INTRODUCTION
Campylobacter jejuni and C. coli remain one of the most important causes of gastrointestinal zoonotic bacterial infections in the Western world including the UK [1]. Poultry is considered the main source for human campylobacteriosis and data collected from various research studies has shown that a large proportion of broiler flocks are colonized at slaughter [Reference McDowell2, Reference Bull3]. Infection with Campylobacter causes no production loss or symptoms in poultry and in all-in/all-out conventional rearing systems flocks do not usually become positive until at least day 20 of the production cycle. It is unknown whether this is due to the protection of maternal antibodies or to introduction of the bacteria into the shed around this time in the crop cycle [Reference Bull3–Reference Sahin5].
There has been little reported evidence of direct carry-over of Campylobacter strains between consecutive flocks in the same shed, although it has been reported occasionally [Reference Evans and Sayers6]. It is more likely that an external reservoir maintains multiple Campylobacter strains, while a shed is empty, which allows for reintroduction of Campylobacter to a new flock. Once introduced to the shed Campylobacter colonizes the flock fully within a few days [Reference Shreeve7].
Campylobacter are able to survive well in the farm environments and have been isolated from soil, water, dust, building surfaces and even air [Reference Bull8–Reference Ellis-Iversen10]. However, once a flock is colonized, the surroundings frequently become contaminated, which can be misinterpreted as a source, if temporality is not considered. For an external environmental reservoir to be considered a source of colonization for a broiler flock, it needs to be contaminated before the flock becomes positive.
Other domestic and wild animals also carry Campylobacter. Cattle, sheep and wild birds are associated with asymptomatic shedding of Campylobacter and may act as environmental reservoirs for poultry or broiler farms [Reference Ellis-Iversen10–Reference Chuma, Hashimoto and Okamoto14].
Partial depopulation, where a proportion of birds are removed from the shed around day 35 to allow the remaining birds to grow larger, is practised by the majority of commercial poultry producers in Great Britain. The entrance of a catching crew and associated equipment is a serious breach of the biosecurity barrier of the poultry shed and the crews and their equipment are frequently contaminated with Campylobacter [Reference Allen15]. Flocks, which have been partially depopulated, tend to be at higher risk of colonization at slaughter [Reference Hald, Rattenborg and Madsen16, Reference Ellis-Iversen17]. Genotyping data has indicated that contaminated catching crew personnel, vehicles and equipment such as crates and modules are considered a potential source of Campylobacter introduction into flocks [Reference Allen15].
The objective of this study was to differentiate between the impact of potential environmental reservoirs of Campylobacter on broiler farms and to identify the ones that are most likely to be associated with positive flocks, when considering the temporal sequence of the exposure and colonization.
METHODS
Flock sampling
All broiler flocks in one shed were monitored on six standard house broiler farms in Great Britain over a period of 1½−2 years. The farms were owned or managed under contract by five large integrated poultry companies and were located in South West England (one farm), South England (two farms), Wales (one farm), central England (one farm) and Scotland (one farm). All farms practised all-in/all-out rearing for each shed and had between four and 12 rearing sheds. Every farm had different areas of potential reservoirs. Farms A and B had horses grazing on a neighbouring farm and Farm C was located on the edge of a small woodland. Farm D had an adjacent dairy unit and their calf housing facility situated ~100 m from the poultry shed and Farm E was situated ~400 m from a lake used by swans living on the surrounding land.
One perimeter shed on each farm was chosen for monitoring. The flocks were sampled 2–4 times during the cycle and in principle sampled at around day 24, at day 35 and again at clearance. At clearance sampling would also include caeca from the abattoir, where 16 pairs of caeca from each batch were collected by the poultry company QA staff and transported under chilled conditions to the laboratory within 24 h. At all visits, pools of six freshly voided faecal or caecal droppings were taken from each of six zones in the monitored shed to cover the house floor. These were then transported to the laboratory within 3 h.
