INTRODUCTION
Infection with hepatitis B virus (HBV) is a problem of global public health importance. The failure of individuals to clear HBV leads to a chronic infection, characterized by carriage of the HBV surface antigen (HBsAg), a marker of infectivity as well as possible progression to end-stage hepatic diseases such as cirrhosis or primary hepatocellular carcinoma [Reference Valsamakis1].
The Regional Office for Europe of the World Health Organization (WHO) has estimated that in Europe each year one million people become infected with HBV, and of these 90 000 evolve to a chronic carrier state and more than 22 000 die of cirrhosis or hepatocellular carcinoma [Reference Roure2]. The prevalence of HBsAg, which is influenced primarily by the age at infection, has been used to categorize endemicity as high (⩾8%), intermediate (2–8%), low (<2%) [Reference Mahoney, Kane, Plotkin and Orenstein3] or very low (<0·5%) [Reference van Damme and Vorsters4]. In Europe, there are wide variations in reported HBsAg carriage, increasing west to east and north to south [Reference Mahoney, Kane, Plotkin and Orenstein3]. This geographical variation is reflected in the estimated mortality from cirrhosis and hepatocellular carcinoma due to HBV, with about 200 attributable deaths annually in northwestern Europe compared to nearly 19 000 in central and eastern Europe [Reference Roure2].
HBV is a possible candidate disease for elimination and ultimately eradication as the reservoir for HBV is entirely human and highly efficacious preventative vaccines are available [Reference Fenner5]. In 1991, the WHO called on all countries to introduce universal HBV vaccination by 1997 [6]. By the end of 2004, universal HBV vaccination programmes were in place in 43 of the 52 countries in the WHO European region [Reference van Damme7]. Some countries in northern and western Europe, where endemicity is low, have not implemented universal HBV vaccination programmes [Reference van Damme and Vorsters4] but rather selective immunization programmes [Reference Mortimer and Miller8, Reference O'Connell9] targeting migrant populations and at-risk groups [Reference Pebody10, Reference Hahne11].
Serological surveillance is a vital tool for assessing the burden of infection and thus the need for a vaccination programme as well as the evaluation of such programmes once they are in place. The use of an algorithm testing for HBsAg as well as antibodies to surface (anti-HBs) and core (anti-HBc) antigens enables serological studies to estimate the prevalence of carriers, resolved infection and vaccinated individuals in a population [Reference Mahoney, Kane, Plotkin and Orenstein3]. Thus, serological data can supplement HBV disease surveillance data, which are limited because of underreporting of acute and chronic cases, the long incubation between infection and disease as well as the large proportion of asymptomatic acute cases, especially in children.
The European Sero-Epidemiology Network (ESEN2), based on the previous ESEN project [Reference Osborne, Weinberg and Miller12], was established in 2001 with the aim of standardizing serological surveillance to eight vaccine-preventable diseases, of which one was HBV infection, in 22 European countries [Reference Nardone and Miller13]. Variations in antibody titres have been reported between laboratories, even those using the same assay [Reference Kafatos14, Reference Andrews15]. Thus, the standardization of assay results was essential to allow international comparisons of HBV serology. We present the comparison of HBV seroepidemiology in the ten participant countries in order to describe the epidemiology of HBV infection in Europe in relation to vaccine policy, to inform the development of the most appropriate control measures and to evaluate vaccination programmes in place.
METHODS
Serum survey collection
As part of ESEN2, ten countries undertook testing for HBV antibody and antigens in sera specimens collected between 1996 and 2003 (Table 1). The sera were obtained either by residual sera collected during routine laboratory testing (6/10 countries) or by population-based random sampling (4/10) (Table 1). Ethical approval was sought from the appropriate national authorities for all collections.
