Impact statement
Given the critical role of mitochondria in cellular and organismal health, MPs/NPs pose a significant threat to mitochondrial health and function. The current evidence underscores the urgency of addressing the pervasive problem of MP/NP pollution, not only for the protection of the environment but also for human health. The information provided here should inspire and guide further research in several directions. The specific molecular mechanisms by which MPs/NPs affect mitochondrial health need to be elucidated. A deeper understanding of these processes could inform the development of strategies to mitigate these effects or be used as biomarkers of exposure or toxicity. In addition, this information should motivate regulators to reassess the environmental and health risks associated with MP/NP pollution, incorporating new knowledge on mitochondrial effects into these assessments. This could help to shape more comprehensive and effective strategies for dealing with plastic pollution, ranging from policies to reduce plastic waste and promote more sustainable materials, to remediation of existing pollution.
Introduction
Background on microplastics (MPs) and nanoplastics (NPs)
During the past 70 years, the use of plastics has increased more than many other products, but the waste of plastics has spread throughout the environment as well (Kayan and Küçük, Reference Kayan and Küçük2020). This has given rise to the term ‘plastic debris’, which is defined as “human-generated solid polymeric material waste that is intentionally or accidentally released into the environment.” The production of plastic products reached approximately 390.7 million tons in 2021, and the negative impacts of persistent plastic waste on aquatic and terrestrial environmental health are of serious concern (Thompson et al., Reference Thompson, Swan, Moore and vom Saal2009; European Commission, 2019; Plastics Europe, 2022). Polyethylene (PE), polypropylene, polystyrene (PS), polyvinyl chloride (PVC), polyethylene terephthalate (PET), and polyurethane used in the production of plastics are attracting more attention because they are produced in large quantities and are widespread in the environment (Vert et al., Reference Vert, Doi, Hellwich, Hess, Hodge, Kubisa, Rinaudo and Schué2012; Revel et al., Reference Revel, Châtel and Mouneyrac2018; European Commission, 2019; Science Advice for Policy by European, 2019; Pinto Da Costa et al., Reference Pinto Da Costa, Rocha-Santos and Duarte2020; Plastics Europe, 2021). Although studies to determine the potential effects of plastic debris on human health have increased exponentially over the past decade, current knowledge is still insufficient to determine the risk. Of all the plastic produced, ~33% is not suitable for recycling and is thrown into the environment within 1 year of production (Koelmans et al., Reference Koelmans, Gouin, Thompson, Wallace and Arthur2014). During the incineration process, which is one of the ways of dealing with plastic waste, toxic chemicals (such as furan and dioxin) that are harmful to human health and the environment are released. Furthermore, many countries have yet to introduce legislation to regulate the recycling of plastic waste, often opting instead for the cheaper and easier route of landfilling (Crawford and Quinn, Reference Crawford and Quinn2017).
Plastic waste accumulates in large quantities in terrestrial, marine, and freshwater ecosystems. Today, plastic debris and the pollution it causes are recognized as one of the most important global environmental threats (Ryan et al., Reference Ryan, Moore, Franeker and Moloney2009; Villarrubia-Gómez et al., Reference Villarrubia-Gómez, Cornell and Fabres2018; European Commission, 2019; Science Advice for Policy by European, 2019; Hale et al., Reference Hale, Seeley, La Guardia, Mai and Zeng2020; Plastics Europe, 2021). It is estimated that ~10% of all plastics produced to date end up as litter in the oceans (Laglbauer et al., Reference Laglbauer, Franco-Santos, Andreu-Cazenave, Brunelli, Papadatou, Palatinus, Grego and Deprez2014). It has been reported that 61–87% of litter ≥5 mm in size and 98–99% of litter <5 mm in size is plastic (plastic film, plastic fibers, polystyrene, plastic pellets) (Tekman et al., Reference Tekman, Gutow, Macario, Haas, Walter and Bergmann2021). Recently, in addition to the visible macro form of plastic waste, microplastic (MP) and nanoplastic (NP) particles have also raised ecotoxicological concerns (Mattsson et al., Reference Mattsson, Jocic, Doverbratt, Hansson and Zeng2018). MPs and NPs can be found as primary and secondary plastic particles, depending on the way they are formed. Primary particles are produced in a fixed size for the purpose, usually in the form of beads, while secondary particles are formed by the degradation of larger plastic materials (McDevitt et al., Reference McDevitt, Criddle, Morse, Hale, Bott and Rochman2017). Secondary MPs/NPs are much more abundant in the environment than primary ones (Hale et al., Reference Hale, Seeley, La Guardia, Mai and Zeng2020). Anthropogenic impacts, environmental factors such as solar radiation (UV photooxidation reactions), wind and waves, and abrasion from car tires are effective in the formation of secondary MP/NP pollution (Andrady, Reference Andrady, Bergmann, Gutow and