Introduction
The late Ediacaran Period witnessed the rise and fall of the Ediacaran macrobiota (~574–539 Ma; Linnemann et al., Reference Linnemann2019; Matthews et al., Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2021), a group of macro-organisms that are complex and soft-bodied. Although some of the Ediacara-type macro-organisms are very likely metazoans (Fedonkin and Waggoner, Reference Fedonkin and Waggoner1997; Bobrovskiy et al., Reference Bobrovskiy, Hope, Ivantsov, Nettersheim, Hallmann and Brocks2018; Chen et al., Reference Chen, Zhou, Yuan and Xiao2019), most of them remain phylogenetically unresolved. The Ediacaran macrobiota consists of three assemblages that may broadly represent three stages of evolution (Waggoner, Reference Waggoner2003), although environmental conditions may have also played a role in their spatio-temporal distribution (Gehling and Droser, Reference Gehling and Droser2013; Grazhdankin, Reference Grazhdankin2014). These stages are exemplified by the Avalon (~574–560 Ma), White Sea (~560–550 Ma), and Nama (~550–539 Ma) assemblages (Waggoner, Reference Waggoner2003; Boag et al., Reference Boag, Darroch and Laflamme2016). Overall, the three assemblages have distinct taxonomic groupings and representative taxa, although a small number of taxa, for example, the frondose fossil Arborea, are known to occur in all three assemblages (Xiao and Laflamme, Reference Xiao and Laflamme2009; Droser et al., Reference Droser, Tarhan and Gehling2017). Like Arborea, the rangeomorph fossil Charnia is also previously known from the Avalon (Hofmann et al., Reference Hofmann, O'Brien and King2008; Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014; Liu et al., Reference Liu, Kenchington and Mitchell2015; Wilby et al., Reference Wilby, Kenchington and Wilby2015), White Sea (Martin et al., Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000; Gehling and Droser, Reference Gehling and Droser2013), and Nama assemblages (Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008), therefore representing one of the longest-ranging genera of Ediacara-type macrofossils in terms of stratigraphic distribution.
Charnia was first described from the Ediacaran Charnian Supergroup in England (Ford, Reference Ford1958). It is perhaps one of the most studied taxa of the Ediacara-type macro-organisms (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Antcliffe and Brasier, Reference Antcliffe and Brasier2008; Dunn et al., Reference Dunn, Liu and Donoghue2018, Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021; Butterfield, Reference Butterfield2022). Different phylogenetic affinities for Charnia have been proposed since its discovery. It was originally described as an alga (Ford, Reference Ford1958) but later classified into the family Charniidae (Glaessner, Reference Glaessner, Moore, Robinson and Teichert1979), which may belong to the class Rangeomorpha of a proposed extinct phylum, the Petalonamae (Pflug, Reference Pflug1970, Reference Pflug1972; Jenkins, Reference Jenkins1985). Charnia and other rangeomorphs have been variously interpreted as pennatulacean cnidarians (Glaessner, Reference Glaessner1959. Reference Glaessner1984; Glaessner and Wade, Reference Glaessner and Wade1966; Gehling, Reference Gehling1991), lichens (Retallack, Reference Retallack1994), fungi (Peterson et al., Reference Peterson, Waggoner and Hagadorn2003), members of the extinct kingdom Vendobionta (Seilacher, Reference Seilacher1992), or members within the total-group Metazoa (e.g., Xiao and Laflamme, Reference Xiao and Laflamme2009; Dunn et al., Reference Dunn, Liu and Donoghue2018; Butterfield, Reference Butterfield2022). Although Charnia and modern sea pens both possess a similar leaf-like shape, ontogenetic analysis reveals that Charnia and modern sea pens may have opposite growth polarities (Antcliffe and Brasier, Reference Antcliffe and Brasier2007, Reference Antcliffe and Brasier2008). Recently, detailed morphological descriptions and functional analyses, coupled with cladistic investigations (Dececchi et al., Reference Dececchi, Narbonne, Greentree and Laflamme2017, Reference Dececchi, Narbonne, Greentree and Laflamme2018), have led to the phylogenetic interpretations of rangeomorphs as stem-group metazoans (Xiao and Laflamme, Reference Xiao and Laflamme2009; Budd and Jensen, Reference Budd and Jensen2017; Darroch et al., Reference Darroch, Smith, Laflamme and Erwin2018; Dunn et al., Reference Dunn, Liu and Donoghue2018), stem-group eumetazoans (Dunn et al., Reference Dunn, Liu and Donoghue2018, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021; Hoyal Cuthill and Han, Reference Hoyal Cuthill and Han2018; Butterfield, Reference Butterfield2022; Runnegar, Reference Runnegar2022), or stem-group cnidarians (Dunn et al., Reference Dunn, Liu and Donoghue2018; Butterfield, Reference Butterfield2022). The feeding strategy of rangeomorphs also remains debated. Several studies have suggested that rangeomorphs, as well as erniettomorphs and dickinsoniomorphs, may have been capable of osmotrophy due to their high surface area to volume (SA/V) ratios (Laflamme et al., Reference Laflamme, Xiao and Kowalewski2009; Sperling and Vinther, Reference Sperling and Vinther2010; Ghisalberti et al., Reference Ghisalberti, Gold, Laflamme, Clapham, Narbonne, Summons, Johnston and Jacobs2014). Liu et al. (Reference Liu, Kenchington and Mitchell2015) argued that osmotrophy in rangeomorphs may have been limited by the availability and recalcitrant nature of dissolved organic carbon. In addition, Butterfield (Reference Butterfield2022) contended that the large size, hence elevated Reynolds and Péclet numbers, of rangeomorphs are not conducive for osmotrophy. Alternative feeding strategies of rangeomorphs include suspension feeding (Butterfield, Reference Butterfield2022) and intracellular symbiosis (Dufour and McIlroy, Reference Dufour and McIlroy2016; McIlroy et al., Reference McIlroy, Dufour, Taylor and Nicholls2021), but these hypotheses have not been critically evaluated on the basis of detailed morphological observation and theoretical modeling.
The occurrence of Charnia in the Shibantan biota was first reported by S. Xiao et al. (Reference Xiao, Chen, Pang, Zhou and Yuan2020), but a detailed description and morphometric assessment were not presented. Here we provide a systematic description of Charnia fossils, including the type species C. masoni Ford, Reference Ford1958 emend. and C. gracilis n. sp., from this Lagerstätte. The fossils are preserved in thin-bedded bituminous limestone, which was deposited in subtidal environment (Q. Xiao et al., Reference Xiao, She, Wang, Li, Ouyang, Cao, Mason and Du2020), from the terminal Ediacaran Shibantan Member of the Dengying Formation in the Yangtze Gorges area of South China. The Shibantan Member is geochronologically constrained to between ~550 Ma and ~543 Ma (Huang et al., Reference Huang, Chen, Ding, Zhou and Zhang2020; Yang et al., Reference Yang, Rooney, Condon, Li, Grazhdankin, Bowyer, Hu, Macdonald and Zhu2021). Thus, these Shibantan specimens not only extend the paleogeographic and paleoenvironmental distributions of Charnia, but also represent one of the youngest fossil records of this genus. They help us to obtain a more comprehensive picture of the rise and fall of the Ediacara-type macro-organisms.