Environmental sampling
At each visit samples were collected from the target flock and from multiple potential Campylobacter reservoirs on the farms including: surroundings of the monitored shed, shed entrance and anteroom, water from drinkers, surface water, mains water, vehicles, farm equipment, other animals in the near vicinity of the shed (including cattle, sheep, horses, rodents, dogs, cats), dead birds stored on site, litter scattered outside the shed. Incoming vehicles and catching crews and their equipment entering the farm regularly were also sampled when present. A core sampling strategy consisted of 60 samples per visit following a predefined sampling protocol. Often more samples were taken, when potential reservoirs such as puddles, vehicles and equipment, or wildlife faeces were present. Six sampling types were used, applying the most appropriate for the reservoir: swabs, overshoes, faeces, insects, or liquid samples. Swabs were taken from areas such as the doors of the house, equipment on site, vehicles, farmer's boots and catching-related items, e.g. crates and modules. An area of approximately 100 cm2 was sampled from each sampling site by using a sterile Readiwipe (Robinson Healthcare Ltd, UK) pre-moistened in a small amount of Maximum Recovery Diluent. Boot swabs in the form of gauze overshoes (Mike Bowden Livestock Service, UK) were used for sampling grass, gravel and concreted areas such as the main drive of the farm, surrounds of the farm, including the front concrete, apron, the anteroom and inside the house to monitor flock status. The gauze shoes were pre-moistened in Maximum Recovery Diluent and worn over plastic overboots (A547, Arnold, UK). Following sampling, the swabs and gauze overshoes were immediately placed individually in modified Exeter Broth (mEB). Samples of effluent, drainage water, puddles, ponds, bore-hole and drinking water were placed in an equal volume of double-strength mEB.
Laboratory analysis
Samples were enriched in mEB before streaking onto modified charcoal cefoperazone deoxycholate agar (mCCDA) and identifying Campylobacter-like colonies. Colonies of presumptive Campylobacter spp. were identified by their morphology. If available three colonies per sample were subcultured onto Blood Agar Base No. 2 Oxoid CM0271 and incubated microaerobically at 41·5°C for 24 h. The following confirmatory tests were performed: cell morphology using a wet preparation or a Gram stain (with dilute carbol fuchsin as the counter stain); oxidase test; lack of growth in air at 25°C after 48 h. A selection of isolates was examined by Oxoid Campy Dry Spot DR0150M (Basingstoke, UK).
Statistical analysis
The Campylobacter status of each potential reservoir and of the flocks was described at sample, visit and flock levels. Multivariable analyses were performed to adjust the association between the Campylobacter status of the flock and that of the environmental reservoirs for confounding by other risk factors and also to account for repeated measurement and time dependency of the data. The outcome of interest was the Campylobacter status of the flock at each visit to the farm and the Campylobacter status of reservoirs were considered as exposure variables. If one or more samples were positive for Campylobacter, the flock or reservoir was classified as positive. Potential reservoirs were classified as risk to the flock at the time of sampling, if they were (1) present and (2) positive. Potential reservoirs were considered no risk to the flock if they were absent or Campylobacter negative. Month of sampling, age, farm, average slaughter age, annual number of crops, length of downtime and depopulation status were considered as potential adjustment factors and confounders.
A Generalized Estimating Equation model (GEE) was applied to assess association between Campylobacter status of potential reservoirs and of the flock. The model was specified with flock as panel variable, using a binomial family and a logit link. A time-dependent autoregressive second-order correlation structure was used specifying visit as time variable and farm was introduced as an additional level of clustering. Robust standard errors were also applied to adjust for other random effects in the data.
Initially, all adjustment variables were included in the model and were then excluded by a stepwise approach, where the least significant variable was removed before the model was rerun. This was continued until all remaining variables were significant or made a significant contribution to the fit of the model (assessed by Wald's χ2). All reservoir variables were then added to the model and removed using the same stepwise approach as for the adjustment variables. When all remaining reservoir variables were significant, the discarded variables (including adjustment variables) were re-introduced one at a time to ensure that no confounding effects were missed and to assess their affect on the fit of the model. Model fit was assessed using Wald's χ2 test. If the fit improved by more than 25% the variable was kept in the model even if non-significant. All statistical analyses and data handling were performed in Stata (StataCorp, USA).
RESULTS
A total of 75 flocks were sampled, but nine were excluded due to incomplete sampling. Of these, six were sampled only once due to outbreaks of avian influenza in other areas of the UK restricting farm access during the study period. Another two flocks were excluded because caeca could not be sampled at clearance and one flock was excluded because the environmental reservoirs were not sampled at clearance. All remaining flocks were included in the analysis.