Klages2015; Wagner et al., Reference Wagner, Hüffer, Klöckner, Wehrhahn, Hofmann and Reemtsma2018). Although the rate and amount of nanofragmentation in nature is unknown, it is predicted that the fragmentation of MP particles with a size >100 nm-5 mm into NP particles with a size of 100 nm would lead to an NP particle concentration > 1014 times higher than the current MP particle concentration (Besseling et al., Reference Besseling, Redondo-Hasselerharm, Foekema and Koelmans2019). Ter Halle et al. (Reference Ter Halle, Jeanneau, Martignac, Jardé, Pedrono, Brach and Gigault2017) detected PVC, PET, PS, and PE polymers with a size of 1–999 nm in ocean surface samples for the first time. Although MP/NP pollution is considered a global problem, their potential risks to human health are far from known with the available data (Thompson et al., Reference Thompson, Olsen, Mitchell, Davis, Rowland, John, McGonigle and Russell2004; Reference Thompson, Moore, Andrady, Gregory, Takada and Weisberg2005). Field studies have shown the presence of MP in a large proportion of living organisms in the food chain (Lusher et al., Reference Lusher, Mchugh and Thompson2013; Reference Lusher, Hollman and Mendoza-Hill2017; Hermsen et al., Reference Hermsen, Mintenig, Besseling and Koelmans2018). MPs have also been detected in bottled water and tap water (Mintenig et al., Reference Mintenig, Int-Veen, Löder, Primpke and Gerdts2017; Kosuth et al., Reference Kosuth, Mason and Wattenberg2018; Mason et al., Reference Mason, Welch and Neratko2018; Mintenig et al., Reference Mintenig, Löder, Primpke and Gerdts2019). Studies have started to reveal that MPs/NPs can trigger physical and chemical toxicity in organisms (Bergmann et al., Reference Bergmann, Gutow and Klages2015; Klages et al., Reference Klages, Gutow and Bergmann2015; Wagner and Lambert, Reference Wagner and Lambert2018). In aquatic organisms, MPs have been shown to cause oxidative stress, genotoxicity, neurotoxicity, developmental delay, reduced reproductive success, and death (De Sá et al., Reference De Sá, Oliveira, Ribeiro, Rocha and Futter2018). In addition, in vivo studies have shown that primary MPs/NPs accumulate in tissues after oral or respiratory exposure (Deng et al., Reference Deng, Yan, Shen, Huang, Ren and Zhang2021; Xu et al., Reference Xu, Ma, Han and Chen2021; Fan et al., Reference Fan, Wei, Hu, Zhang, Yang, Du, Zhu, Sun, Oh and Gu2022; Meng et al., Reference Meng, Zhang, Wang, Gonzalez-Gil, Vrouwenvelder and Li2022; Yang et al., Reference Yang, Zhu, Zhou, Pan, Nan, Yin, Lei, Ma, Zhu, Chen, Han, Ding and Ding2022; Jeong et al., Reference Jeong, Baek, Koo, Park, Ryu, Kim, Zhang, Chung, Dogan, Choi, Um, Kim, Lee, Jeong, Shin, Lee, Kim and Lee2022a).
Exposure and toxicity of MPs/NPs
In humans, to determine whether exposure to plastic particles poses a public health risk, it is first necessary to understand the exposure to these substances and the hazards associated with exposure. Humans are exposed to MPs/NPs primarily through oral and dermal routes, inhalation, and during medical procedures (Prata et al., Reference Prata, da Costa, Lopes, Duarte and Rocha-Santos2020). The fact that MPs have been detected in human feces and tissues (placenta, lung, and whole blood) in clinical studies and are beginning to be associated with disease suggests that the potential effects of MP/NP exposure should be taken seriously (Schwabl et al., Reference Schwabl, Köppel, Königshofer, Bucsics, Trauner, Reiberger and Liebmann2019; Amato-Lourenço et al., Reference Amato-Lourenço, Carvalho-Oliveira, Júnior, Dos Santos Galvão, Ando and Mauad2021; Ragusa et al., Reference Ragusa, Svelato, Santacroce, Catalano, Notarstefano, Carnevali, Papa, Rongioletti, Baiocco, Draghi, D’Amore, Rinaldo, Matta and Giorgini2021; Jenner et al., Reference Jenner, Rotchell, Bennett, Cowen, Tentzeris and Sadofsky2022; Leslie et al., Reference Leslie, van Velzen, Brandsma, Vethaak, Garcia-Vallejo and Lamoree2022; Yan et al., Reference Yan, Liu, Zhang, Zhang, Ren and Zhang2022). While the detection of human MP exposure is still very new, the extent of NP exposure is unfortunately not yet known due to the lack of methods to detect particles of this size (European Commission, 2019; Science Advice for Policy by European, 2019).
Plastic particles smaller than 150 μm can enter the system by crossing the intestinal epithelium; NPs smaller than 100 nm can easily be taken into the cell and pose a threat to humans (EFSA, 2016; Celebi Sözener et al., Reference Celebi Sözener, Cevhertas, Nadeau, Akdis and Akdis2020). in vitro studies have shown that primary PS MPs/NPs are taken into cells, reduce cell viability, trigger apoptosis, alter reactive oxygen species (ROS) production, mitochondrial membrane potential (MMP), and function (Prietl et al., Reference Prietl, Meindl, Roblegg, Pieber, Lanzer and Fröhlich2014; Forte et al., Reference Forte, Iachetta, Tussellino, Carotenuto, Prisco, De Falco, Laforgia and Valiante2016; Wu et al., Reference Wu, Wu, Liu, Wang and Chen2019; Xu et al., Reference Xu, Halimu, Zhang, Song, Fu, Li, Li and Zhang2019a; Li et al., Reference Li, Xu, Zhang, Halimu, Li, Li, Gu, Zhang and Wang2022; Wang et al., Reference Wang, Shi, Gao, Zhang, Zhao, Wang, Zhang and Chen2022a; Sun et al., Reference Sun, Wen, Zhang, Fu, Yuan, Kuang, Kuang, Huang, Zheng and Zhang2023b).