Materials and methods
The Ediacaran succession in the Yangtze Gorges area of South China consists of the Doushantuo and Dengying formations (Fig. 1.1). The Dengying Formation consists of three lithostratigraphic members: the Hamajing, Shibantan, and Baimatuo members, in ascending order (Fig. 1.2). It is constrained by two CA-ID-TIMS zircon U–Pb ages of 551.1 ± 0.7 Ma (Condon et al., Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005) and 550.1 ± 0.6 Ma (Yang et al., Reference Yang, Rooney, Condon, Li, Grazhdankin, Bowyer, Hu, Macdonald and Zhu2021) from the uppermost Miaohe Member, which is regarded as the uppermost Doushantuo Formation (Xiao et al., Reference Xiao, Bykova, Kovalick and Gill2017; Zhou et al., Reference Zhou, Xiao, Wang, Guan, Ouyang and Chen2017; but see An et al., Reference An, Jiang, Tong, Tian, Ye, Song and Song2015), and a SIMS zircon U–Pb age of 543.4 ± 3.5 Ma from the Baimatuo Member (Huang et al., Reference Huang, Chen, Ding, Zhou and Zhang2020).
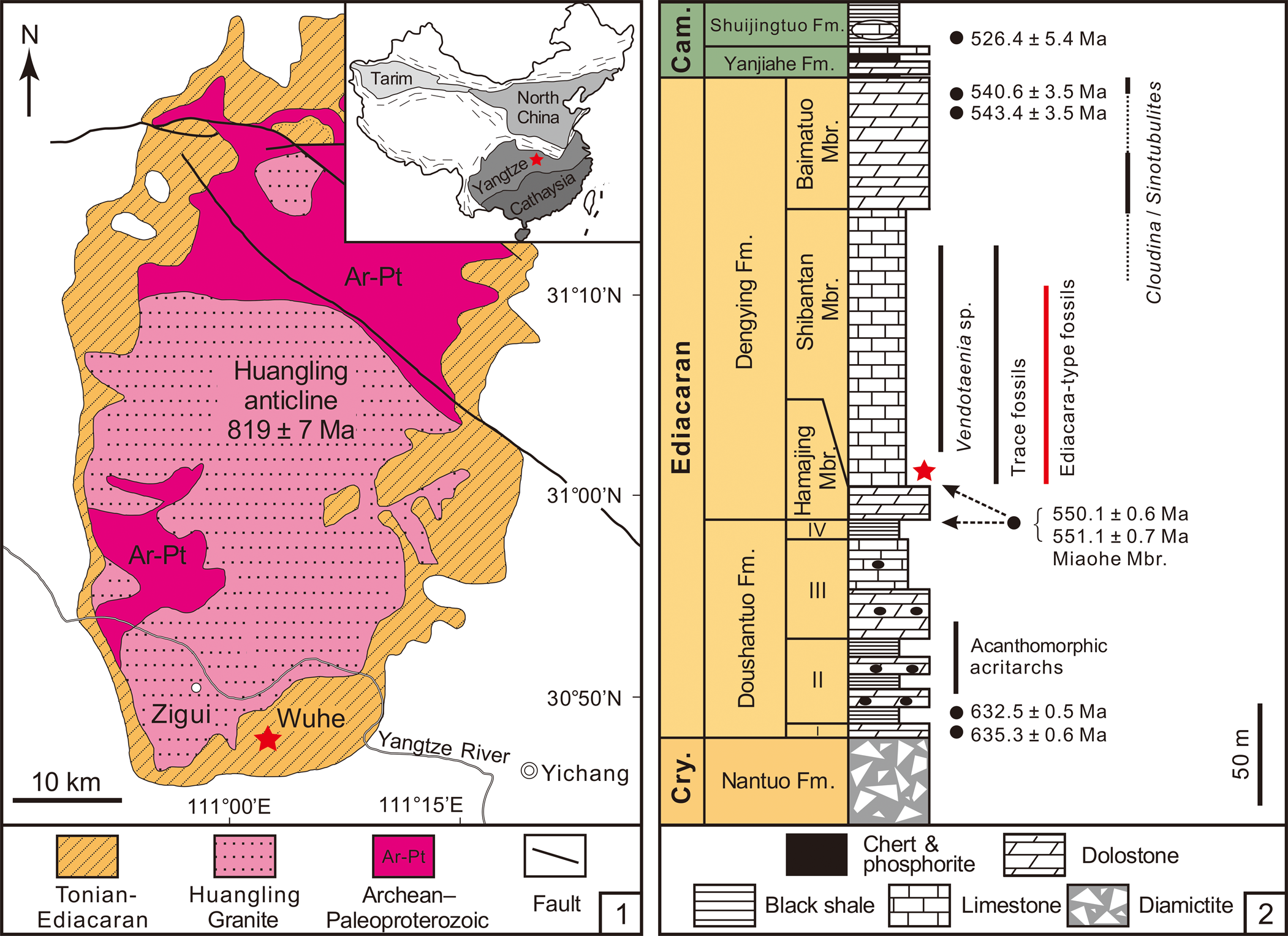
Figure 1. (1) Geological map of the Wuhe section in the Yangtze Gorges area with location of the Wuhe quarry (marked with a red star) and the Huangling anticline in the Yangtze Gorges area. Inset map shows the location of the Huangling anticline (red star) and major tectonic terranes in China. (2) Generalized stratigraphic column of the Ediacaran succession in the Yangtze Gorges area, South China, showing stratigraphic distribution of fossils and U–Pb radiometric ages. Star marks the stratigraphic occurrence of Charnia in the Shibantan limestone. Modified from S. Xiao et al. (Reference Xiao, Chen, Pang, Zhou and Yuan2020) and Wu et al. (Reference Wu, Chen, Pang, Wang, Wan, Zhou and Yuan2021). Geochronometric data sources: Condon et al. (Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005) and Yang et al. (Reference Yang, Rooney, Condon, Li, Grazhdankin, Bowyer, Hu, Macdonald and Zhu2021) for the lower Doushantuo Formation and Miaohe Member; Huang et al. (Reference Huang, Chen, Ding, Zhou and Zhang2020) and Zhang et al. (Reference Zhang, Chang, Chen, Wang, Feng, Steiner, Yang, Mason, She and Yan2022) for the Baimatuo Member; Okada et al. (Reference Okada, Sawaki, Komiya, Hirata, Takahata, Sano, Han and Maruyama2014) for the Cambrian Shuijingtuo Formation. Cry. = Cryogenian; Fm. = Formation; Mbr. = Member; Cam. = Cambrian.
