First, the acidifying mechanism of ingested free sulfate and sulfate produced from sulfur amino acids after protein ingestion are two different processes. In urine, the excretion of SO42 − reflects the oxidation of sulfur amino acids methionine, cysteine or cystine of dietary or endogenous proteins and is accompanied by the generation of 2 mEq H+ per mmol sulfur oxidisedReference Lemann and Relman4–Reference Lemann, Bushinsky and Hamm7. In contrast, when calcium sulfate or calcium chloride is ingested at levels to allow for equivalent absorption of sulfate and their cations, there is no ‘net acid’ intakeReference Whiting and Cole8. The acid effect of ingested CaCl2 is due to a much greater absorption of Cl− than CaReference Gamble, Blackfan and Hamilton9. Similarly, calcium sulfate given intravenously is neutral. When ingested, fractional sulfate absorption is higher that that of Ca and the type of anionic exchange determines its effect on the acid–base balanceReference Oh10. The acid load in that case is metabolically different from sulfate derived from absorbed amino acids and endogenous protein, as protons released during sulfur oxidation must be added to sulfate excreted in urine. Thus we cannot say that there exists ‘a commonly accepted consensus to attribute acidifying property of sulfate’ when sulfate originates from inorganic salts or from organic compounds.
Second, I disagree with the claim that ‘it is well known that sulfate is well absorbed and excreted in urine because this anion cannot be metabolised or retained’. In our studyReference Arnaud and Welsch11 cited by Brandolini et al. Reference Brandolini, Guéguen, Boirie, Rousset, Bertière and Beaufrère1, it was shown that 7 % of sulfate from a water containing 1479 mg per litre was incorporated and in urine and stool between 30 and 60 % was in the form of conjugates or bound to organic compounds. There are hundreds of sulfur-containing compounds in the human body12 and sulfated oligosaccharides have important biological roles, their unique structure contributing to recognition by a receptorReference Hooper, Manzella and Baenziger13. Proteochondroitin sulfate plays a major role in the mechanical support of cartilage; its functions are dependent on the high charge of the sulfate and any decrease in the sulfation might be expected to affect the structure and stability of the cartilageReference Blinn, Dibbs, Hronowski, Vokonas and Silbert14. Sulfate is the fourth most abundant anion in the human plasma, and circadian variations of serum inorganic sulfate levels have been shown in healthy volunteersReference Hoffman, Wallace and Verbeeck15. Mean plasma levels of 0·29–0·35 mmol sulfate/l are reported in infants and adult subjects with no dependence on age and sexReference Kock, Schneider, Delvoux and Greiling16. Higher values are reported in newborns, suggesting that the elevated serum sulfate levels in the newborn fulfil the needs for important biological functions including connective tissue synthesisReference Cole and Scriver17. Sulfate requirements for the growing fetus are high and thus the needs during pregnancy are not adequately assessed12. Free sulfate is used for the biosynthesis of 3′-phosphoadenosine-5′-phosphosulfate; this pool of active sulfate is small in man as compared with animal species, so that efficient sulfate conjugations are maintained by its continuous provision for xenobiotic elimination and hormone activation. Sulfate was also suggested to mediate the therapeutic effect on osteoarthritis of glucosamine sulfateReference Hoffer, Kaplan, Hamadeh, Grigoriu and Baron18.
Third, I also disagree with their comment on the impact of fluid intake on mineral balance: ‘it must be recommended to drink less water in order to preserve bone mineral mass’. Any increase of water excretion or diuresis is accompanied by intra- and extracellular fluid electrolytes, particularly an elevated excretion of CaReference Atchley, Loeb, Richards, Benedict and Driscoll19. It was shown that urea saline diuresis induced a linear increase in the clearance of CaReference Better, Gonick, Chapman, Varrady and Kleeman20 and extracellular volume expansion also augments Ca excretionReference Blythe, Gitelman and Welt21. Drinking 0·5 litres of distilled water produces a significant increase of ionised plasma Ca concentration and an inverse reduction of parathyroid hormone secretionReference Guillemant, Le, Delabroise, Arnaud and Guillemant22. A similar effect was reported with a mineral water containing 9 mg Ca/l and the suppressive effect was more important in the morning, less pronounced at noon and disappeared in the afternoonReference Guillemant, Accarie, de La Guéronnière and Guillemant23. While the risk of mineral disturbances after the ingestion of distilled, deionised or low-mineralised water is not perceived in European countries, it is discussed in countries such as China (Hong Kong) and the Philippines where more than 60 % of bottled drinking water sold is distilled. The German Nutrition Society published the advice that the ‘exclusive consumption of pure water (distilled) may lead, according to the dietary intake, to a depletion of the body minerals’24. It is thus true to advise ‘to drink less (distilled or low-mineralised) water in order to preserve bone mineral mass’ but the optimal content of mineral in water and beverages to prevent bone loss has not been investigated. Total water intake, particularly in the case of polydipsia–polyuria, increases Ca losses and leads to osteoporosisReference Mercier-Guidez25. During intense physical exercise, an increased concentration of the bone marker of osteoclastic bone resorption is observed from 30 min after the start of the exercise and up to 2 h after the end of the exercise while this effect is suppressed when the consumption of mineral water with a low Ca content is replaced by Contrex with 486 mg Ca/lReference Guillemant, Accarie, Peres and Guillemant26. These studies show that fluid and water intake affect Ca metabolism and bone turnover. The WHO released a report on nutrients in drinking water examining the relationship between water hardness and health, which may lead to the establishment of minimum health-based future WHO guideline values and an international symposium on the Health Aspects of Magnesium and Calcium in Drinking Water has been organised (24–26 April 2006; Baltimore, MD, USA) to evaluate the evidence and the needs for research before a decision can be taken.