When considering the routes of human exposure to NPs and MPs, the respiratory and digestive systems are the first areas of concern. However, it clearly demonstrates that MPs/NPs, which are small in size, can overcome biological barriers in humans and circulate through the blood system to access other tissues (Leslie et al., Reference Leslie, van Velzen, Brandsma, Vethaak, Garcia-Vallejo and Lamoree2022). It has also been reported that NPs can cross the air-blood barrier in the lung and enter the bloodstream (Prata et al., Reference Prata, da Costa, Lopes, Duarte and Rocha-Santos2020), while primary exposure to PS NPs can cause lung damage (Wu et al., Reference Wu, Wang, Zhao, Sun, Xu, Che, Pan, Wu and Shen2023). In addition, primary PS NPs have been shown to accumulate in the brain by crossing the blood–brain barrier after intravascular injection (Yang et al., Reference Yang, Chang, Tsai, Chen, Tseng and Lo2004). Mice exposed to primary PS NPs had a significant increase in blood glucose, glucose intolerance, and insulin resistance. PS NPs exacerbated STZ-induced type 2 diabetes (Wang et al., Reference Wang, Wei, Xu, Wang, Gao, Han, Wang and Chen2023). PS NPs induced Parkinson’s disease-like neurodegeneration in mice, so NP exposure should be carefully considered as a neurological health risk (Liang et al., Reference Liang, Huang, Zhong, Li, Ye, Wang, Zhang, Meng, Lin, Du, Hu, Wu, Sui, Yang and Huang2022). Mitochondrial dysfunction, endoplasmic reticulum (ER) stress, oxidative stress, and lysosomal membrane damage are observed in diseases such as neurodegenerative diseases, inflammation, metabolic stress, oxidative stress, diabetes, cardiovascular diseases, gastrointestinal diseases, kidney and lung diseases, skin diseases, aging, and cancer (Calì et al., Reference Calì, Ottolini and Brini2011; Herst et al., Reference Herst, Rowe, Carson and Berridge2017; Ryter et al., Reference Ryter, Rosas, Owen, Martinez, Choi, Lee, Elias and Choi2018; Burgos-Morón et al., Reference Burgos-Morón, Abad-Jiménez, Martínez de Marañón, Iannantuoni, Escribano-López, López-Domènech, Salom, Jover, Mora, Roldan, Solá, Rocha and Víctor2019; Shacham et al., Reference Shacham, Sharma and Lederkremer2019; Xu et al., Reference Xu, Di, He, Wang, Ma, Sun, Li, Wang, Shen, Fang, Feng and Shen2019b; Rana, Reference Rana2020; Lee et al., Reference Lee, Yi, Moon, Yoon and Park2022). The organelles mitochondria, endoplasmic reticulum, and lysosome, which play an important role in the pathophysiology of these diseases, are also targets of MP/NP toxicity (Lim et al., Reference Lim, Ng, Zou, Lu, Chen, Bay, Shen and Ong2019; Wang et al., Reference Wang, Lee, Hsu, Chiu, Huang, Huang, Chia, Lee, Y-F and Chiu2021; Halimu et al., Reference Halimu, Zhang, Liu, Zhang, Wang, Gu, Zhang, Dai, Zhang, Zhang and Xu2022). In this review, we will discuss studies revealing the effects of MPs/NPs on mitochondria.
Mitochondrial toxicity of MPs/NPs
Impact of MPs/NPs on mitochondrial structure and function
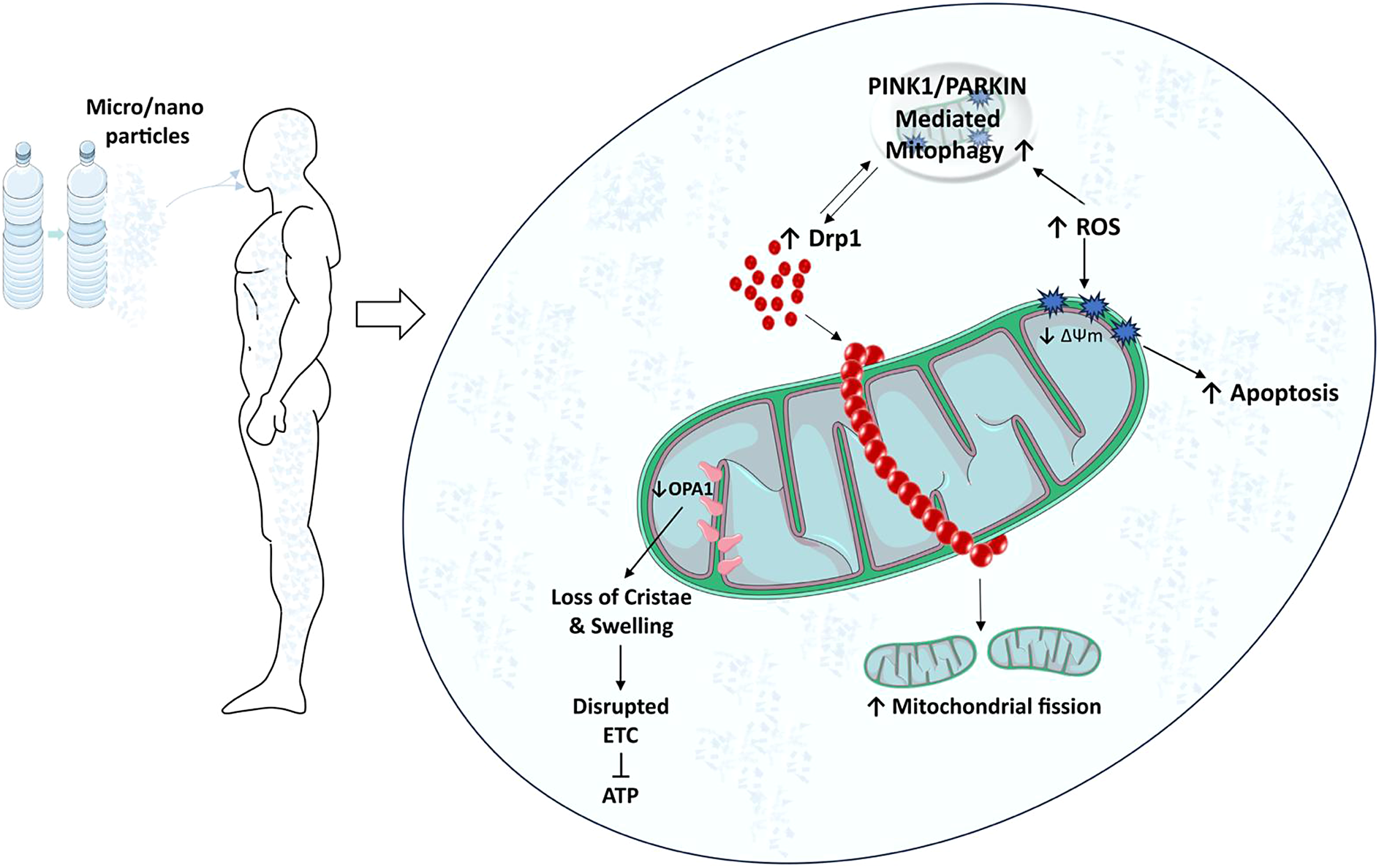
Due to their size and surface properties, MPs/NPs can physically interact with cellular structures, including mitochondria. Often referred to as the ’powerhouses of the cell’, mitochondria are key organelles responsible for ATP production through oxidative phosphorylation. In addition to energy production, they regulate several cellular processes, including calcium homeostasis, apoptosis, and the generation of reactive oxygen species (ROS). Exposure to these pollutants can induce mitochondrial damage and dysfunction, disrupting normal cellular operations and potentially leading to cell death. There is evidence that these particles can penetrate cell membranes and accumulate inside cells, possibly targeting mitochondria (Lee et al., Reference Lee, Yi, Moon, Yoon and Park2022). This penetration appears to be facilitated by NP small size, allowing them to cross biological barriers more easily than larger particles (Yang et al., Reference