INTRODUCTION
Severe sepsis and septic shock in the emergency department (ED) represent a significant health care burden that is associated with high morbidity and mortality.Reference Cohen, Vincent and Adhikari 1 , Reference Wang, Shapiro, Angus and Yealy 2 In 2013, sepsis accounted for more than $23.7 billion of total United States (US) hospital costs.Reference Torio and Moore 3 It has been estimated that one patient arrives to the ED with this clinical presentation in the US every minute, with a mortality rate ranging from 25 to 50%.Reference Wang, Shapiro, Angus and Yealy 2 , Reference Torio and Moore 3 Antimicrobial therapy plays a major role in the treatment of severe sepsis and septic shock, yet there are several other factors that influence mortality outcomes, including fluid resuscitation and hemodynamic stability.Reference Rivers, Nguyen and Havstad 4 In septic patients, appropriate selection of antimicrobial therapy based on suspected pathogens, source of infection, and patient-specific factors has been associated with reduced mortality.Reference Barie, Hydo, Shou, Larone and Eachempati 5
The Surviving Sepsis Campaign for the management of severe sepsis and septic shock recommends the administration of broad-spectrum antimicrobial therapy within 1 hour of septic shock recognition.Reference Dellinger, Levy and Rhodes 6 These recommendations are largely based on moderate or low grades of supporting evidence. A retrospective study in the intensive care unit (ICU) showed that each 1 hour of delay in initiation of effective antimicrobial therapy after the onset of hypotension was associated with a 7.6% increase in mortality.Reference Kumar, Roberts and Wood 7 Other studies have found inconsistent results concerning the association between prompt administration of antimicrobials in sepsis and mortality.Reference Puskarich, Trzeciak and Shapiro 8 - Reference Burnham and Kollef 23 Regardless of these inconsistent findings, initiation of antimicrobial therapy is generally considered a life-saving component in the resuscitation of a septic patient.Reference Gaieski, Mikkelsen and Band 24 Due to the complex issues and competing demands in the ED, administration of antimicrobials within the one-hour timeframe can be a challenging task to accomplish.Reference Kanji and Dumaresque 25 Current guidelines recommend storing premix antimicrobials (PMAs) in the ED to avoid delays in administration if possible.Reference Dellinger, Levy and Rhodes 6 It is theorized that PMAs in the ED would decrease the preparation time required by pharmacy or nursing staff to compound the intravenous (IV) antimicrobial agents and therefore provide earlier accessibility, although this recommendation appears to be solely based on expert opinion.Reference Dellinger, Levy and Rhodes 6 There are inadequate data directly exploring the relationship between PMA use and administration time.
The primary objective of this study was to evaluate the impact of antimicrobial preparation on time to administration of the first as well as the complete empiric antimicrobial regimen in septic patients located in the ED. These data are required to better understand the potential value of PMAs on reducing time to antimicrobial administration and improving patient outcomes.
METHODS
This single-center, retrospective, cohort study was performed in the ED of a 472-bed academic medical center. The ED is a Level I trauma center and has an annual census of approximately 70,000 patients. Commonly used medications, including several antimicrobials, are stored in six automated dispensing cabinets (ADCs) in the ED. Additionally, the pharmacy department provides other medications not available in the ADCs 24 hours a day. This study was granted approval status by Upstate Medical University’s institutional review board based on its retrospective/observational design.
Adult ED patients with a diagnosis of sepsis were identified between June 1, 2015 and August 31, 2015 via a query of diagnosis codes in the electronic medical record. Adult patients with a primary diagnosis code of sepsis, severe sepsis, or septic shock with the initiation of antimicrobial therapy while in the ED were included in the study. Patients were reviewed consecutively and included if they received recommended empiric antimicrobial treatment. Recommended antimicrobial treatment was defined as any antimicrobial regimen recognized by nationally accepted guidelines for the empiric management of typical pathogens for the suspected source of infection and local hospital susceptibility patterns.Reference Tunkel, Hartman and Kaplan 26 - Reference Solomkin, Mazuski and Bradley 32 If the suspected source of infection was unclear or unknown, piperacillin–tazobactam and vancomycin was defined as an acceptable initial empiric regimen. Patients were excluded if an antimicrobial was administered outside the ED location, such as the ICU, or if pertinent data were missing from the medical record.