The Hamajing and Baimatuo members are both characterized by dolostones with peritidal structures, such as tepees, karstification structures, and dissolution vugs (Duda et al., Reference Duda, Zhu and Reitner2016; Y. Ding et al., Reference Ding, Chen, Zhou, Guo, Huang and Zhang2019; S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020). The Shibantan Member consists of 100–150 m dark gray, medium- to thin-bedded bituminous limestone with diagenetic chert nodules and bands (Chen et al., Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014; S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020). Fine and crinkled laminae are dominant in the Shibantan Member, but hummocky cross-beds, rip-up clasts, intraclastic breccias, and graded beds are also common. Indeed, intraclastic breccias and graded beds in the Shibantan Member are interpreted as tempestites that were laid down in a subtidal environment between fair-weather and storm-wave bases (Q. Xiao et al., Reference Xiao, She, Wang, Li, Ouyang, Cao, Mason and Du2020). These tempestites may have contributed to the rapid burial of the soft-bodied Ediacara-type macrofossils (Q. Xiao et al., Reference Xiao, She, Wang, Li, Ouyang, Cao, Mason and Du2020). The wrinkled surfaces and dark crinkled micro-laminae in the Shibantan Member, interpreted as evidence for microbial mats (Chen et al., Reference Chen, Zhou, Meyer, Xiang, Schiffbauer, Yuan and Xiao2013; Meyer et al., Reference Meyer, Xiao, Gill, Schiffbauer, Chen, Zhou and Yuan2014), may have played an important role in the preservation of the soft-bodied Ediacara-type macrofossils (Gehling, Reference Gehling1999; Callow and Brasier, Reference Callow and Brasier2009; Laflamme et al., Reference Laflamme, Schiffbauer, Narbonne and Briggs2011). These microbial mats, probably constructed mainly by cyanobacteria, have close associations with trace fossils from the Shibantan Member and may suggest that they provided oxygen oases as well as nutrients for the trace makers (Chen et al., Reference Chen, Zhou, Meyer, Xiang, Schiffbauer, Yuan and Xiao2013; Meyer et al., Reference Meyer, Xiao, Gill, Schiffbauer, Chen, Zhou and Yuan2014; W. Ding et al., Reference Ding, Dong, Sun, Ma, Xu, Yang, Peng, Zhou and Shen2019; Xiao et al., Reference Xiao, Chen, Zhou and Yuan2019).
The Charnia fossils described here were collected from the Shibantan limestone at the Wuhe quarry (30.789°N, 111.051°E; Fig. 1.1), where a number of diverse fossils are preserved (S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020). The Shibantan biota consists of algal fossils, Ediacara-type macrofossils, trace fossils, and some problematic fossils (S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020). Recently, biomineralizing tubular fossils have also been discovered in the Baimatuo Member (Liang et al., Reference Liang, Cai, Nolan and Xiao2020; Zhang et al., Reference Zhang, Chang, Chen, Wang, Feng, Steiner, Yang, Mason, She and Yan2022) and also likely in the upper Shibantan Member (Chen et al., Reference Chen, Zhou, Zhang, Wei and Zhang2016), but these tubular fossils may be younger than the soft-bodied Ediacara-type macrofossils from Wuhe quarry. There are several frondose genera among the Ediacara-type macrofossils of the Shibantan biota (S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020), including Arborea, Rangea, Pteridinium (Chen et al., Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014; Wang et al., Reference Wang, Pang, Chen, Wan, Xiao, Zhou and Yuan2020), and Charnia (herein). The Charnia specimens were collected from the basal Shibantan Member (Fig. 1.2) with known stratigraphic orientation (to determine whether fossils are preserved on the top or bottom bedding surface). Specimens were photographed with a Nikon D810 digital camera, and measurements of the specimens were carried out on these photos using ImageJ software. Specimens from Charnwood Forest in the United Kingdom (Wilby et al., Reference Wilby, Kenchington and Wilby2015; Dunn et al., Reference Dunn, Liu and Donoghue2018, Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021), Newfoundland in Canada (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Hofmann et al., Reference Hofmann, O'Brien and King2008; Liu et al., Reference Liu, McIlroy, Matthews and Brasier2013, Reference Liu, Kenchington and Mitchell2015; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019), Sekwi Brook in Northwest Canada (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014), the White Sea region and Olenek Uplift, Siberia in Russia (Sokolov and Fedonkin, Reference Sokolov and Fedonkin1984; Runnegar and Fedonkin, Reference Runnegar, Fedonkin, Schopf and Klein1992; Grazhdankin and Bronnikov, Reference Grazhdankin and Bronnikov1997; Martin et al., Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000; Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008), Flinders Ranges, South Australia (Glaessner and Wade, Reference Glaessner and Wade1966; Nedin and Jenkins, Reference Nedin and Jenkins1998; Gehling and Droser, Reference Gehling and Droser2013), and Oulongbuluke terrane in Northwest China (Pang et al., Reference Pang, Wu, Sun, Ouyang, Yuan, Shen, Lang, Wang, Chen and Zhou2021) were also measured for comparison (Table 1). Measurements on Newfoundland specimens were conducted on retrodeformed photos from cited sources to account for tectonic deformation of the sediments.
Table 1. Biometric data for morphology comparison of Charnia specimens from the Shibantan biota and other localities.
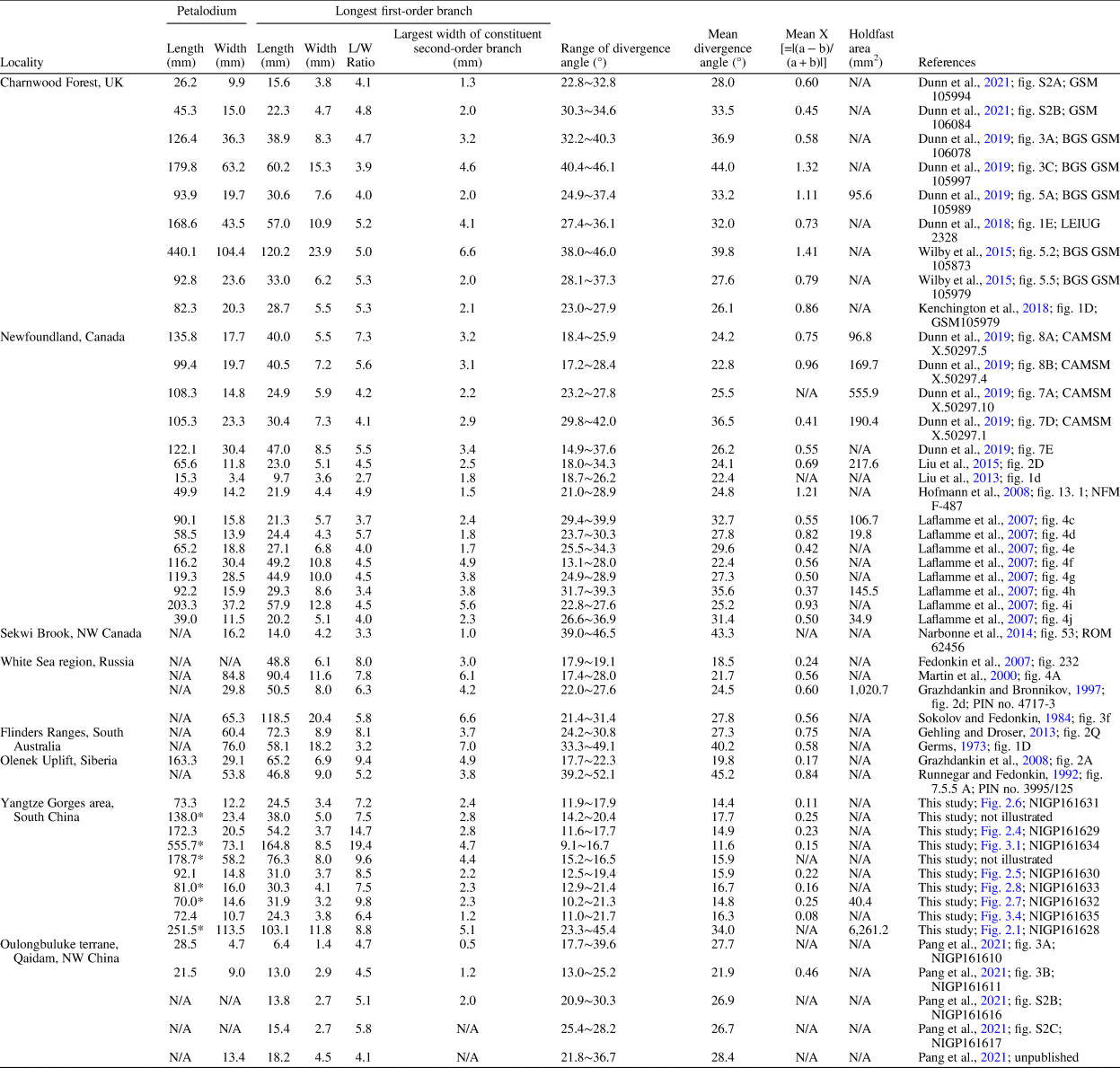
*Incomplete petalodium.