Fourth, the sentence ‘a woman drinking 1 litre of CaSO4-rich water daily would have bone mineral density equivalent to a woman 7 years younger who drinks only Ca-poor water’ is correct. Due to the solubility of Ca in water, only sulfate-rich water can reach concentrations higher that 400 mg/l. The names of the brands of mineral waters consumed in the EPIDOS study were listedReference Gillette-Guyonnet, Andrieu, Nourhashemi, De La Guéronnière, Grandjean and Vellas27 and only two waters have contents above 400 mg/l: Contrex (480 mg/l) and Vittel Hépar (563 mg/l). As shown in Fig. 1, there is a direct relationship for still mineral waters between Ca and sulfate contents while bicarbonate concentration does not change. Such correlation between higher Ca concentration and sulfate as the principal counter-ion in water was reported recentlyReference Heaney28. The comparison of short-term administration of 500 mg Ca from either a CaSO42 − -rich mineral water, a CaSO42 − solution or a calcium carbonate pharmaceutical preparation on plasma Ca and intact parathyroid hormone as well as Ca and creatinine in urine leads to the conclusion that sulfate does not increase Ca urinary excretionReference Fardellone, Bellony, Texier, Brazier, Delabroise, Arnaud and Sebert29.
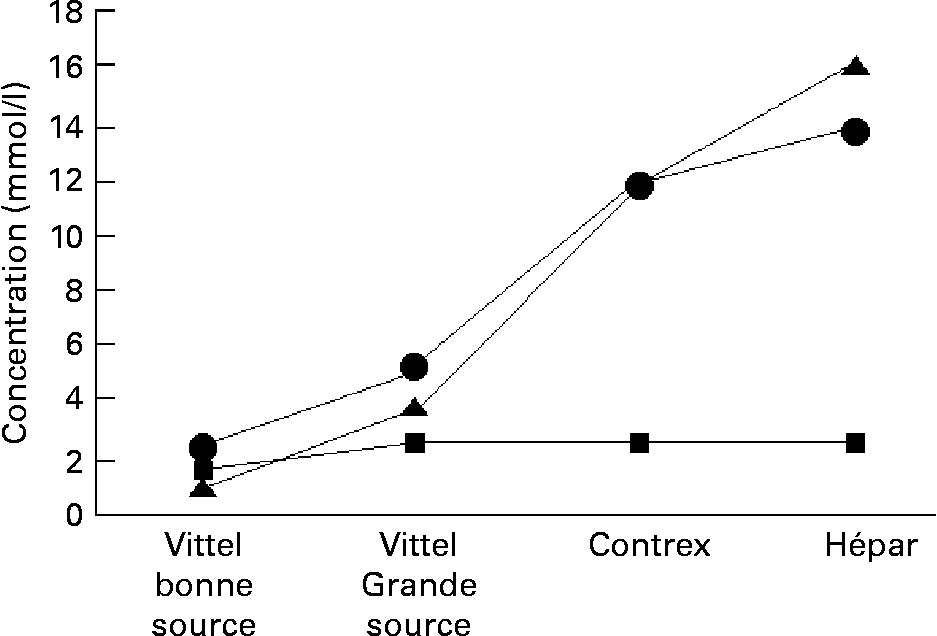
Fig. 1 Ca (●), sulfate (▲) and bicarbonate (■) concentrations of some French natural still mineral waters.