Yang, Cheng, Chen, Liu, Yin, Pu and Liang2021). Once inside the cell, MPs/NPs can disrupt mitochondrial structure. Studies in mice have shown that exposure to PS MPs can cause significant morphological changes in mitochondria, such as swelling and loss of cristae (Lin et al., Reference Lin, Tong, Xue, Qianru, Xinyu, Zhe, Zhikun and Shu2022a). Building on the structural disruption mentioned above, these physical interactions may also induce functional abnormalities in mitochondria, further contributing to their toxicity. As the powerhouse of the cell, mitochondria play a critical role in maintaining cellular energy homeostasis (Lee et al., Reference Lee, Zeng, Drew, Sallam, Martin-Montalvo, Wan, Kim, Mehta, Hevener, de Cabo and Cohen2015). Recent evidence suggests that PS NPs can impair mitochondrial energy production capacity by disrupting the electron transport chain, leading to reduced ATP synthesis (Trevisan et al., Reference Trevisan, Voy, Chen and Di Giulio2019; Lin et al., Reference Lin, Zhang, Wang, Su, Song, Wu, Yang, Wong, Cai and Zheng2022b; Reference Li, Guo, Niu, Shang, Chang, Sun, Zhang, Shen and Xue2023a). The impairments observed in mitochondrial function are not limited to energy production but also extend to other vital processes such as signaling, mitochondrial dynamics, mitophagy, calcium homeostasis, and apoptosis. In in vitro (Table 1) and in vivo (Table 2) studies, decreased mitochondrial membrane potential (ΔΨm) was observed after PS, PVC and PET MPs/NPs (Wu et al., Reference Wu, Wu, Liu, Wang and Chen2019; Wang et al., Reference Wang, Bai, Ning, Fan, Sun, Fang, Wu, Li, Duan, Zhang, Liang and Gao2020; Chen et al., Reference Chen, Chen, Lin, Chen, Jiang and Lin2022; Florance et al., Reference Florance, Chandrasekaran, Gopinath and Mukherjee2022; Halimu et al., Reference Halimu, Zhang, Liu, Zhang, Wang, Gu, Zhang, Dai, Zhang, Zhang and Xu2022; Li et al., Reference Li, Xu, Zhang, Halimu, Li, Li, Gu, Zhang and Wang2022; Liu et al., Reference Liu, Hou, Wang and Yang2022; Salimi et al., Reference Salimi, Alavehzadeh, Ramezani and Pourahmad2022; Zhang et al., Reference Zhang, Zhang, Duan and Wang2022a; Chen et al., Reference Chen, Xu, Liu, Mei, Wang and Shi2023; Koner et al., Reference Koner, Florance, Mukherjee and Chandrasekaran2023; Zhang et al., Reference Zhang, Li, Yu, Ye, Li, Zhang, Wang, Li, Ji, Gao and Dong2023). In a study by Sun et al., exposure to PS NPs (size 20 nm) resulted in collapse of ΔΨm, an event associated with the activation of cellular apoptosis, at TM3 mouse Leydig cells (Sun et al., Reference Sun, Wen, Zhang, Fu, Yuan, Kuang, Kuang, Huang, Zheng and Zhang2023b). This finding was reinforced by studies showing increased expression of apoptosis-related proteins in cells exposed to MPs/NPs, providing further evidence that these particles may interfere with the role of mitochondria in the regulation of apoptosis (Li et al., Reference Li, Ma, Ye, Tang, Liang, Liang and Xiao2021; Wang et al., Reference Wang, Zhang, Sun, Wang and Gong2022b; Li et al., Reference Li, Xu, Peng, Tang, Chi, Li and Li2023b). In addition, the interaction of MPs/NPs with mitochondria could also affect cellular calcium homeostasis, as calcium is critical for several mitochondrial functions, including ATP production, this disruption could have significant consequences for cellular health. Research has shown that PS nanoparticles with a size of 20 nm can increase intracellular calcium levels, possibly by disrupting mitochondrial calcium handling at SHSY-5Y human neuroblastoma cells and protozoan Tetrahymena thermophila which has a strong ability to ingest particles (Meindl et al., Reference Meindl, Kueznik, Bosch, Roblegg and Frohlich2015; Wu et al., Reference Wu, Guo, Liu, Yang and Miao2021). The impact of MPs/NPs on mitochondrial structure and function potentially links to various pathologies, highlighting the importance of elucidating the mechanistic pathways of these interactions for understanding systemic and long-term health effects.