Repository and institutional abbreviation
Specimens of Charnia illustrated in this study are reposited in Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (NIGPAS), with NIGPAS museum catalog numbers (prefix NIGP-) provided for each specimen.
Systematic paleontology
Genus Charnia Ford, Reference Ford1958, emended Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019
Type species
Charnia masoni Ford, Reference Ford1958
Charnia masoni Ford, Reference Ford1958, emended
Figure 2.1, 2.2
- Reference Ford1958
Charnia masoni Ford, p. 212, pl. 13, fig. 1.
- ?Reference Glaessner1959
Charnia sp.; Glaessner, p. 1472, text-fig. 1b.
- ?Reference Glaessner and Daily1959
Rangea?; Glaessner in Glaessner and Daily, p. 387, pl. 46, fig. 2.
- Reference Glaessner1961
Charnia sp.; Glaessner, p. 75, text-fig.
- Reference Glaessner1962
Charnia sp.; Glaessner, p. 484, pl. 1, fig. 4 (non fig. 5).
- Reference Ford1962
Charnia masoni; Ford, fig. 4 (non fig. 5).
- Reference Glaessner and Wade1966
Rangea grandis; Glaessner and Wade, p. 616, pl. 100, fig. 5.
- Reference Germs1973
Glaessnerina grandis; Germs, p. 5, fig. 1D.
- Reference Sokolov1976
Charnia ex gr. masoni; Sokolov, p. 141, text-fig.
- Reference Sokolov1977
Charnia ex gr. masoni; Sokolov, p. 441.
- Reference Fedonkin1978
Charnia sp.; Fedonkin, fig. 3 (9).
- Reference Glaessner, Moore, Robinson and Teichert1979
Charnia masoni; Glaessner, p. A99, fig. 12 (3).
- Reference Fedonkin1981a
Charnia masoni; Fedonkin, p. 66, pl. 3, figs. 5, 6, pl. 29, fig. 1.
- Reference Fedonkin1981a
Zolotytsia biserialis; Fedonkin, p. 67, pl. 3, fig. 7.
- Reference Fedonkin1981b
Charnia masoni; Fedonkin, p. 100.
- Reference Sokolov and Brekhovskikh1981
Charnia masoni; Sokolov and Brekhovskikh, p. 3, text-fig.
- Reference Glaessner and Walter1981
Glaessnerina grandis; Glaessner and Walter, fig. 6.11C.
- Reference Fedonkin1983a
Charnia masoni; Fedonkin, pl. 1, fig. 1.
- Reference Fedonkin1983b
Charnia masoni; Fedonkin, fig. 37.
- Reference Sokolov and Fedonkin1983
Charnia masoni; Sokolov and Fedonkin, p. 13, fig. 9.
- Reference Sokolov1984
Charnia masoni; Sokolov, p. 6, text-fig.
- Reference Sokolov and Fedonkin1984
Charnia masoni; Sokolov and Fedonkin, fig. 3f.
- Reference Glaessner1984
Charnia cf. C. masoni; Glaessner, fig. 2.21B.
- Reference Glaessner1984
Charnia masoni; Glaessner, fig. 2.21A.
- Reference Glaessner1984
Glaessnerina grandis; Glaessner, fig. 2.21C.
- Reference Fedonkin, Sokolov and Iwanowski1985
Charnia masoni; Fedonkin, p. 99, pl. 12, fig. 4, pl. 13, figs. 2–4.
- Reference Jenkins1985
Charnia cf. C. masoni; Jenkins, fig. 7C.
- Reference Jenkins1985
Charnia masoni; Jenkins, fig. 7B.
- Reference Fedonkin1987
Charnia masoni; Fedonkin, pl. 15.
- Reference Preiss1987
Glaessnerina grandis; Preiss, fig. E.
- Reference Fedonkin, Sokolov and Iwanowski1990
Charnia masoni; Fedonkin, p. 110, pl. 12, fig. 4, pl. 13, figs. 2–4.
- Reference Fedonkin, Lipps and Signor1992
Charnia masoni; Fedonkin, figs. 28–30.
- Reference Runnegar, Fedonkin, Schopf and Klein1992
Charnia masoni; Runnegar and Fedonkin, fig. 7.5.5A, 7.5.10A.
- Reference Fedonkin and Bengtson1994
Charnia masoni; Fedonkin, fig. 2A, B.
- Reference Boynton and Ford1995
Charnia grandis; Boynton and Ford, p. 168, fig. 1.
- Reference Jenkins, Davies, Twidale and Tyler1996
Glaessnerina grandis; Jenkins, p. 35, fig. 4.1.
- Reference Grazhdankin and Bronnikov1997
Charnia masoni; Grazhdankin and Bronnikov, fig. 2a, d.
- Reference Ford1999
Charnia grandis; Ford, p. 231, figs. 1, 3.
- Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000
Charnia; Martin et al., fig. 4A.
- Reference Grazhdankin2004
Charnia; Grazhdankin, fig. 2.
- Reference Narbonne2004
Charnia-like frond; Narbonne, fig. 3D.
- Reference Narbonne, Dalrymple, Laflamme, Gehling and Boyce2005
Charnia masoni; Narbonne et al., pl. 1L.
