for newly diagnosed patients with mania. In these studies, the percentages of bipolar patients with subcortical hyperintensities varied from 55% to 18%, whereas the percentages in normal controls varied from 17% to 0%. This variation could have resulted from several factors. In particular, risk factors for the formation of hyperintensities, especially advancing ageing and hypertension, may not have been controlled for sufficiently rigorously. However, our study suggests that a major cause of the variation is likely to be the inclusion of poor prognosis patients in the study. Studies based on hospital populations tend to overrepresent the more chronic patients with long illness histories who suffer frequent relapse. It is notable that the study of Strakowski et al (Reference Strakowski, Woods and Tohen1993), which failed to find significant differences between controls and patients, considered patients only in their first episode of manic illness. This factor alone is likely to reduce significantly the proportions of poor outcome patients included in their study because only approximately 10-20% of all bipolar patients go on to develop a treatment-resistant disorder (Reference Cole, Scott and FerrierCole et al, 1993). Furthermore, Strakowski's study excluded patients with a family history of bipolar disorder, thereby eliminating those with a genetic predisposition that could be associated with the development of organic lesions. Dupont et al (Reference Dupont, Jernigan and Butters1990) noted that bipolar patients with WMLs had significantly more hospitalisations, higher Hamilton Rating Scale for Depression scores and received more neuroleptics than patients without WMLs. All of these measures of poor outcome may confirm the current finding that WMLs are associated with a poorer prognosis. This is further suggested by the observation of a follow-up 1 year later, that approximately half of Dupont's patients with WMLs failed to recover. Further support for this hypothesis is provided by studies in elderly, unipolar subjects that have linked DWMLs to outcome. Individuals with DWMLs are reported to respond less well to in-patient treatment, including electroconvulsive therapy (Reference Hickie, Scott and MitchellHickie et al, 1995), and to relapse more rapidly (Reference O'Brien, Ames and ChiuO'Brien et al, 1998).
Although we have classified outcome on the basis of a clinical assessment and level of functioning, others, for example McGlashan (Reference McGlashan1984) in his outcome studies at Chestnut Lodge, have described outcome on a more systematic basis using five dimensions: hospitalisation, employment, social activity, symptoms and global functioning, rated 0-4. With the exception of social activity, these were recorded for our patients. In retrospect, our poor outcome patients would score 0 or 1, 0, no data, 0 and 0, respectively, on each of these dimensions. In contrast, the corresponding scores of our good outcome patients would be 4, 3-4 (including house-work as employment), no data, 3-4 and 4, respectively. This reflects the study design to maximise the contrast between good and poor outcome patient groups.
Periventricular white matter lesions
The frequency of PVWMLs in our study did not differ significantly between the good and the poor outcome groups and normal controls. The frequency of all PVWMLs was high, at 57%, 67% and 47%, respectively, for the good and poor prognosis groups and controls. These rates are comparable with other studies (Reference Altshuler, Curran and HauserAltshuler et al, 1995). However, interpretation of the presence of PVWMLs is problematic. Studies (Reference Sze, De Armond and Brant-ZawadzkiSze et al, 1986; Reference Leifer, Buonanno and RichardsonLeifer et al, 1990) suggest that the hyperintense caps seen at the end of lateral ventricles on T2-weighted scans are a normal finding, as is the presence of a thin white hyperintense line around the ventricle margins. When we rated our scans with these appearances as normal, the differences between the groups increased but nevertheless still failed to reach statistical significance at the 5% level. Failure to find significantly different numbers of good and poor outcome patients and controls with PVWMLs may be a consequence of the low power of a study based on 44 subjects. We are uncertain whether some PVWMLs are related to a poor outcome, and further studies are indicated.
Medication
The medication taken by good and poor outcome groups was similar and reflected the recent trend in bipolar management to focus upon treatment with mood stabilisers. Thus, 10, 6 and 0 of the 14 good outcome patients received lithium, carbamazepine and/or valproate, respectively, whereas the corresponding numbers were 12, 8 and 5 for the 15 poor outcome patients. Four patients in the good outcome group received lithium/anticonvulsant combinations whereas nine poor outcome patients received this combination.
It is improbable that lithium will give rise to WMLs. Good and poor outcome groups had broadly similar usage of these drugs and yet had markedly different DWML frequencies, the good group being similar to the controls, who were drug free. Only patients received medication, yet PVWMLs were found in considerable numbers in the controls.
With the exception of sodium valproate usage, the most striking differences between the groups was the number of patients in the poor outcome compared to the good outcome group receiving neuroleptics (6 v. 2), antidepressants (6 v. 2) or anticholinergics (4 v. 0). There are insufficient patients receiving different drugs to warrant a statistical analysis of the data, especially in view of the pharmacological heterogeneity of the different neuroleptics or antidepressants. There is, however, little evidence in the literature that either neuroleptic or anti-depressant usage results in WMLs.