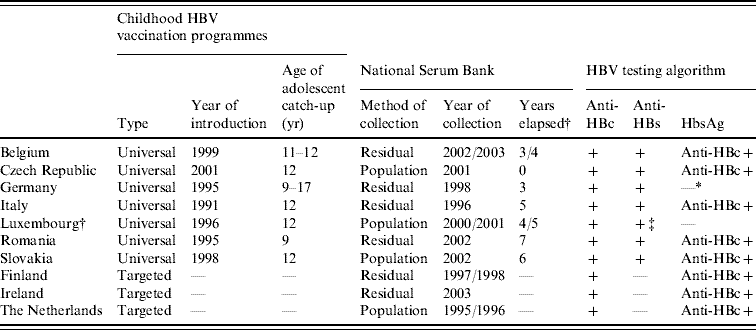
Table 1. Childhood vaccination policies for hepatitis B, national serum bank collection and serological testing algorithms employed in the ten participant countries in ESEN2
* Years elapsed between introduction of vaccination programme and serum bank collection.
† All anti-HBc samples were tested for HBsAg but data unavailable.
‡ All samples tested for anti-HBs antibody, of which only positives were tested for anti-HBc antibodies.
Sera were evenly distributed between males and females and were geographically representative of each country. Project guidelines recommended that >3000 samples should be tested from all age groups, of which two-thirds were from those aged <20 years [Reference Osborne, Weinberg and Miller12]. Sera were collected in all age groups except in Belgium (only those aged <20 years), Germany (from 17 to ⩾60 years) and Luxembourg (from 4 to ⩾60 years) (Table 2). Furthermore, smaller than recommended sample sizes were collected in the Czech Republic (2644 samples tested), Ireland (2535) and Romania (1338) (Table 2).
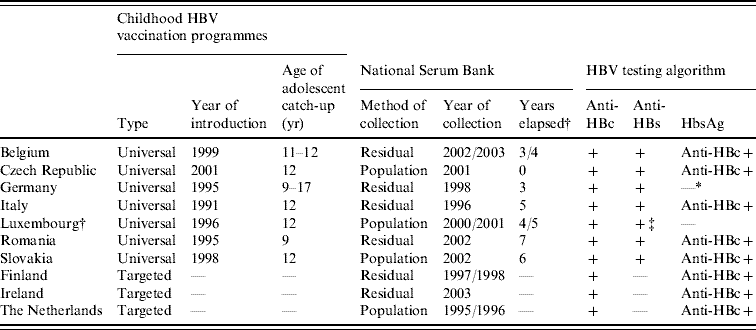
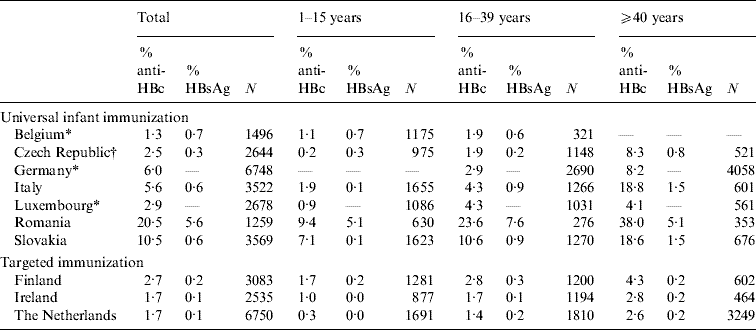
Table 2. Age-specific seroprevalence of anti-HBc-positive and HBsAg-positive samples in ten ESEN2 countries, 1996–2003
* Samples collected from limited age ranges: Belgium 1–20 years; Germany >16 years; and Luxembourg >3 years.
† Czech Republic compiled the serum bank at the same time as the introduction of universal infant immunization (2001).
Organizational analysis
In March 2002, a standardized questionnaire requesting historical information regarding the HBV vaccination programmes was sent to all national representatives. Reported infant HBV vaccination coverage was updated with data reported to the WHO, accessed from the ‘Health For All’ database in August 2007.
Assay standardization
The methodology and results of the qualitative and quantitative standardization of the antibody and antigen results have been described in detail elsewhere [Reference Kafatos14, Reference Kafatos, Andrews and Nardone16]. In brief, the designated reference centre (Hellenic Centre for Disease Control, Athens, Greece) prepared a panel of 172 sera, which were tested using the Abbott AxSYM system (Abbott Laboratories, Abbott Park, IL, USA) for three markers of HBV infection or vaccination: anti-HBs, anti-HBc and HBsAg. The panel included negative, equivocal, low and high positive anti-HBs and anti-HBc antibody titres, thereby enabling the quantitative standardization of the antibody responses. In contrast, only qualitative standardization of HBsAg results was performed [Reference Kafatos14].