A single reviewer, among multiple members of our research team, reviewed and extracted data from electronic medical records using a standardized data collection form. The reviewer collected demographic, clinical, and antimicrobial data. Periodic reviews of the data throughout the data collection process were performed by members of the research team to ensure proper and accurate data retrieval techniques. Patient demographic data included patient age, weight, and gender. Clinical data included admission serum creatinine (SCr), volume of IV fluids given in the ED, use of vasopressors in the ED, ICU admission, and length of hospital stay (LOHS). Antimicrobial data included drug name, time of antimicrobial order by physician and time of antimicrobial administration, antimicrobial storage site (ADC vs. pharmacy), and antimicrobial preparation (premix vs. non-premix). An antimicrobial agent was considered premixed if it was stored in an ED ADC in a preparation that did not require reconstitution and/or dilution prior to administration. An empiric regimen was considered premixed if all administered antimicrobials met the premixed definition. PMAs were used if the particular antimicrobial was commercially and readily available in the ADC located in the ED. Non-premix antimicrobials prepared by pharmacy personnel were used if the selected antimicrobial at the selected dose was not commercially available as a PMA.
All collected data were entered into a database created with IBM SPSS version 22.0. Descriptive statistics, including n (%), mean±standard deviation (SD), and median (interquartile range [IQR]) were used to summarize data. Student’s t-test and the Mann–Whitney U test were respectively used for comparisons of normally and non-normally distributed continuous data. The chi-square test for independence or Fisher’s exact test was used for comparisons of categorical data. All tests were two-tailed, and a p value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS 22.0.
RESULTS
Ninety-seven patients were included in the final analysis. Fifty-two percent (50/97; 52%) were female, and the average age (year) and weight (kg) ± SD were 56± 19 years and 77±25 kg, respectively. Mean SCr (μmol/L)±SD was 1.4±1.4, and on average 964 mL± 706 mL of IV fluid was administered in the ED. Eighteen of 97 patients (19%) required vasopressors, and 32% (31/97) required an ICU admission. Mean LOHS (days)±SD was 9.2±10.4 days. Common infection sources included skin and soft tissue (25/97; 26%), urinary infection (19/97; 20%), health care-associated lung infection (18/97; 19%), and community-acquired lung infection (17/97; 18%). There were no statistically significant demographic or clinical differences between comparison groups.
Table 1 describes the antimicrobials used in 97 empirical regimens. In total, 165 antimicrobials were administered. Among the most commonly utilized were vancomycin (41/165; 25%), third- or fourth-generation cephalosporins (39/165; 24%), and piperacillin–tazobactam (38/165; 23%). Most patients received more than one antimicrobial (59/97; 61%), while 6.2% (6/97) received more than two antimicrobials. Premix antimicrobials were used 55% of the time (91/165), and 62% (60/97) of the empirical regimens contained at least one or more non-premixed antimicrobial. Premixed antimicrobials were commonly the first- (58/97; 50%) and second-administered antimicrobials (31/59; 53%). Non-premixed antimicrobials were more commonly administered third (4/6; 67%). The first antimicrobial was administered within 1 and 3 hours of physician order entry for 75% (73/97) and 97% (94/97) of patients, respectively. The final antimicrobial of the patient’s empirical regimen was administered within 1 and 3 hours of physician order entry in 49% (47/97) and 84% (81/97) of patients, respectively.
Table 1 Antimicrobials administered in the ED

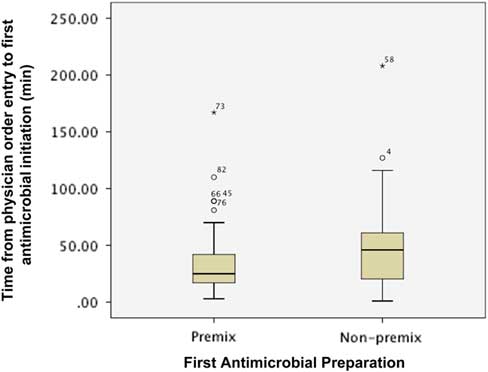
Figure 1 describes the impact of preparation on the time from order entry until first antimicrobial administration. The first antimicrobial was initiated sooner when available as a PMA preparation (median (IQR): premix 25 minutes (16.5-42.3) vs. non-premix 46 minutes (20-102, p=0.027). However, the proportion of patients with the first antimicrobial initiated within 1 (premix 79.3% vs. non-premix 69.2%, p=0.338) and 3 hours (premix 98.3% vs. non-premix 94.9%, p=0.563) of physician order entry was similar between groups.