Age and white matter lesions
It has been emphasised that age is an important determinant of the prevalence of WMLs. Woods et al (Reference Woods, Yurgelun-Todd and Benes1990) reported that the prevalence of both DWMLs and DVWMLs in bipolar subjects but not in controls increased after the age of 30 years. In contrast, Altshuler et al (Reference Altshuler, Curran and Hauser1995) reported that the prevalence of only PVWMLs in bipolar subjects but not in controls increased after the age of 30 years. This difference may result from the lack of control for cardiac risk factors in Woods' study, especially in view of the likelihood that subcortical lesions are vascular in origin. In our bipolar subjects, we found no significant effect of age upon the prevalence of DWMLs (ANCOVA: F=0.02, P=0.88), whereas the PVWMLs increased with age (ANCOVA: F=5.57, P=0.007). The mean age of our poor outcome group (42.1, s.d.=13.9 years) was comparable to that of the controls (41.9, s.d.=12.6 years) but less than that of the good outcome group (47.4, s.d.=10.1 years). Hence, it is improbable that differences in the ages of groups could contribute significantly to the increased prevalence of DWMLs in the poor outcome groups. Age similarities rather than group differences (F=2.73, P=0.08) account for the similarities in the prevalence of PVWMLs in patient groups and controls.
The presence of DWMLs distinguishes poor outcome from good outcome patient groups and controls. Nevertheless, these abnormalities were found in only 47% of the poor prognosis patient group. The aetiology of treatment resistance therefore appears to be multi-factorial. Preliminary investigations suggest that cerebral dysrhythmias (Reference Cole, Scott and FerrierCole et al, 1993) are associated with treatment resistance. These observations may link to the growing evidence that poor outcome patients respond much better to anticonvulsants than to lithium. Some of the poor outcome treatment-resistant patients were clinically unwell at the time of scanning and it is possible that differences between the good and poor outcome groups reflect differences in current mental state. However, there is evidence (Reference Dupont, Jernigan and ButtersDupont et al, 1990) that WMLs observed in bipolar patients are stable over time. Nevertheless, it would seem prudent to re-examine patients with DWMLs in the future when they are clinically euthymic to establish whether the lesions reflect the clinical state of the patient or more fundamental abnormalities of cerebral architecture.
APPENDIX
Patient selection criteria
The inclusion criteria are:
-
(a) DSM-IV bipolar affective disorder;
-
(b) age 20-65 years;
-
(c) two episodes minimum and a history of illness for at least 3 years.
Patients were then divided into two groups:
-
(a) Good outcome group (A):
-
(i) return to premorbid level of functioning between illness;
-
(ii) currently euthymic for at least 8 weeks;
-
(iii) if prescribed, good response to lithium.
-
-
(b) Poor outcome group (B):
-
(i) symptomatic non-recovery for at least the past 2 years;
-
(ii) any period of well-being in the past 2 years for 8 weeks or less;
-
(iii) failure to regain premorbid functioning during periods of well-being.
-
The exclusion criteria are:
-
(a) Psychiatric: evidence of cognitive decline; other Axis I comorbid condition; bipolar disorder other than type I or type II; learning disabilities.
-
(b) Neurological: cerebrovascular disease; neuro-degenerative disorders; head injury with concussion; epilepsy; idiopathic parkinsonism; systemic illness with cerebral consequences; focal neurological signs on examination.
-
(c) Medical: hepatic disorder; cardiovascular disorder; renal failure; hypertension (blood pressure >150/100 untreated or any treated hypertension); endocrine disorder (excluding corrected hypothyroidism).
-
(d) Pharmacological: medication (corticosteroids, antihypertensives); alcohol dependence or misuse; illicit drug use or solvent misuse.
-
(e) Radiological: metal implants.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ The presence of deep subcortical white matter lesions (DWMLs) on MRI in bipolar patients indicates a poorer clinical outcome.
-
▪ We would suggest that any patient found to have DWMLs early in the course of illness might benefit from intense therapeutic input to optimise outcome.
-
▪ When examining a research paper on bipolar disorder, the clinical features of the patient group require careful scrutiny.
LIMITATIONS
-
▪ The difficulties of identifying and recruiting poor outcome bipolar patients means that the study is necessarily of limited size and should be replicated by a larger multicentre study.
-
▪ There is no consensus on reliable and meaningful definitions of outcome in bipolar disorder.
-
▪ The study requires replication.
ACKNOWLEDGEMENTS
We wish to thank Mrs M. Cheek for patiently typing this manuscript, all the staff at the Department of Neuroradiology, Newcastle General Hospital, for their time and courtesy when scanning the subjects and, finally, Newcastle City Health Trust for a grant to cover the costs of MRI.





eLetters
No eLetters have been published for this article.