These panels were distributed to participant laboratories where they were tested for anti-HBs, anti-HBc and HBsAg with the enzyme immunoassay normally used by the participating laboratory [Reference Kafatos14]. For each antibody marker of HBV infection (i.e. anti-HBs and anti-HBc), national results of testing the reference panel were plotted against those of the reference centre and a regression was performed. The equation of the line-of-best-fit was used as a standardization equation so that local titres could be converted to standard titres. The standardizations of the assays were evaluated quantitatively by determining the fit of the equation using R 2 (coefficient of determination) and qualitatively by assessing the level of concordance in identifying positive, negative and equivocal results [Reference Kafatos14, Reference Kafatos, Andrews and Nardone16].
In two countries (Germany and The Netherlands), the serum banks had been tested over a year before the distribution of the reference panel. A method of back-standardization, described in detail elsewhere [Reference Kafatos, Andrews and Nardone16], was performed in these countries in order to standardize their results to project units. In brief, about 150 sera, representative of the different defined statuses (e.g. resolved infection, vaccinated) were randomly selected from the national serum bank and forwarded to the reference centre for testing for the three serological markers of HBV infection. A regression analysis, as described above, was performed on the two sets of data in order to obtain the appropriate standardization equation [Reference Kafatos14].
Main serum bank testing
Each national serum bank was tested using the same validated assay as was used for the reference panel. For the anti-HBc and anti-HBs antibody results, the country-specific standardization equations were used to convert the local quantitative results of the serum survey into standardized reference laboratory units. The reference laboratory cut-offs were used to re-classify qualitatively the standardized quantitative results as negative, equivocal or positive. As testing of the reference panel for HBsAg showed 100% concordance [Reference Kafatos14], the HBsAg result was taken as that reported by the national participant.
National HBV testing algorithms
The HBV testing algorithms employed often reflected whether a universal childhood HBV vaccination programme was in place in those countries (Table 1). Most countries with a vaccination programme tested all samples for anti-HBc and anti-HBs, and then went on to test anti-HBc-positive samples for HBsAg. Germany tested all samples for anti-HBc and anti-HBs, but data regarding HBsAg were unavailable due to insufficient volumes of sera for back-standardization. Luxembourg tested all samples for anti-HBs, and then tested anti-HBs-positive samples for anti-HBc, but no testing for HBsAg was undertaken.
The HBV testing algorithm for countries without a universal childhood vaccination programme was to test all samples for anti-HBc, and then only test anti-HBc-positive samples for HBsAg (Table 1). In these countries, all anti-HBc negative samples were assumed to be anti-HBs negative as the level of vaccination was very low.
Although the participant countries employed different HBV testing algorithms, we were able to categorize all sera into four groups: no history, vaccinated (only anti-HBs positive), resolved HBV infection (anti-HBc and anti-HBs positive) and acute infection or chronic carriers (anti-HBc positive and HBsAg positive). It should be noted that the testing algorithms employed would have misclassified as ‘no history’ those who were chronic carriers in Luxembourg as well as successfully vaccinated individuals in Finland, Ireland and The Netherlands.
RESULTS
At the time of the sera collections, three countries (Finland, Ireland, The Netherlands) had only a targeted vaccination programme of at-risk individuals (e.g. men who have sex with men, or injecting drug users) as well as screening pregnant women for HBsAg (Table 1). The remaining seven countries had introduced universal HBV immunization of infants, and all had included recommendations for adolescent catch-up vaccination, ranging from age 9 years (Romania) to age 17 years (Germany). In six of these seven countries, the main serum banks were collected after the introduction of the mass infant immunization, and one (Czech Republic) at the same time (Table 1).