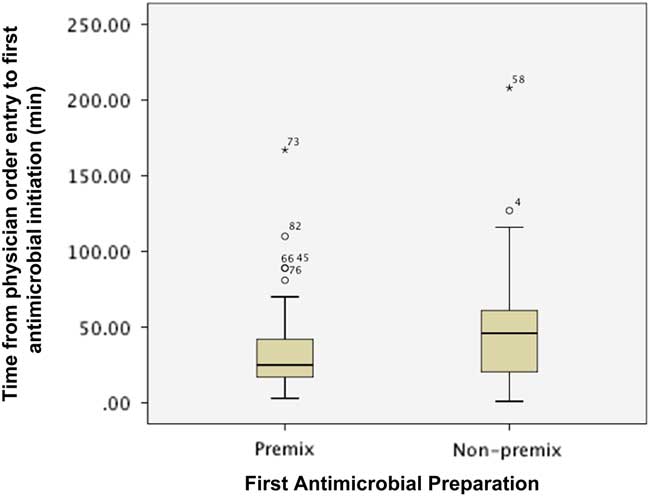
Figure 1 Impact of preparation on time from physician order entry to administration of first antimicrobial agent. * indicates the extreme value.
Figure 2 describes the impact of preparation on the time from order entry until administration of the final antimicrobial agent of an empirical regimen. The final antimicrobial agent was administered at a similar time for empirical regimens containing at least one or more antimicrobial agents, regardless of whether the drug was available as a premix or non-premix preparation (median (IQR): premix 69 min (21-115) vs. non-premix 65 min (38.5-133.8); p=0.455). The proportion of patients with their final antimicrobial agent administered within 1 (premix 48.6% vs. non-premix 48.3%, p=1.000) and 3 hours (premix 86.5% vs. non-premix 81.6%, p=0.587) of physician order entry was similar between groups.
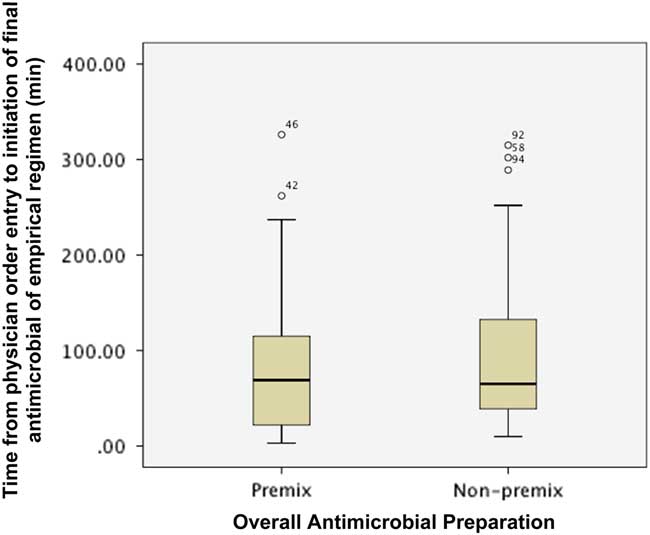
Figure 2 Impact of preparation on time from physician order entry to administration of final antimicrobial of an empirical regimen.
DISCUSSION
The Surviving Sepsis Campaign guidelines recommend administration of effective antimicrobials within the first hour of recognition of severe sepsis (grade 1C) and septic shock (grade 1B).Reference Dellinger, Levy and Rhodes 6 This recommendation is mostly based on a retrospective study that showed that each 1-hour delay in the administration of effective antimicrobial therapy was associated with a 7.6% increase in mortality in septic shock.Reference Kumar, Roberts and Wood 7 An antimicrobial was considered effective if it had in-vitro activity against the causative pathogen. More recent information also suggests that delays in antimicrobial administration also increases mortality in sepsis and severe sepsis. Liu and colleaguesReference Liu, Fielding-Singh and Greene 33 performed a retrospective study in a cohort of more than 35,000 patients with sepsis, severe sepsis, and septic shock. Each hour delay in antibiotic administration was associated with an 11% increased risk for in-hospital mortality risk with severe sepsis (OR 1.08; 95% CI 1.02-1.14; p=0.01). Sisk and colleaguesReference Sisk, Kashiouris and Pedram 34 performed a retrospective case-control study of ED patients with sepsis and noted that early antibiotic administration was associated with lower 28-day mortality (17.1 vs. 22.8%, p=0.01).