- Reference Grazhdankin, Maslov, Mustill and Krupenin2005
Charnia; Grazhdankin et al., fig. 3d.
- Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007
Charnia masoni; Laflamme et al., p. 252, fig. 4A–J.
- Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Charnia cf. C. masoni; Fedonkin et al., fig. 276 (partim).
- Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Charnia cf. C. masoni; Fedonkin et al., figs. 304, 314 (partim).
- Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Charnia masoni; Fedonkin et al., p. 265, fig. 354.
- Reference Hofmann, O'Brien and King2008
Charnia masoni; Hofmann et al., p. 16, fig. 13.1, 13.2.
- Reference Hofmann, O'Brien and King2008
Charnia grandis?; Hofmann et al., p. 16, fig. 14.
- Reference Bamforth and Narbonne2009
Charnia masoni; Bamforth and Narbonne, fig. 7.5.
- Reference Wilby, Carney and Howe2011
Charnia masoni; Wilby et al., figs. 2A, 3A.
- Reference Grazhdankin, Reitner and Thiel2011
Charnia masoni; Grazhdankin, fig. 3a–d.
- Reference Liu, McIlroy, Matthews and Brasier2012
Charnia masoni; Liu et al., figs. 4b, 5a.
- Reference Liu, McIlroy, Matthews and Brasier2012
Charnia aff. C. masoni; Liu et al., fig. 4a.
- Reference Liu, McIlroy, Matthews and Brasier2013
Charnia aff. C. masoni; Liu et al., fig. 1d.
- Reference Liu, McIlroy, Matthews and Brasier2013
Charnia masoni; Liu et al., fig. 2a–d.
- Reference Gehling and Droser2013
Charnia sp.; Gehling and Droser, fig. 2Q.
- Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014
Charnia cf. C. masoni; Narbonne et al., p. 215, fig. 5.1–5.4.
- Reference Noble, Condon, Carney, Wilby, Pharaoh and Ford2015
Charnia masoni; Noble et al., fig. 4A, G.
- Reference Wilby, Kenchington and Wilby2015
Charnia masoni; Wilby et al., fig. 5.
- Reference Liu, Kenchington and Mitchell2015
Charnia masoni; Liu et al., fig. 2D.
- Reference Liu, Matthews and McIlroy2016
Charnia masoni; Liu et al., fig. 3D.
- Reference Antcliffe, Liu, Menon, McIlroy, McLoughlin and Wacey2017
Charnia masoni; Antcliffe et al., fig. 4E.
- Reference Dunn, Liu and Donoghue2018
Charnia masoni; Dunn et al., figs. 1E, 3.
- Reference Kenchington, Harris, Vixseboxse, Pickup and Wilby2018
Charnia masoni; Kenchington et al., figs. 1A, D, 7E (partim).
- Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019
Charnia masoni; Dunn et al., p. 16, figs. 1–3, 5–8, 10.
- Reference Liu and Dunn2020
Charnia masoni; Liu and Dunn, fig. 4d.
- Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2021
Charnia masoni; Matthews et al., fig. 2B.
- Reference McIlroy, Dufour, Taylor and Nicholls2021
Charnia masoni; McIlroy et al., fig. 1b.
- Reference Pang, Wu, Sun, Ouyang, Yuan, Shen, Lang, Wang, Chen and Zhou2021
Charnia masoni; Pang et al., figs. 3A–C, S2A–E.
- Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021
Charnia masoni; Dunn et al., figs. 1 (A-2), S1–S2.
- Reference Butterfield2022
Charnia masoni; Butterfield, figs. 1a, 2.
- Reference McIlroy, Hawco, McKean, Nicholls, Pasinetti and Taylor2022
Charnia masoni; McIlroy et al., fig. 10f.
Holotype
LEIUG 2328, from Bed B (Wilby et al., Reference Wilby, Carney and Howe2011), North Quarry, Charnwood Forest, UK.
Emended diagnosis
Charnia with ovate to parallel-sided petalodium consisting of sigmoidal first-order branches emanating alternately at an acute angle, typically >20°. First-order branches composed of series of near-rectangular second-order branches arranged acutely to almost perpendicularly to the first-order branches.
Occurrence
Shibantan Member, Dengying Formation, Yangtze Gorges area, South China (S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020); Bradgate Formation, Charnian Subgroup, Charnwood Forest, UK (Wilby et al., Reference Wilby, Carney and Howe2011); Drook, Briscal, Mistaken Point, Trepassey, and Fermeuse formations, Newfoundland, Canada (Narbonne, Reference Narbonne2004; Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Hofmann et al., Reference Hofmann, O'Brien and King2008; Liu et al., Reference Liu, McIlroy, Matthews and Brasier2012; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019; Matthews et al., Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2021); Nadaleen Formation (previously known as “June Beds,” Moynihan et al., Reference Moynihan, Strauss, Nelson and Padget2019), northwestern Canada (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014); Khatyspyt Formation, Olenek Uplift, north-central Siberia, Russia (Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008); Verkhovka Formation, the White Sea region, Russia (Martin et al., Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000); Rawnsley Quartzite, Flinders Ranges, South Australia (Gehling and Droser, Reference Gehling and Droser2013).
Description
Only one specimen in our collections can be assigned to this species. The specimen is characterized by a uniterminal bifoliate frond comprising an incompletely preserved ovate petalodium connected to a discoidal holdfast at the basal end by a stem (Fig. 2.1). The incomplete petalodium is 113.5 mm wide, and its preserved length is 251.5 mm. Petalodium is composed of strongly constrained (sensu Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) first-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) or primary branches, which emanate alternately on each side of the zig-zag central axis at an angle of 23.3–45.4° (mean = 34.0°). The longest first-order branch is 103.1 mm long and 11.8 mm wide. First-order branches are inclined toward the apex of the frond and are more or less sigmoidal in shape. First-order branches at the apical end of the petalodium are poorly preserved but seem to be shorter than those at the proximal end. First-order branches appear to be rotated and furled (sensu Brasier et al., Reference Brasier, Antcliffe and Liu2012) or single-sided (sensu Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009). First-order branches are composed of about a dozen rectangular to near-rectangular second-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) or secondary branches. Second-order branches in the longest first-order branch of each specimen are 4.3–5.1 mm (mean = 4.7 mm, n = 5) wide and arranged perpendicularly to the first-order branch. Third-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) are not observed. The stem is twisted and 158.8 mm long (measured from the base of petalodium to the center of the holdfast). The holdfast is discoidal, 87.7 mm in diameter, with a few filiform-texture structures in the middle (Fig. 2.2).
Materials
One specimen (NIGP161628) from Shibantan Member, Dengying Formation at Wuhe quarry.