Fifth, more surprising, the ‘potential toxic effect of hydrogen sulfide on colonic mucosa’ that was not relevant to both their study and my comments leads Brandolini et al. to conclude that I ‘occulted this hypothesis’. Since 1993, a large number of studies have been published on sulfate-reducing bacteria and colonic sulfur metabolism, health and safety. Major research progresses were obtained since the hypothesis that H2S may be involved in the aetiopathogenesis of inflammatory bowel disease was publishedReference Roediger and Nance30. H2S cannot be ignored as the main constituent associated with halitosis and responsible for the unpleasant odour of the human flatusReference Suarez, Springfield and Levitt31. The title of a recent review ‘Hydrogen sulfide: from the smell of the past to the mediator of the future?’Reference Moore, Bhatia and Moochhala32 draws our attention to the relatively high concentrations of endogenously produced H2S that have been observed in the brain of human subjects showing to act as a neuromodulatorReference Eto, Ogasawara, Umemura, Nagai and Kimura33–Reference Chen, Jhee and Kruger35 as well as to its properties as a vasodilator compoundReference van Zwieten36. Sulfate found in the colon may come from unabsorbed dietary sulfate and sulfur amino acids, taurine, and sulfur-containing food additives such as sulfur dioxide, sulfites and carrageenan12. To discriminate the metabolic fate of inorganic sulfate from water and dietary sulfate, we conducted a study on patients proctocolectomised for ulcerative colitis under a strictly controlled diet and drinking 0·5 litres of a sulfate-rich water containing 7·7 mmol (740 mg) inorganic sulfate. Sulfate absorption from water is similar to that observed when sulfate is consumed from food taken over the whole dayReference Normén, Arnaud, Carlsson and Andersson37. Inorganic sulfate supplementation of the drinking water (16·7 mmol; 1600 mg/l) in mice showed in the short (7 d) and long term (1 year) that this supplementation did not increase intestinal sulfate or H2S concentrations, suggesting that inorganic sulfate is not an important modulator of colonic H2SReference Deplancke, Finster, Graham, Collier, Thurmond and Gaskins38. Several human studies confirmed that proteins were far superior to sulfate as substrates for the production of faecal H2SReference Levine, Ellis, Furne, Springfield and Levitt39 and that differences in dietary intake of sulfate are unlikely to be responsible for the higher free sulfate in ulcerative colitis patients. Pitcher et al. Reference Pitcher, Beatty and Cummings40 suggest that increased bacterial desulfation of secreted colonic mucin releases more free sulfate and is contributory to the observed reduction in mucus gel thickness, probably due to cleavage of disulfide bonds, and the consequent loss of barrier function in ulcerative colitis. Because cysteine and cystine in protein are less absorbed from the upper small intestine, standard therapy for ulcerative colitis patients has included restriction of foods such as milk, eggs and cheese, which are significant sources of dietary sulfurReference Roediger41. Finally, evidence on the role of sulfate in the aetiology of ulcerative colitis is inconclusive12 and there is little evidence to implicate dietary components in the aetiology or pathogenesis of ulcerative colitisReference Carter and Lobo42, while Ohge et al. Reference Ohge, Furne, Springfield, Sueda, Madoff and Levitt43 qualified as a speculation that H2S induces colonic mucosa injury.
Finally, Brandolini et al. Reference Brandolini, Guéguen, Boirie, Rousset, Bertière and Beaufrère1 indicated that subjects of their study had to drink, per d, either 400 ml milk or 1 litre of a CaSO4-rich mineral water, but it is not mentioned how the subjects drank the milk or water. If they drink glasses of 200 ml, they get a 240 mg dose of Ca for the milk twice per d and 96 mg for water, five times per d. With the dose-dependent absorption of CaReference Blanchard and Aeschlimann44, 48 % (230 mg) and 68 % (326 mg) of the dose will be absorbed from milk and water, respectively. This difference of 96 mg Ca/d intake in favour of the water diet may explain an excess of 14 mg urinary Ca excretion. In a study with controlled fluid and dietary intakes, Ca absorption from milk was 20 % greater on a 6-fold divided-dose regimen when compared with a single daily dose. On divided doses, a greater net retention of Ca leads to a positive balance of +43 mg/d and the mean urinary Ca excretion was increased by up to 60 mg/dReference Kales and Phang45. Just an uncontrolled ingestion of unbalanced fluid intake can explain more that the difference reported by Brandolini et al. Reference Brandolini, Guéguen, Boirie, Rousset, Bertière and Beaufrère1