Table 1. Summary of in vitro studies assessing the effects of MP/NP exposure on mitochondrial function

Table 2. Summary of in vivo studies assessing the effects of MP/NP exposure on mitochondrial function

Induction of mitochondrial ROS by MPs/NPs
One of the critical consequences of the interaction of MPs/NPs with mitochondria is the induction of ROS. ROS are chemically reactive molecules that can be a by-product of normal metabolic processes, but when produced in excess they can lead to oxidative stress, causing damage to DNA, proteins, and lipids in cells (Thannickal and Fanburg, Reference Thannickal and Fanburg2000). MPs/NPs have been reported to trigger the overproduction of ROS in mitochondria. A study by Li et al. showed that exposure to PS NPs with a diameter of 21.5 nm increased mitochondrial ROS levels in human hepatocellular carcinoma cells in a dose-dependent manner (Li et al., Reference Li, Guo, Niu, Shang, Chang, Sun, Zhang, Shen and Xue2023a). Similar findings were observed in fish gills exposed to 1–5 μm sized MPs, where elevated levels of mitochondrial ROS were associated with a significant increase in lipid peroxidation, a marker of oxidative damage (Santos et al., Reference Santos, Luzio, Felix, Bellas and Monteiro2022). Furthermore, this ROS-induced oxidative stress may exacerbate mitochondrial dysfunction by damaging mitochondrial proteins and disrupting ΔΨm, thereby amplifying the detrimental effects of MPs/NPs on mitochondrial function (Wang et al., Reference Wang, Lee, Hsu, Chiu, Huang, Huang, Chia, Lee, Y-F and Chiu2021; Li et al., Reference Li, Guo, Niu, Shang, Chang, Sun, Zhang, Shen and Xue2023a). Adding to this complexity, shape also appears to play a role in the interaction of these pollutants with cellular systems. Spherical and fiber/fragment-shaped PS MPs and NPs reduced intracellular H2O2 levels attributable to mitochondrial stress responses such as increased mitochondrial DNA content, footprint, and morphology in Caco-2 cells (Saenen et al., Reference Saenen, Witters, Hantoro, Tejeda, Ethirajan, Van Belleghem and Smeets2023).
Effects of MPs/NPs on mitochondrial dynamics
MPs/NPs can also affect mitochondrial dynamics, a process that is critical for maintaining mitochondrial function and overall cellular health. Mitochondrial dynamics involves the balanced processes of mitochondrial fission and fusion, which are necessary for cell survival, adaptation to metabolic changes, and removal of damaged mitochondria (Youle and van der Bliek, Reference Youle and van der Bliek2012). In general, Fis1, Mff, MiD49, MiD51, and dynamin-associated protein 1 (Drp1) are involved in mitochondrial fission (Bleazard et al., Reference Bleazard, McCaffery, King, Bale, Mozdy, Tieu, Nunnari and Shaw1999; Mozdy et al., Reference Mozdy, McCaffery and Shaw2000; Tieu and Nunnari, Reference Tieu and Nunnari2000). In mammals, Drp1 is usually distributed in the cytosol and some of it is found in the form of a dot on the outer membrane of mitochondria (Smirnova et al., Reference Smirnova, Griparic, Shurland and van der Bliek2001). During fission, dynamin homologs are transported to the Drp1 outer membrane knuckle region by intermediary proteins (Fis1, Mff, MiD49, and MiD51), where they form large homomultimetric structures that spirally envelop the mitochondria (Atkins et al., Reference Atkins, Dasgupta, Chen, Mewburn and Archer2016). Mitochondrial fission also plays an active role in the even distribution of mitochondria to daughter cells during cell division, as well as in the transport of the organelle to energy-demanding sites in the cell, such as neuronal axons and lamellipods. Recent studies revealed that MPs/NPs may disrupt this balance and cause abnormal mitochondrial dynamics. A study in human liver cells showed that exposure to PS NPs led to increased mitochondrial fission, as evidenced by a significant increase in the expression of the fission protein Drp1 and p-Drp1 (Li et al., Reference Li, Guo, Niu, Shang, Chang, Sun, Zhang, Shen and Xue2023a). Excessive fission is often associated with mitochondrial fragmentation and cell death, suggesting a potential pathway for NP-induced toxicity. Conversely, NPs may also interfere with mitochondrial fusion, a process necessary for the sharing of mitochondrial DNA and other essential components. Mitochondrial fusion is a complex process in which two neighboring organelles are connected to each other and two independent membranes (inner and outer membranes of mitochondria) are fused in harmony without any significant loss of mitochondrial proteins (e.g. cytochrome c) that could lead to cell death. In mammals, Mfn1 and Mfn2 proteins called mitofusins are involved in outer membrane fusion, while OPA1 protein is involved in inner membrane fusion. Fu et al. found that exposure to amino-functionalized PS NPs increased the mRNA expression level of MFN2 (mitochondrial fusion-related gene) in Human Umbilical Vein Endothelial Cells (HUVECs) (Fu et al., Reference Fu, Fan, Xu, Wang, Hu and Jin2022). Another study conducted on human bone marrow-derived mesenchymal stem cells (hBM-MSCs) also revealed that surfactant-free amine-functionalized PS NPs and PS NPs with decreased cross-linking density (DPS-NPs) led to upregulation of MFN2 expression and downregulation of FIS1 (mitochondrial fission related gene) expression (Im et al., Reference Im, Kim, Jo, Yoo, Kim, Park, Hyeon, Yi and Bhang2022). Interestingly there were opposite results regarding OPA1 levels in mouse and chicken experiments when exposed