Remarks
Three species of the genus Charnia—Charnia grandis Glaessner and Wade, Reference Glaessner and Wade1966, Charnia wardi Narbonne and Gehling, Reference Narbonne and Gehling2003, and Charnia antecedens Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007—have previously been erected in addition to its type species Charnia masoni Ford, Reference Ford1958. However, C. grandis is considered a junior synonym of C. masoni (Wilby et al., Reference Wilby, Carney and Howe2011; Brasier et al., Reference Brasier, Antcliffe and Liu2012), whereas C. wardi and C. antecedens were subsequently reassigned to Trepassia Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009 and Vinlandia Brasier et al., Reference Brasier, Antcliffe and Liu2012, respectively. This Shibantan specimen is tentatively classified in the genus Charnia due to its constrained and alternately arranged first-order branches as well as its single-sided rangeomorph units. The divergence angle of first-order branches of this specimen, 34.0° on average, is similar to that of the C. masoni specimens from other localities and much larger than that of the other Shibantan Charnia specimens (Table 1). First-order branches of this specimen are more curved than those of the other Shibantan Charnia specimens (Table 1). Its ovate petalodium and sigmoidal first-order branches also share similarities with other C. masoni specimens elsewhere. Taking all these factors into account, we choose to assign this specimen to C. masoni.
This specimen bears a twisted stem and a relatively large holdfast (Fig. 2.1), which are rare in previously reported Charnia specimens. The stem, together with the proximal end of the petalodium, seems to have been affected by water currents. The holdfast bears filiform textures in the middle (Fig. 2.2), somewhat different from Hiemalora-like holdfasts, which have radially arranged tentacle-like structures around the rim of the central disc (e.g., Chen et al., Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014, fig. 4; Shao et al., Reference Shao, Chen, Zhou and Yuan2019, fig. 2). The filiform textures could represent drag structures generated by uprooting of the holdfast (Tarhan et al., Reference Tarhan, Droser and Gehling2010), consistent with the twisting of the stem. However, there seem to be at least two sets of filiform textures that are perpendicular to each other, an observation not easily accounted for by uprooting. Alternatively, the filiform textures may be wrinkles resulting from the compression of an originally three-dimensional bulbous holdfast. They are also broadly similar to the radial and concentric bands and filamentous mesh present in an Ediacaria disc from the White Sea region, which was interpreted as possible “skeletal” structure by Luzhnaya and Ivantsov (Reference Luzhnaya and Ivantsov2019).
Charnia gracilis new species
Figures 2.3–2.8, 3
- ?Reference Sokolov1972a
Rangea sibirica; Sokolov, p. 50.
- ?Reference Sokolov and Sokolov1972b
Rangea sibirica; Sokolov, pl. 1, fig. 3.
- ?Reference Glaessner, Moore, Robinson and Teichert1979
Glaessnerina sibirica; Glaessner, p. A99, fig. 12 (1).
- ?Reference Glaessner1984
Glaessnerina sibirica; Glaessner, fig. 2.21D.
- ?Reference Nedin and Jenkins1998
Charnia masoni; Nedin and Jenkins, p. 315, fig. 1.
- Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007
Charnia sp.; Fedonkin et al., fig. 232 (partim).
- Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008
Charnia masoni; Grazhdankin et al., fig. 2A.
- Reference Grazhdankin2014
Charnia masoni; Grazhdankin, fig. 2.3.
- Reference Xiao, Chen, Pang, Zhou and Yuan2020
Charnia sp.; S. Xiao et al., fig. 4f.
Holotype
NIGP161629, from Shibantan Member, Dengying Formation, Yangtze Gorges area, South China, illustrated in Fig. 2.4.

Figure 2. (1) Charnia masoni from the Shibantan limestone preserved in positive relief, bed sole view; black arrow points to twisted stem and white arrow points to discoidal holdfast; (2) magnified view of rectangle in (1), with black and white rectangles marking two different orientations of filiform structures on the holdfast; NIGP161628. (3, 4) Holotype of C. gracilis n. sp. from the Shibantan limestone preserved in positive relief, bed sole view: (3) magnified view of rectangle in (4), showing well-preserved second-order branches and possible third-order branches (arrow); note the straight first-order branches with small divergence angles in (4); NIGP161629. (5) Specimen of C. gracilis n. sp. with complete petalodium, preserved in positive relief, bed sole view; arrowhead points to apex of petalodium; NIGP161630. (6) Specimen of C. gracilis n. sp. preserved in positive relief, bed sole view; NIGP161631. (7) Incomplete specimen of C. gracilis n. sp. preserved in positive relief, bed sole view; arrow points to a possible and faintly preserved holdfast; NIGP161632. (8) Incomplete specimen of C. gracilis n. sp. preserved in positive relief, bed sole view; NIGP161633. (1) Scale bar = 5 cm; (2) scale bar = 2 cm; (3–8) scale bars = 1 cm.
Diagnosis
A Charnia species characterized by a slender petalodium consisting of relatively long, thin, and straight first-order branches that have a parallel-sided blade-like shape. First-order branches emanate alternately from the central axis at an acute angle, typically ≤20°. First-order branches are composed of a series of rectangular or rhomboid second-order branches arranged acutely to perpendicularly to the first-order branches.
Occurrence
Shibantan Member, Dengying Formation, Yangtze Gorges area, South China; Khatyspyt Formation, Olenek Uplift, north-central Siberia, Russia (Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008); Verkhovka Formation, the White Sea region, Russia (Fedonkin et al., Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007); possible occurrence in Rawnsley Quartzite, Flinders Ranges, South Australia (Nedin and Jenkins, Reference Nedin and Jenkins1998).
Description
Specimens are characterized by a centimeter- to decimeter-scale, uniterminal bifoliate frond comprising a nearly parallel-sided and spicate petalodium tapering gradually at the apical end (Figs. 2.3–2.8, 3). There are four specimens in our collection that bear a completely preserved petalodium, which is 72.4–172.3 mm long (mean = 102.5 mm, n = 4) and 10.7–20.5 mm wide (mean = 14.6 mm, n = 4). Incomplete petalodia of five other specimens can be measured for maximum width, which varies from 14.6 mm to 73.1 mm (mean = 37.0 mm, n = 5), and their preserved length varies from 70.0 mm to 555.7 mm (mean = 204.7 mm, n = 5). Two additional specimens are too incompletely or poorly preserved to allow reliable measurements of petalodium width and length; thus, they are not included in the measurement data. The petalodium is composed of about a dozen strongly constrained (sensu Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) first-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). First-order branches emanate alternately on each side of the central axis at an angle of 9.1–21.7°, with the average divergence angle (11.6–17.7° for nine specimens) usually <20°. Opposing first-order branches are offset by half a branch width, forming a zig-zag central suture (e.g., Fig. 2.4). First-order branches are inclined toward the apex of the frond and are nearly straight, although they can be slightly curved at both proximal and distal ends, leading to a blade-like shape. Proximal first-order branches are usually more curved than distal ones. The longest first-order branch usually occurs near the middle of the petalodium, 24.3–164.8 mm long (mean = 52.8 mm, n = 9) and 3.3–8.5 mm wide (mean = 4.8 mm, n = 9). First-order branches are composed of about a dozen second-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) arranged parallel to one another. The widest second-order branches in the longest first-order branch are 1.2–4.7 mm (mean = 2.8 mm, n = 9) wide. Second-order branches vary in shape and orientation in specimens of different sizes. In smaller specimens (e.g., Fig. 2.3–2.8), second-order branches are generally near rectangular and arranged more or less perpendicularly to the first-order branch, whereas in larger specimens (e.g., Fig. 3.1, 3.2), second-order branches are rhomboidal and arranged more acutely to the first-order branch. The number of second-order branches in each first-order branch seems to remain more or less constant (Fig. 4.1), whereas the average size of second-order branches in each first-order branch increases by ~14 times as the length of the first-order branch increases ~eight times (Fig. 4.2). The shape of second-order branches can also vary from rectangular or rhomboidal in the central region to trigonal or trapezoidal in the proximal and distal regions of the first-order branch (e.g., Fig. 2.3). Rangeomorph units of both first-order branches and second-order branches are rotated and furled (sensu Brasier et al., Reference Brasier, Antcliffe and Liu2012) or single-sided (sensu Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009). Third-order branches (sensu Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021) are barely discernable in some second-order branches, characterized by obliquely arranged ridges (e.g., Figs. 2.3, 3.2). First-order and third-order branches are inclined toward the apex of the frond. A discoidal holdfast is faintly preserved in one specimen (Fig. 2.7), ~7.3 mm in diameter. A Charnia gracilis n. sp. specimen (Fig. 3.4) is preserved together with a Helminthoidichnites-like trace fossil on the same bedding surface (Fig. 3.3). A possible juvenile specimen of C. gracilis is in alignment with another poorly preserved, taxonomically unidentifiable frond (Fig. 3.5), indicating common orientation of tethered, erect epibenthic organisms by water currents.