Five of the farms were visited between 31 and 37 times during the 2-year study period, which comprised all flocks raised in the monitored sheds in that period. The remaining farm was sampled for a period of 1½ years and visited 22 times. A total of 195 visits were made and 66 flocks and their environment were sampled between one and four times. The flocks were slaughtered at an average age of 41·5 days (range 36·5–48 days) and the farms produced an average of 6·9 crops annually, ranging between 6 and 8.
A total of 11 516 samples were collected from flocks on six farms between the 23 February 2006 and 8 April 2008 and analysed for presence of Campylobacter. The number of samples collected on each farm ranged from 1382 to 2164. Samples originating from chicken and incoming sources were more likely to be positive than environmental samples taken on the farm itself (Table 1).
Table 1. Samples collected on six poultry farms over a period of 2 years
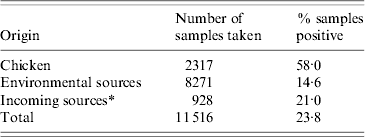
* All vehicles and catching crews and their equipment.
The prevalence of Campylobacter in chicken samples appeared to decrease in early spring only to be followed by a steep increase through spring, peaking in October (Fig. 1). Another increase was observed in November/December. A very distinct dip in prevalence was observed in May, before the beginning of a steady increase until October. A small increase in positive environmental samples was also observed at the beginning of the increase in positive flocks in April to May. However, the positivity of environmental sources fluctuated slightly throughout the year. Positivity of samples from incoming sources also showed an increase starting in April before the increase in positive chicken samples. However, a drop in prevalence of samples from incoming sources in July and August was observed, where only very few of the 60 samples from these sources were positive.
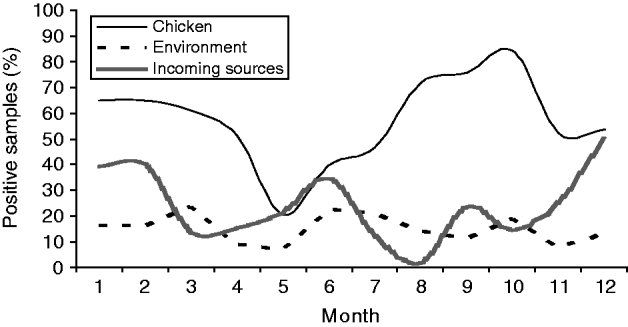
Fig. 1. The monthly prevalence of Campylobacter-positive samples from three different sources on poultry farms.
The flocks were Campylobacter positive at 109 (56%) of the 195 sampling occasions and the environment was positive on 148 (76%) of the visits. Positive samples from incoming sources were detected on 47 (24%) occasions. Prevalence within each of the reservoirs is shown in Table 2. Little variation was found in the proportion of positive flocks sampled in winter: 65% [95% confidence interval (CI) 46–82]; summer: 67% (95% CI 54–80) and autumn: 57% (95% CI 44–71). However, the number of visits, at which the flocks were positive, was significantly lower in spring: 39% (95% CI 26–52, P=0·026).
Table 2. Detection of Campylobacter in environmental sources at 195 visits over a 2-year period on six poultry farms
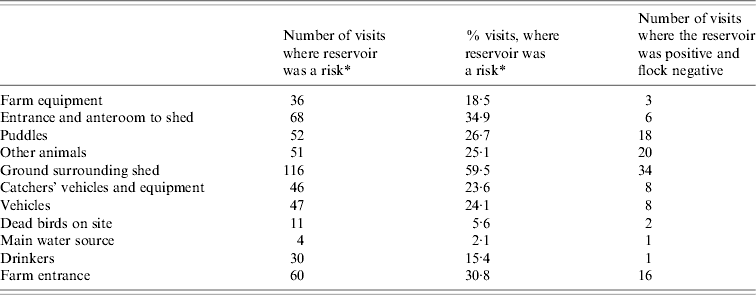
* Risk=reservoir is present and Campylobacter positive.