to PS MP’s. It was found that after GC-2 mouse cells were exposed to PS MP’s for 24 h, both mRNA and protein expression levels of OPA1 were increased along with Drp1 (Liu et al., Reference Liu, Hou, Wang and Yang2022). However, in another study conducted on chickens, it was shown that after 42 days of exposure to PS MP’s, mRNA and protein expression levels of OPA1 were decreased along with Mfn1 and Mfn2 suggesting a decrease at mitochondrial fusion. Conversely, Drp1 mRNA and protein expression levels were increased suggesting an increase in mitochondrial fission (Zhang et al., Reference Zhang, Yin, Wang, Wang, Lu, Zhao and Xing2022b). These conflicting findings underscore the complexity of MP interactions within biological systems and highlight the species-specific responses to PS MP exposure, which may affect mitochondrial dynamics in diverse ways.
Induction of mitochondrial unfolded protein response (UPRmt) by MPs/NPs
MPs/NPs may also exert their toxic effects by disrupting the mitochondrial unfolded protein response (UPRmt), a protective cellular mechanism that is activated in response to the accumulation of misfolded proteins in mitochondria (Xu et al., Reference Xu, Hua, Rui and Wang2022). The UPRmt plays a critical role in maintaining mitochondrial proteostasis, thereby contributing to overall mitochondrial health and functionality. Due to their ability to induce oxidative stress and disrupt mitochondrial function, MPs/NPs may lead to protein misfolding within mitochondria. A study by Liu and Wang showed that exposure to PS NP particles with a size of 100 nm significantly increased the expression of HSP6, a marker of the UPRmt, in Caenorhabditis elegans (Liu and Wang, Reference Liu and Wang2021). This suggests that NPs may lead to protein misfolding and subsequent activation of the UPRmt. However, chronic activation of the UPRmt, as may occur with continuous or repeated exposure to NPs, may become maladaptive. Prolonged activation of the UPRmt has been associated with mitochondrial dysfunction (Lin et al., Reference Lin, Schulz, Pellegrino, Lu, Shaham and Haynes2016). Therefore, MPs/NP-induced activation of the UPRmt may represent another mechanism of their cellular toxicity. In addition, disruption of the UPRmt may have further implications for mitochondrial dynamics, as protein homeostasis is crucial for maintaining balanced fission and fusion processes. Thus, the interaction of MPs/NPs with the UPRmt could add another layer of complexity to their impact on mitochondrial health.
Effects of MPs/NPs on mitophagy
Another important aspect to consider in the interaction between MPs/NPs and mitochondria is the process of mitophagy, the selective degradation of damaged mitochondria by autophagy. This mechanism plays an important role in maintaining cellular homeostasis by removing dysfunctional mitochondria and recycling their components (Onishi et al., Reference Onishi, Yamano, Sato, Matsuda and Okamoto2021). In the context of MP/NP-induced mitochondrial damage, the PINK1/Parkin pathway plays a pivotal role. Upon mitochondrial depolarization or damage, PINK1, a kinase, stabilizes on the outer mitochondrial membrane. This stabilization signals the recruitment of Parkin, an E3 ubiquitin ligase and once Parkin is recruited, it ubiquitinates various mitochondrial proteins (Mfn1, Mfn2, Drp1, and TOM20) marking the damaged mitochondria for degradation (Gegg and Schapira, Reference Gegg and Schapira2011; Wang et al., Reference Wang, Song, Du, Tian, Yue, Liu, Li, Wang, Zhu, Cao, Zhou and Chen2011; Yoshii et al., Reference Yoshii, Kishi, Ishihara and Mizushima2011). This selective autophagy process, crucial for cellular health, ensures the removal of dysfunctional mitochondria, thereby preventing potential cellular damage induced by MPs/NPs. A study by Xu et al. found that PS NPs with the size of 100 nm accumulated in mitochondria and induced PINK1/Parkin-mediated mitophagy in mice, likely as an effort to eliminate mitochondria damaged by oxidative stress and mitochondrial dysfunction (Xu et al., Reference Xu, Ma, Peng, Gan, Wang, Chen, Han and Chen2023). This observation is consistent with the known role of mitophagy as a response to stressful conditions, such as ROS overproduction (Onishi et al., Reference Onishi, Yamano, Sato, Matsuda and Okamoto2021). However, continuous or high-level activation of mitophagy could be detrimental. Prolonged stimulation of mitophagy, especially in the absence of effective biogenesis to replace degraded mitochondria, could lead to overall loss of mitochondrial mass and function, contributing to further cellular stress and even cell death (Kubli and Gustafsson, Reference Kubli and Gustafsson2012). Furthermore, the involvement of mitophagy highlights the interconnectedness of the different mitochondrial responses to MP/NP exposure. These findings also emphasize the complex and potentially detrimental effects of MP/NP pollution on mitochondrial health and cellular function, including disruptions in energy production, increased oxidative stress, and induction of apoptotic pathways. The urgent need for targeted research to fully understand the extent of MP/NP toxicity, the implementation of stricter pollution controls to reduce exposure, and the development of innovative solutions to remove existing pollutants from the environment is underscored by these negative outcomes. This will help protect public health and biodiversity. Mitochondrial biogenesis, the formation of new mitochondria within the cell, is another