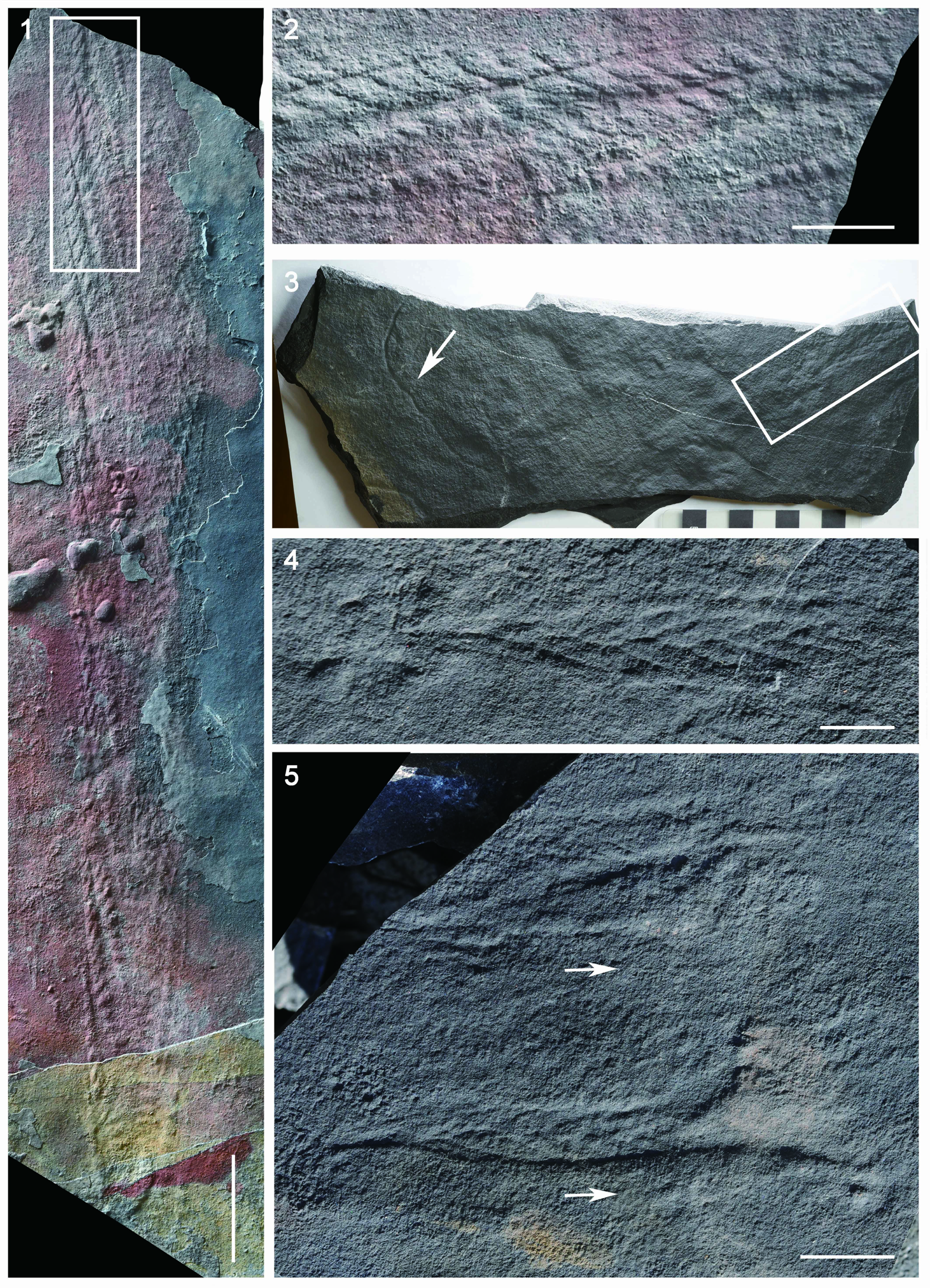
Figure 3. Charnia gracilis new species from the Shibantan limestone. (1, 2) The longest specimen of C. gracilis n. sp. in the Shibantan limestone with an incomplete petalodium and well-preserved second-order branches, positive relief, bed sole view; (2) magnified view of the rectangle in (1), showing rhomboidal second-order branches and inclined third-order branches; NIGP161634. (3, 4) A juvenile specimen of C. gracilis n. sp. marked by rectangle in (3), positive relief, bed sole view; the specimen is preserved together with a Helminthoidichnites-like trace fossil, marked by arrow in (3); (4) magnified view of rectangle in (3); NIGP161635. (5) A possible juvenile specimen of C. gracilis n. sp. (bottom) and another poorly preserved unnamed frond (upper) preserved in positive relief, bed sole view. Arrows point to inferred direction of water current that felled and aligned the specimens. NIGP161636. (1) Scale bar = 5 cm; (2, 5) scale bars = 2 cm; (3, 4) scale bars = 1 cm.