Of the ten participant countries, the highest prevalence of previous exposure to HBV infection (i.e. anti-HBc positive) was observed in Romania, where 20·5% of sera were anti-HBc positive (Table 2). The percentage exposed increased with age; from 9·4% in children (1–15 years) to 38·0% in older adults (⩾40 years) (Table 2). Similarly, the prevalence of HBsAg was highest in Romania (5·6%), although there was no increase in prevalence by age group (χ2=2·7, P=0·25). The seroprofile for Romania demonstrates that although the reported coverage of infants with HBV vaccination was high (>95% of infants), a large percentage of children had no markers of vaccination (Fig. 1). For example, the reported vaccine coverage of those aged 3–4 years was 98%, but only 63% had serological evidence of vaccination (i.e. anti-HBc negative and anti-HBs positive). Just under 4% of this age group had been exposed to HBV infection, of which a third (or 1% of the total) were HBsAg positive (Fig. 1).

Fig. 1. HBV seroprofiles showing reported HBV vaccine coverage in infants (□–––□) and as a histogram the prevalence of samples tested with no history (□), anti-HBs-positive only (![]() ), anti-HBc positive (
), anti-HBc positive (![]() ) and HBsAg positive (▪)* in ten ESEN2 countries, 1996–2003 (* in Germany these samples were anti-HBc positive and anti-HBs negative). Universal infant vaccination programme: Belgium, Czech Republic, Germany, Italy, Luxembourg, Romania, Slovakia. Targeted vaccination programme: Finland, Ireland, The Netherlands.
) and HBsAg positive (▪)* in ten ESEN2 countries, 1996–2003 (* in Germany these samples were anti-HBc positive and anti-HBs negative). Universal infant vaccination programme: Belgium, Czech Republic, Germany, Italy, Luxembourg, Romania, Slovakia. Targeted vaccination programme: Finland, Ireland, The Netherlands.
Of the remaining nine countries, only Slovakia showed previous exposure to HBV infection in the population >10% (10·5%). The proportion of anti-HBc-positive samples increased with age, from 7·1% in children to 18·6% in older adults (Table 2). The proportion of HBsAg-positive individuals in the population was <1% (0·6%) and similarly increased with age, from 0·1% in children to 1·5% in older adults (χ2=15·2, P<0·001) (Table 2). Children aged ⩽4 years would have been targeted by the HBV vaccination of infants introduced with a high reported coverage (>95%), and among 1-year-olds 85% were anti-HBs positive (Fig. 1). In contrast, despite the introduction of a catch-up vaccination campaign of 12-year-olds, <15% of adolescents in the 12–15 years age group had serological evidence of vaccination (Fig. 1).
In Italy, the overall prevalence of past exposure to HBV infection was 5·6%, with much higher prevalence of anti-HBc being observed in those aged ⩾40 years (18·8%) than in the younger adult age groups (1·9% and 4·3% respectively) (Table 2, Fig. 1). The carriage of HBsAg also increased with age, from 0·1% in children to 1·5% in older adults (χ2=17·8, P<0·001) (Table 2). HBV vaccination of both infants and adolescents was well implemented in Italy as >80% of recently targeted age groups (i.e. 1–3 and 13–15 years) were anti-HBs positive.
In Germany, sera were collected from adults only. Past exposure to hepatitis B was 6·0%, and as in Italy, higher anti-HBc was observed in those aged ⩾40 years (8·2%) than in the younger adult age groups (2·9%) (Table 2, Fig. 1). No HBsAg data were reported from Germany, but the proportion of anti-HBc positive-/anti-HBs-negative samples (some of which would have been HBsAg positive) increased with age, from 0·7% (19/2680) in younger adults to 1·5% (67/6058) in older adults (χ2=11·47, P<0·001). Of the participants in Germany targeted by the vaccination recommendation implemented in 1995 (17–20 years age group at the time of the serum collection), only 9·6% were anti-HBs positive (Fig. 1).
The percentage of the population with a past exposure to HBV infection was <5% in the remaining six countries (Belgium, Czech Republic, Finland, Ireland, Luxembourg, The Netherlands), ranging from a minimum of 1·7% in Ireland and The Netherlands to 2·9% in Luxembourg (Table 2). The age-specific prevalence of past infection remained <5% even in the oldest age groups except in the Czech Republic where 8·3% of older adults (i.e. ⩾40 years) had serological evidence of past exposure to HBV infection.