critical cellular process that could be disrupted by exposure to MPs/NPs. Mitochondrial biogenesis is essential for replacing damaged mitochondria and adjusting the mitochondrial population within a cell to meet changing metabolic demands (Kubli and Gustafsson, Reference Kubli and Gustafsson2012). Disruption of this process can have a significant impact on cellular health, potentially leading to energy depletion, increased oxidative stress, and increased susceptibility to cell death. Exposure to environmental stressors, such as MPs/NPs, could potentially trigger such disruptions. However, a latest study by Jeong et al. revealed that mitochondrial biogenesis was increased at PS MPs and chromium exposed freshwater flea, Daphnia magna, compared to chromium only treated group suggesting that MPs expel chromium from cells (Jeong et al., Reference Jeong, Lee, Sayed, Jeong, Zhou, Lee and Byeon2022b). The group exposed to chromium-only showed a decrease in PGC-1a gene expression and an increase in Drp1 gene expression, indicating that chromium may cause mitochondrial dysfunction. However, exposure to both MPs and chromium resulted in increased PGC-1a expression and decreased Drp1 expression, suggesting a potential mitigating effect on mitochondrial dysfunction compared to chromium exposure alone. While the available study provides initial insights into the potential impacts of MPs/NPs on mitochondrial biogenesis in freshwater fleas, including their intriguing role in mitigating the effects of heavy metals, it should be emphasized that this research does not extend to human data. Consequently, a comprehensive understanding and broader conclusions regarding such effects in humans necessitate further in-depth studies.
Conclusion
The toxicity of MPs/NPs is a serious environmental and public health problem that is not yet fully understood. The unique physicochemical properties of these particles, including their small size and large surface area, enable them to penetrate biological membranes and accumulate in various organs where they can induce a range of adverse effects. This review has highlighted one particular area of concern - the effects of exposure to MPs/NPs on mitochondria, a critical cellular organelle responsible for energy production and several other vital functions.
Evidence suggests that MPs/NPs can induce mitochondrial dysfunction, primarily through the generation of oxidative stress, which damages mitochondrial components and impairs mitochondrial function. This can result in reduced ATP production, which can disrupt cellular processes and lead to cell death. MPs/NPs have also been found to physically interact with mitochondria, causing structural damage and contributing to functional impairment. These effects can in turn trigger a cascade of cellular responses, from inflammation to apoptosis, contributing to the overall toxicity of MPs/NPs. In addition, exposure to MPs/NPs may disrupt the dynamic processes that maintain mitochondrial health, including mitochondrial dynamics and the UPRmt. Also, emerging research suggests that MPs/NPs could disrupt mitochondrial biogenesis, potentially leading to a decrease in mitochondrial mass and further impairing cellular health and function. Such a chain of detrimental effects highlights the importance of understanding the impact of MP/NP exposure on mitochondria, not only in terms of cellular health but also considering potential systemic effects and long-term effects on organismic health.
Knowledge gaps and future perspectives
Mitochondrial damage and dysfunction are related to numerous health conditions, suggesting that exposure to MPs/NPs could have far-reaching effects on human health. Therefore, it is crucial to investigate the potential impact of MPs and NPs on human cells to raise awareness of this issue and take necessary precautions. The analytical methods used are inadequate to measure the concentration of NPs in the environment and organisms and therefore little is known about the importance of NPs for human health (Science Advice for Policy by European, 2019). Therefore, it is important to first develop analytical methods that can analyze not only MPs but also NPs, which will enable a full understanding of human exposure.
The type of PS NPs that have been shown to cause adverse effects in human and animal cells in in vitro and in vivo studies are primary ones. Primary MPs/NPs have a smooth surface and uniform shape (uniform; nanobeads). Secondary MPs/NPs, on the other hand, are formed in a wide variety of shapes compared to those of primary origin (Koelmans et al., Reference Koelmans, Besseling, Shim, Bergmann, Gutow and Klages2015; Lei et al., Reference Lei, Liu, Song, Lu, Hu, Cao, Xie, Shi and He2018). At the same time, when primary PS NPs are released into the environment, their structures deteriorate and their properties change after a certain period of time like secondary particles (Im et al., Reference Im, Kim, Jo, Yoo, Kim, Park, Hyeon, Yi and Bhang2022). The shapes of secondary MP/NP particles are amorphous and it has been shown that the negative effects of particles without smooth surfaces on the cell are more than those with smooth surfaces (Qin et al., Reference Qin, Xia, Yuan and Wang2022; Völkl et al., Reference Völkl, Jérôme, Weig, Jasinski, Meides, Strohriegl, Scheibel and Freitag2022). Therefore, the effects of secondary MPs/NPs, which are more abundant in the environment, need to be investigated and studies need to be designed to realistically assess human exposure.