The delivery of effective antimicrobials in sepsis, severe sepsis, and septic shock is complicated by the uncertainty of the causative organism at the time of recognition. In these situations, clinicians must select antimicrobials with activity against common organisms and organisms with high mortality risk.Reference Dellinger, Levy and Rhodes 6 Ani and colleagues performed a retrospective analysis of more than 5,000,000 cases of severe sepsis.Reference Ani, Farshidpanah, Bellinghausen stewart and Nguyen 35 Gram-negative bacteria were the most common causative organism (51.5% of cases), and resistant gram-negative organisms such as Pseudomonas species (17.6% of gram-negative cases) were frequently observed. Gram-positive infections were not uncommon, representing 45.6% of cases, and methicillin-resistant Staphylococcus aureus (MRSA) was associated with an increased mortality risk (HR 1.38, 95% CI 1.33-1.44). Unfortunately, no single antimicrobial has activity against all of these organisms, and multiple antimicrobials are frequently required to provide the highest chance for effective treatment. The administration of multiple antimicrobials can be logistically difficult, and administration delays can occur. To enhance the likelihood that antimicrobials are infused promptly, the Surviving Sepsis Campaign guidelines recommend using PMA preparations.Reference Dellinger, Levy and Rhodes 6 This recommendation is logical, as non-PMA preparations require nursing or pharmacy staff to compound the antimicrobial agent, which has the potential to cause treatment delays. However, the impact of PMA preparations on administration times in sepsis has not been previously assessed.
To the best of our knowledge, our study is the first to address PMA preparation as an independent factor in antimicrobial administration time for septic patients in the ED. PMA preparations were associated with a significant reduction in time to administration for the first antimicrobial agent. Though the proportion receiving their first antibiotic within an hour was not statistically greater with PMA preparations, this was likely a function of sample size, as the absolute difference between groups was approximately 10%. The clinical significance of this observation is uncertain. Considering the variance in bacterial aetiology in sepsis, severe sepsis, and septic shock, time to administration of appropriate empirical antimicrobials is likely a better assessment of initial management, and many patients will require prompt administration of several antibiotics to achieve empirically appropriate treatment. PMAs were not associated with more rapid administration of appropriate empirical treatment (median (IQR): premix 69 minutes (21-115) vs. non-premix 65 minutes (38.5-133.8); p=0.455) or more frequent administration of appropriate empirical treatment within 1 (premix 48.6% vs. non-premix 48.3%, p=1.000) and 3 hours (premix 86.5% vs. non-premix 81.6%, p=0.587) of physician order entry.
Like many others, our institution uses vancomycin and piperacillin–tazobactam as the primary antimicrobial agents for the empiric treatment of sepsis, severe sepsis, and septic shock, especially when the source is unknown initially. Both of these antimicrobials are available as premix preparations and are stored in all ADCs, including in the ED. These PMAs have some limitations that potentially constrain their utility in the management of sepsis, severe sepsis, and septic shock at our institution, most notably that there are conflicting reports concerning y-site compatibility as well as recent changes in our local piperacillin–tazobactam susceptibility patterns. We recognize these limitations but elected not to change our current practice because we do not carry a premix alternative for piperacillin–tazobactam. While there may be other contributions to administration delays of antimicrobial agents, our study primarily focuses on the impact of PMAs as one aspect of antimicrobial administration in this critically ill patient population.
LIMITATIONS
Our study has several important limitations to consider. First, it was performed at a single-center ED and may therefore not be generalizable to other institutions or settings. Other institutions may not have access to PMA preparations in their ADCs for ease of administration and may have different ED protocols. Second, our retrospective observational study design and relatively small sample size without an a-priori power analysis limited our ability to determine a causal relationship between PMA preparation administration time and clinical outcomes such as mortality. Lastly, we primarily focused on one practical element, antimicrobial preparation, that may impact antimicrobial administration time. We did not specifically characterize each patient’s severity of illness using a severity-of-illness scale. However, the frequency of vasopressor use and ICU admission were similar whether PMAs or non-PMAs were given, potentially indicating a similar illness severity. Finally, there may be several other factors that may delay antimicrobial administration in septic patients that were not accounted for in our study.
CONCLUSIONS
Our study aimed to address whether PMA preparations decreased administration time in septic patients. We found that PMA preparations significantly reduced time to administration of the first antimicrobial agent for septic patients treated in the ED, but time to administration of subsequent antimicrobials was not improved. Strategies, including the availability of PMAs in ADCs in the ED, coupled with other schematics, may further reduce the time to antimicrobial administration in septic patients in the ED. Further research over a longer period of time in various settings is needed to quantify the impact of PMA preparations on antimicrobial administration time and clinical outcomes such as mortality.
Acknowledgements. To the pharmacy staff and Emergency Department at Upstate University Hospital for their gracious assistance and support in the operations of this project.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit https://doi.org/10.1017/cem.2017.33