In Belgium, where only samples from those aged <20 years were tested, nearly 80% of the youngest children (i.e. <4 years) were anti-HBs positive, although coverage was reported as 60% in this age group. Levels of anti-HBs in those aged 11–12 years targeted by the catch-up recommendations were much lower, but reached 70–80% in those aged 14–15 years (Fig. 1). A large proportion of children aged between 4 and 11 years expressed serological markers of vaccination, many of whom would not have been targeted by an HBV vaccination campaign. In Luxembourg, not only was there an important percentage of children with serological markers of HBV vaccination in age groups not targeted by HBV vaccination, but also in those age groups that were targeted by HBV vaccination, the proportion of children with serological markers of vaccination was much higher than the reported coverage of 49% in 4-year-olds (Fig. 1).
No universal HBV vaccination policies were in place in Finland, Ireland and The Netherlands (Table 1) and in these countries levels of surface antigen carriage were lower than countries with universal programmes (Table 1). In children, HBsAg carriage was reported to be 0% in Ireland and The Netherlands and 0·2% in Finland. In Finland, carriage in children was higher than in Italy and Slovakia (0·1% respectively) and in young adults was higher than in the Czech Republic (0·2%, Table 1).
No gender differences were observed in any of the countries for past exposure to HBV (i.e. anti-HBc positive) in young adults (aged 16–39 years). Statistically significant gender differences in HBsAg carriage were reported only Romania (9·9% males, 2·9% females; χ2=6·27, P=0·01). In the five countries with testing algorithms providing serological evidence of vaccination, young women were more likely to be anti-HBc negative and anti-HBs positive than men in Germany and in Slovakia; although only in Germany would any of this age group (17–20 years) have been targeted by a catch-up immunization programme.
DISCUSSION
We report for the first time a study comparing HBV seroepidemiology in ten European countries, in which possible inter-assay and inter-laboratory variations have been controlled for by a standardization procedure [Reference Kafatos14, Reference Kafatos, Andrews and Nardone16]. Thus, international comparisons of the epidemiology of HBV and in particular the performance of national immunization, control and prevention programmes can be made. The ten countries participating in this study were not randomly selected and they were representative of Europe in terms of geography, vaccination policies and HBV epidemiology [Reference Roure2, Reference Mahoney, Kane, Plotkin and Orenstein3].
Of the participant countries, the burden of infection was highest in Romania where prevalence of HBsAg carriage (5·6%) was in the intermediate range of endemicity (i.e. 2–8%). Levels of carriage were very much lower in those age groups targeted by the vaccination programme than in those immediately older. However, despite the universal childhood vaccination programme, there is evidence of ongoing transmission of HBV in younger birth cohorts and the reported coverage is much higher than indicated by the serosurvey. In Slovakia and Italy, we have presented serological evidence of the impact of the childhood vaccination programmes as there was high prevalence of anti-HBs in targeted birth cohorts. However, the evaluation of HBV vaccination programmes is best done by a comparison of the prevalence of vaccinated individuals at different time-points, as age and cohort effects can contribute to changes in the prevalence of individuals with past history of infection [Reference Dominguez17, Reference Da Villa18].
In three countries (Italy, Romania, Slovakia), the proportion serologically identified as vaccinated was lower than the coverage reported in infants. This may be accounted for in older age groups as antibody decay following vaccination has been shown to be rapid within the first year, declining more slowly thereafter [Reference Jilg, Schmidt and Deinhardt19, Reference Gesemann and Scheiermann20]. Anamnestic responses, conferring immunity to HBV infection, have been reported in individuals with low or undetectable anti-HBs [Reference West and Calandra21, Reference Banatvala and Van Damme22], and a report by a European consensus group recommended that no booster doses for immunocompetent children and adolescents [23]. Thus, differences in the reported vaccine coverage and observed prevalence of vaccinated individuals may be of minimal public health importance if vaccine coverage estimates are accurate, which may not be so for all countries. In Belgium, the prevalence of those with serological evidence of vaccination was higher than the coverage reported for many ages, this might be because national coverage was not always measured annually (H. Theeten, personal communication). In Romania, discrepancies between reported vaccine coverage and observed serological correlates of protection were noted not only for HBV, but also for other infections such as measles [Reference Andrews24].