In addition, the studies in the literature were conducted with commercially available PS-type MPs/NPs. However, MPs/NPs in the environment also consist of other types of plastic polymers other than PS. Therefore, the effects of MPs/NPs composed of these types of plastic polymers on the mitochondria should be investigated as well.
Furthermore, given the wide range of plastic types, sizes, shapes, and chemical compositions present in the environment, research should also focus on investigating whether and how these different factors modulate the effects of MPs/NPs on mitochondria and other cellular components.
The field of MPs/NPs research, particularly in relation to their effects on mitochondria, is still evolving. Existing studies have primarily used in vitro models, and more in vivo and human epidemiological research is needed to validate these findings and gain a more nuanced understanding of these interactions and their implications for organismal health. Such studies will provide a more realistic understanding of exposure levels, uptake mechanisms, and physiological consequences of MP and NP exposure. Moreover, further work is needed to clarify the molecular mechanisms underlying the effects of MPs/NPs on mitochondria and to determine the extent to which these effects contribute to the overall toxicity of these pollutants. Research in this area could help to inform risk assessments and guide the development of strategies to mitigate the effects of NP and MP pollution.
In the production of plastics, some additives (UV stabilizers, antioxidants, plasticizers (such as phthalate diester), colorants, fillers, etc.) are added to the products along with the polymer (Murphy, Reference Murphy2001; Ventrice et al., Reference Ventrice, Ventrice, Russo and De Sarro2013; ECHA, 2018). There are many studies revealing the effects of these chemicals added to plastics on animals and humans (Gray Jr et al., Reference Gray, Ostby, Furr, Price, Veeramachaneni and Parks2000; Frederiksen et al., Reference Frederiksen, Skakkebaek and Andersson2007; Lyche et al., Reference Lyche, Gutleb, Bergman, Eriksen, Murk, Ropstad, Saunders and Skaare2009; Svensson et al., Reference Svensson, Hernández-Ramírez, Burguete-García, Cebrián, Calafat, Needham, Claudio and López-Carrillo2011; Ding et al., Reference Ding, Xu, Mao, Chen, Qiu, Yang, Zhao, Xu, Feng and Wu2021). NP/MP act as vectors for toxic chemical contaminants and pathogenic microbes by sorbing to their surfaces and cavities (Rai et al., Reference Rai, Sonne, Brown, Younis and Kim2022). MPs/NPs have certain properties that facilitate their ability to adsorb various environmental pollutants. In this way, they increase exposure to these chemicals along with themselves (Sun et al., Reference Sun, Shi, Li, Gao and Liu2023a). The combined effects of these chemicals need to be taken into account when elucidating the effects of MPs/NPs on mitochondria and other cell components.
This extensive body of information emphasizes the importance of increasing awareness among individuals, communities, industries, and policymakers about the potential health risks associated with MP and NP pollution. These risks include respiratory problems, endocrine disruption, and other long-term health effects. There is an urgent need for comprehensive research to better understand the impacts of plastic pollution. Effective waste management practices should be implemented to reduce pollution at the source. Policies aimed at minimizing the production and use of plastic products are necessary to protect human health and the environment. This awareness should be channeled into individual action and policy development aimed at reducing plastic waste and promoting sustainable alternatives. In addition, further research is crucial to fill gaps in our understanding of the impacts of MPs/NPs on human health, particularly the long-term effects. More comprehensive studies are needed to better characterize human MPs/NPs exposure to elucidate their mechanisms of action in our bodies, and to identify potential strategies to mitigate their impacts. The public should also be aware that these findings are based on experimental models and while they indicate potential risks, the actual human health outcomes from real-world exposure scenarios might differ, which further underscores the need for ongoing research in this field. These potential risks underscore the urgency to better understand the precise mechanisms of MP and NP toxicity and to develop effective strategies to mitigate their presence in our environment. The collective effort towards these goals will necessitate cross-disciplinary collaboration encompassing environmental science, toxicology, public health, policymaking, and more.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/plc.2024.6.
Author contribution
F.D.Y. and M.A.A. contributed equally to the conception, design, and writing of the review article. They conducted the literature search, analyzed the selected articles, and synthesized the information in the review. Both authors participated in drafting and revising the manuscript. They read and approved the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interest
The authors declare no conflicts of interest.





Comments
Dear Professor Steve Fletcher,
Attached is the invited manuscript titled “Mitochondria as a Target of Micro- and Nanoplastic Toxicity” by Fulya DAL YÖNTEM and Müfide AYDOĞAN AHBAB that we would like you to consider for publication in Cambridge Prisms: Plastics. This manuscript is based on a review of research studies on the effects of microplastics and nanoplastics on mitochondrial health.
We are looking forward to hearing from you soon.
With kind regards,
*Correspondence to: Müfide AYDOĞAN AHBAB
Address: University of Health Sciences Türkiye, Hamidiye Vocational School of Health Services, Selimiye Mah. Tıbbiye Cad., No:38, 34668 Üsküdar, İstanbul, Türkiye
Tel: +90 216 777 94 00
Fax: +90 216 418 96 20
E-mail: [email protected]; [email protected]