The visits were carried out at different events in the flock cycle and six visits were made at placement, 108 were made before any depopulation, 19 were made after partial depopulation but before clearance had occurred and 61 flocks were sampled at clearance either on farm or at the abattoir or both. More flocks were positive on the farm at clearance and/or at the processing plant (89%) than at any other production cycle event. None of the six flocks tested were found positive at placement and sampling at this event was thus abandoned. A total of 38% were positive before partial depopulation and 65% were positive after partial depopulation. In total 83% of sampling occasions identified Campylobacter-positive flocks, where partial depopulation had previously taken place, whereas only 43% were positive if no depopulation had occurred. Age and depopulation status were strongly associated and on visits where the flock had not previously been depopulated the birds were on average 28 days old, whereas the average age for previously depopulated flocks was 42 days. All flocks older than 41 days (35 sampling occasions) had been partially depopulated.
Not surprisingly, the Campylobacter status of the surrounding ground and external surfaces of the shed were highly correlated with the status of all other environmental reservoirs and was thus considered a proxy rather than an actual reservoir. Because of the correlations, the variable was left out of the model to avoid disguising weaker, but primary reservoirs.
The presence of contaminated drinkers, contaminated shed entrances or anterooms and other infected animals were found to increase the likelihood of positive flocks (Table 3).
Table 3. The association between Campylobacter colonization of broiler flocks and environmental reservoirs, incoming sources and other risk factors
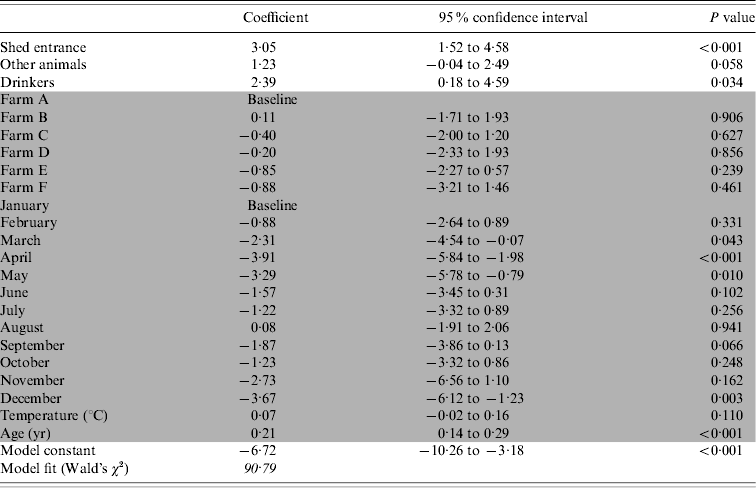
Shaded cells indicate adjustment variables.
The variation between farms was not significant, but inclusion of the variable improved the model fit by 47%. It is likely that the farm variable is a proxy measure for differences in management between the individual farms. A lower risk of positive flocks was observed in April, May and December, but positive flocks were not associated with any of the other months. The risk of a Campylobacter-colonized flock increased significantly as the birds got older. The fit of the model comparing predicted prevalence to observed prevalence was acceptable with an R 2=0·769.
No direct or confounding effects were found by the partial depopulation status, once the data was adjusted for age. No association was found between colonization of the flock and contamination of environmental sources including: farm equipment, puddles, surface water, mains water, main entrance to farm, vehicles, catching crew and their equipment or facilities for dead bird storage on site.
Of the reservoirs associated with positive poultry flocks, other animals were significantly more likely to be positive, before the flock than drinkers or the shed entrance (Fig. 2). When interpreting the results from Table 3 and Figure 2, it appeared that drinkers and the shed entrance were more likely to be contaminated by the flock, where other animals may harbour Campylobacter between flocks and act as a persistent reservoir of Campylobacter on the farms.
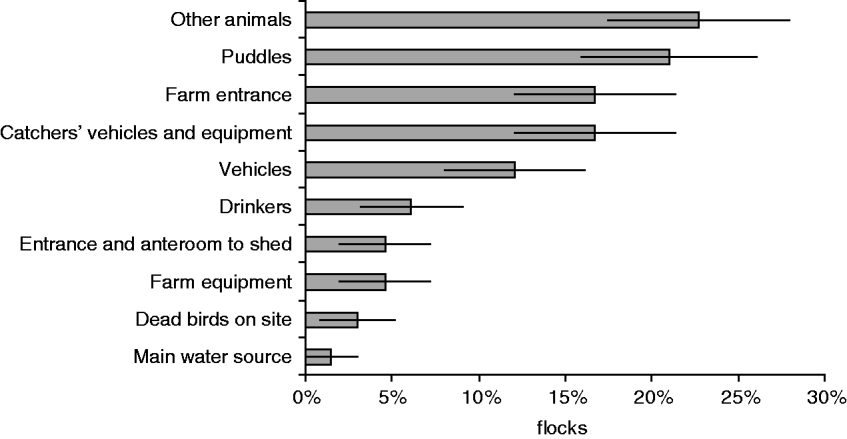
Fig. 2. The proportion of flocks where different reservoirs were positive before the poultry (n=66).