Routine immunization of infants will eventually result in a community with a broad-based immunity. However, in many European countries, most infection occurs in young adults so that a stand-alone infant immunization programme will take several decades to eliminate HBV infection in the community [Reference Mahoney, Kane, Plotkin and Orenstein3]. Many European countries, and all the countries with a childhood vaccination programme participating in this study, have included a catch-up component of older children and adolescents in order to more quickly eliminate the infection from the community [Reference van Damme and Vorsters4]. The official catch-up policy in adolescents varied from a recommendation to vaccinate, as in Germany, to a campaign entailing mandatory vaccination as in Italy [Reference FitzSimons25, Reference Mele, Stroffolini and Zanetti26]. In three of the five countries (Germany, Slovakia, Romania), adolescents appear to have been poorly targeted by these catch-up campaigns as evinced by the low proportion of anti-HBs positives in this age group. These findings highlight the difficulties in targeting such a population [Reference FitzSimons25, Reference Zuckerman and Langer27].
Countries with a targeted rather than a universal HBV vaccination programme have cited their very-low HBV endemicity to justify their policy [Reference van Damme7]. In this study, the lowest overall prevalence of HBsAg carriage was reported in the three countries with targeted HBV vaccination programmes, although similar prevalence of HBsAg carriage was reported in the Czech Republic where an immunization campaign had been recently implemented. Within Europe, the migration of individuals from high- to low-endemicity countries is having an impact on HBV [Reference Hahne11, Reference Zuckerman28]. Targeted programmes require continued refining and improved immunization options to ensure good coverage in at-risk groups [Reference Hahne11]. In The Netherlands, an additional programme targeting children with at least one parent coming from a middle- or high-endemic country was introduced in 2003 (H. de Melker, personal communication). Nonetheless, the difficulties of implementing targeted vaccination programmes was one of the reasons for the change in vaccination policy recently announced in Ireland [29] and is cited by those advocating the introduction of a universal programme [Reference Zuckerman28, Reference Banatvala, Van Damme and Emiroglu30].
There are some limitations to our study. Two countries did not provide HBsAg testing data (Germany, Luxembourg), which meant that HBsAg-positive samples could not be identified. In Germany, we reported a prevalence of 1·3% (86/6748) anti-HBc-positive and anti-HBs-negative samples which represent both HBsAg carriers as well as isolated anti-HBc. In the original study, a mean weighted prevalence of HBsAg carriage of 0·6% was reported [Reference Thierfelder31]. The ‘isolated anti-HBc’ are individuals whose anti-HBs response may have waned, have an occult chronic HBV infection without detectable HBsAg or represent a false-positive reaction [Reference Gandhi32]. Such samples, although they represent a minority of samples in the sera collections reported here, have been categorized as evidence of a past infection.
Another limitation is that we compared HBV seroepidemiology between countries who compiled serum banks either by residual sera collection or community sampling. Community-based surveys of HBV infection are recommended, as the results are more representative and can be generalized to the population [Reference Qirbi and Hall33]. However, in schoolchildren in Australia, the HBV seroprevalence estimated from residual sera or probability sampling was similar [Reference Kelly34]. Other serological studies conducted as part of ESEN2 also did not report any correlation between reported seroprevalence and methods of sera collection [Reference Nardone35].
We have demonstrated the utility of serological surveillance in assessing the need for HBV vaccination programmes and their contribution to the evaluation of such programmes where they have been implemented. Serological surveillance, when undertaken in a coordinated and standardized manner and interpreted in the context of data of vaccination programmes and vaccination coverage has provided valuable information for the comparative evaluation of vaccine programmes internationally. Therefore, such initiatives should play an important role in public health in Europe.
ACKNOWLEDGEMENTS
This work was undertaken with funding from the European Commission (contract number QLK2-CT-2000-00542) and from national governments.
DECLARATION OF INTEREST
None.