Discussion
Our study showed that the presence of other animals carrying Campylobacter on the poultry farm were associated with positive poultry flocks. Furthermore, other animals appeared to be the environmental source most likely to be contaminated with Campylobacter, before Campylobacter was isolated from the monitored broiler flock. Other animal species have been found to frequently carry and shed C. jejuni/C. coli. Cattle, sheep and dogs have in particular been associated with shedding of Campylobacter and thereby contaminating their environment [Reference Moreno12, Reference Oporto13, Reference Ellis-Iversen18]. The presence of other animal species on farm or staff attending other farm animals have previously been reported as a risk factor for Campylobacter-positive broiler flocks [Reference Kapperud19, Reference Van de Giessen20]. MLST has indicated that some Campylobacter strains may exhibit some host specificity, but the common genotypes are rarely found to be exclusive to any domestic animal species. In contrast, many strains are reported in several species [Reference Zweifel21]. Molecular epidemiology on one of the farms in our study showed that calves in boxes near to the monitored poultry shed, were colonized with the same strains as the broilers [Reference Ridley22]. Furthermore, a molecular link between Campylobacter in a broiler flock and cattle grazing in a nearby field owned by the same farmer has also been reported [Reference Ridley23]. Our study did not distinguish between wildlife and domestic animals, but wildlife has also been reported to carry Campylobacter [Reference Chuma, Hashimoto and Okamoto14, Reference Brown24]. It is likely that other animals close to the poultry sheds may act as a persistent reservoir on the farm and once infected, maintain the burden of Campylobacter needed to re-infect future flocks.
Contaminated drinkers were found to be associated with Campylobacter-positive poultry flocks, even though the drinkers were only contaminated after the birds were colonized. This may suggest that drinkers may have been a fomite harbouring the bacteria and thereby increased the risk of contact by multiple birds. The contaminated water drinkers would then act as a distributor of bacteria within a flock rather than an introduction vehicle. This is further supported by the limited number of times the main water supply was found to be positive during our study period. Chlorination of drinking water has been identified in risk-factor studies as a protective intervention, which could potentially inhibit or slow the colonization of broilers in a flock [Reference Ellis-Iversen10, Reference Kapperud25–Reference Sparks27]. Campylobacter are sensitive to chlorine compounds and the disinfectant could slow the dissemination within a flock, which may even inhibit establishment of a particular strain as dominant in the flock. Poor water trough hygiene has also been reported to increase the risk of Campylobacter in cattle herds and even though the exact mechanism of the identified risk remains unknown, farmers should ensure that drinkers are cleaned and disinfected between flocks [Reference Ellis-Iversen18].
In general, Campylobacter can survive for a long time in water, and puddles were found to be positive very often also often before the flock was identified as positive. However, puddles are not always present and cannot be considered a consistent reservoir, and the positivity of puddles may reflect the general level of cleanliness and contamination of surroundings on farms. The shed surrounds were significantly associated with positive poultry flocks. However, the contamination of these appeared to be highly dependent on the status of other reservoirs, which were disguised if tested in the same statistical model. It is likely that the contamination of the general surrounds is a step in the pathway from a reservoir to the flock (or reverse) rather than an actual reservoir itself. Disinfecting the surroundings during the crop cycle, e.g. around day 25 of the crop cycle may reduce the risk of introduction into the shed.
Contamination of the shed entrance and anteroom is also likely to be a step in the pathway between the flock and a potential persistent or incoming reservoir on the farm. Contamination of this area can occur in both directions as this is likely to be one of the first areas contaminated after the flock is infected and indeed on three occasions it was found to be contaminated before the flock. Because the anteroom and main doors are critical barriers into the shed, special attention to this area must be given when cleaning and disinfecting the shed between flocks.
Partial depopulation is known to be a risk factor for Campylobacter colonization of poultry flocks, because of the major breach of the shed's biosecurity barrier [Reference Hald, Rattenborg and Madsen16, Reference Ellis-Iversen17]. It was not possible to assess the exact risk of partial depopulation on the colonization of broiler flocks, because the practice was closely linked with age. Flocks that had been partially depopulated were consistently older at slaughter and it was difficult to differentiate between partial depopulation and older birds as the most important risk factor. In our study, all flocks where birds remained in the houses after 41 days of age had been partially depopulated, thus it was not possible to measure the effect of depopulation separately from the effect of age. Nevertheless, age was a stronger risk and provided a better model fit suggesting it is a better predictor of Campylobacter status of the flocks than partial depopulation. Similar issues may have disguised any effect of contaminated catching crews. Catching crews and equipment were often found to be contaminated with Campylobacter as they arrived on the farm and only 11 of the flocks were negative before the contaminated crew arrived on the farm. Nevertheless, catchers and their crew or other vehicles coming on to the farm were not significantly associated with flock positivity, potentially because a large proportion of flocks were already positive upon the arrival of the catching teams or that they were never partially depopulated and thus, not exposed to the risk.
The prevalence of Campylobacter-positive flocks has been reported to be seasonal in other Northern European countries with a distinct peak in the summer months [Reference Guerin28–Reference Patrick30]. In Great Britain, the seasonality appears less distinct and not as convincing, but a review of previously collected data in Great Britain showed that the prevalence in flocks without partial depopulation showed an increased risk in late summer and early autumn, whereas flocks which had been previously depopulated showed no seasonal pattern [Reference Ellis-Iversen17]. In our study, a steep visual increase in colonized flocks began in April and peaked in October. Once adjusted for farm variation, age of birds, and environmental reservoirs of importance, the increased prevalence in summer months was not significantly different from the winter or autumn prevalence, but a significantly low prevalence was observed in March, April and May. Our study monitored only six farms, which is relatively small size and thus, results should not be considered representative of the whole Great Britain broiler population. Nevertheless, this significant drop in Campylobacter-positive broiler flocks has been reported previously from other studies in Great Britain [Reference Ellis-Iversen17], but the reasons behind it are unexplored.
Despite a modest sample size, we were able to adjust for some variation between farms, which improves the confidence in our results. The reason for inclusion of a limited number of farms was a trade-off to ensure detailed information through repeated sampling and investigation of multiple reservoirs to investigate temporal exposure. The variation between farms was not significant, but of importance to the fit of the model. It is likely that this variable captured differences between farms, such as farming practices, feed, water supply, hygiene and biosecurity status, which have previously been found to be associated with Campylobacter-positive flocks.
The ideal analysis tool to assess temporality of exposure in association analysis would be a survival model, where time to infection would be the outcome of interest. Unfortunately, few of these models could account for the multiple layers of clustering present in our data and when accounted for, the sample size was too small to apply this type of analysis. Instead we applied a GEE model, which accounts for dependencies in the data on all levels including farm and flock and can adjust for multiple confounders. It also enabled us to account for time dependency and repeated sampling of flocks by specifying a time-dependent correlation matrix and we consider the model fit-for-purpose and have confidence in the outputs.
The most likely persistent reservoir associated with Campylobacter colonization of flocks in our study were other animals on the farm. The contamination of drinkers in houses was also associated with flock colonization, but was more likely to play a role in distribution of Campylobacter within the flock than introduction or as a persistently infected reservoir. Contaminated shed entrances and anterooms were also closely associated with colonized birds, but the temporal sequence in the association is not clear. The anterooms and entrance were more likely to be a step in between a potential reservoir and the flock or even contaminated by positive flocks. Nevertheless, cleaning and disinfection of anterooms, doors and drinkers should be considered an important part when cleaning between batches.
ACKNOWLEDGEMENTS
We thank the farmers for allowing us to monitor their farms for this extended period of time. We also thank staff at CERA VLA for data management, especially Mary O'Mara. We are very grateful for assistance of technical staff at SAC and University of Bristol for farm sampling and microbiological analyses. This work was funded by Defra under project OZ0610.
DECLARATION OF INTEREST
None.