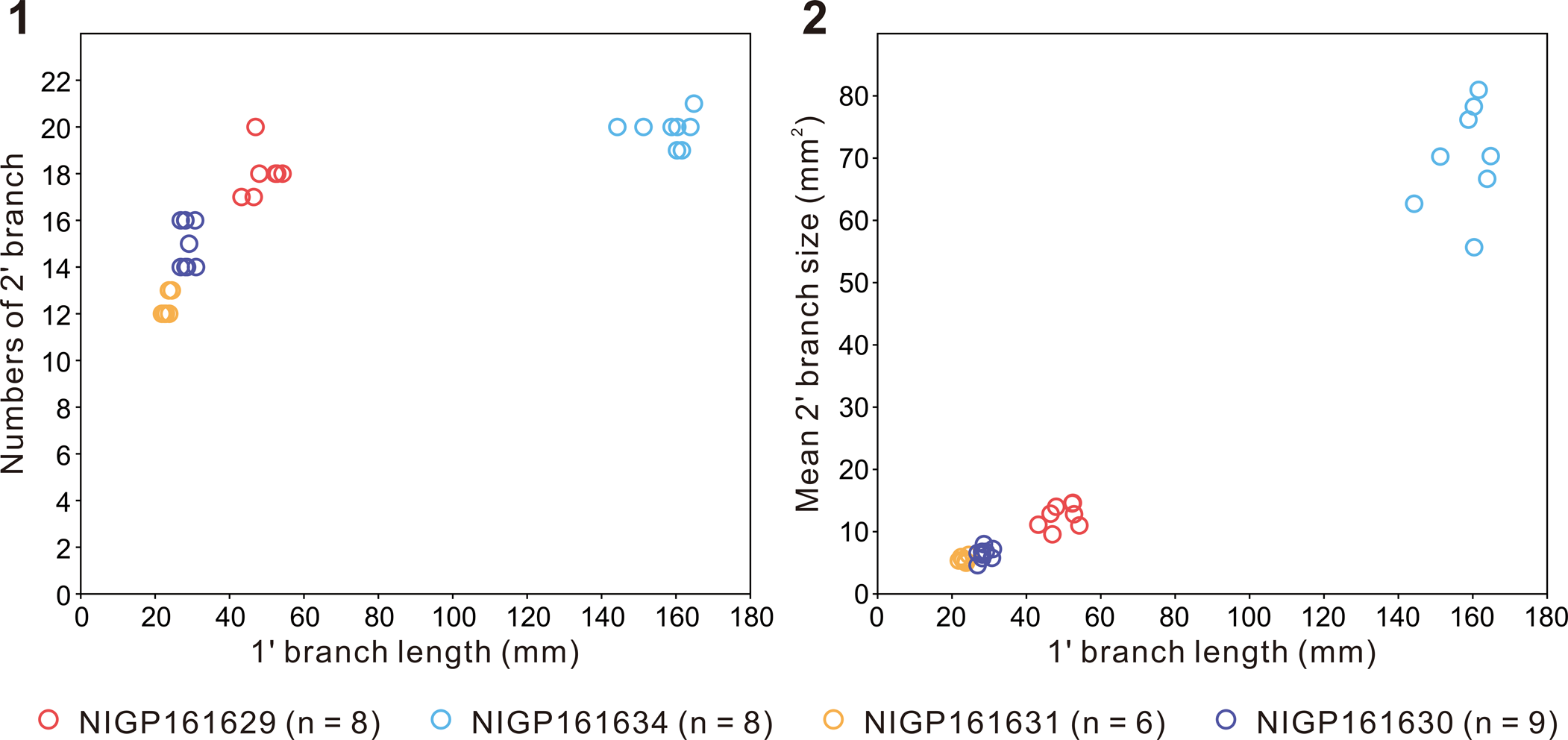
Figure 4. Measurements of several specimens of Charnia gracilis new species with well-preserved second-order branches (NIGP161629; NIGP161630; NIGP161631; NIGP161634). (1) Cross-plot of first-order branch (1' branch) length versus number of constituent second-order branches (2' branches); (2) Cross-plot of first-order branch length versus average size of constituent second-order branches. The number of second-order branches in each first-order branch is evaluated by dividing the length of the first-order branch by the average width of constituent second-order branches that are measurable.
Etymology
From gracilis (Latin, slender), in reference to the slender shape of the petalodium as well as the first-order branches.
Materials
Eleven specimens in total, from Shibantan Member, Dengying Formation at Wuhe quarry.
Remarks
The Shibantan specimens possess diagnostic features of the genus Charnia, including single-sided rangeomorph units, strongly constrained first-order branches, and a zig-zag midline. Biometric plots also show that these Shibantan specimens share similarities with Charnia specimens from other localities (Fig. 5; Table 1). For example, the petalodium length versus width (Fig. 5.1), as well as the width of the longest first-order branch versus the largest width of second-order branch in the longest first-order branch (Fig. 5.2), is similar between the Shibantan specimens and C. masoni from Charnwood Forest, UK (Wilby et al., Reference Wilby, Kenchington and Wilby2015; Dunn et al., Reference Dunn, Liu and Donoghue2018, Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021), C. masoni from Newfoundland, Canada (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Hofmann et al., Reference Hofmann, O'Brien and King2008; Liu et al., Reference Liu, McIlroy, Matthews and Brasier2013, Reference Liu, Kenchington and Mitchell2015), Charnia cf. C. masoni from Sekwi Brook, NW Canada (Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014), C. masoni and C. gracilis n. sp. from the White Sea region, Russia (Sokolov and Fedonkin, Reference Sokolov and Fedonkin1984; Grazhdankin and Bronnikov, Reference Grazhdankin and Bronnikov1997; Martin et al., Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000), C. masoni from Flinders Ranges, South Australia (Germs, Reference Germs1973; Gehling and Droser, Reference Gehling and Droser2013), C. masoni and C. gracilis from Olenek Uplift, Siberia (Runnegar and Fedonkin, Reference Runnegar, Fedonkin, Schopf and Klein1992; Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008), and C. masoni from Oulongbuluke terrane, NW China (Pang et al., Reference Pang, Wu, Sun, Ouyang, Yuan, Shen, Lang, Wang, Chen and Zhou2021). Thus, it is reasonable to assign the Shibantan specimens to the genus Charnia. However, there are several notable differences between the Shibantan specimens and C. masoni. Relative to C. masoni specimens from other localities, the Shibantan specimens have first-order branches that are slenderer, longer, and straighter, taper more gradually toward the distal end, have a higher length/width ratio (Fig. 5.3, 5.4), and present a blade-like rather than a sigmoidal shape (Fig. 5.5, 5.9). In addition, the first-order branches are straight and blade-like in the Shibantan specimens but sigmoidal in C. masoni. This difference can be quantified using a new morphologic descriptor defined as X = |(a − b)/(a + b)|, where “a” and “b” are the angles between the diagonal line and borderlines at the distal end of first-order branches (Fig. 5.9). It can be shown that the parameter X effectively distinguishes the sigmoidal (X ≥ 0.3 for C. masoni) or straight (0 ≤ X < 0.3 for C. gracilis) first-order branches (Fig. 5.5). Finally, the mean divergence angle of the first-order branches in the Shibantan specimens, ranging between 12° and 18°, is much lower than in C. masoni specimens elsewhere (Fig. 5.4, 5.5). Considering these morphological disparities as likely interspecific variations, we choose to erect a new species, and we use the mean divergence angle of the first-order branches to differentiate C. gracilis (≤20°) and C. masoni (>20°).
Grazhdankin et al. (Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008) reported Charnia masoni from the Khatyspyt Formation in Siberia (Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008, fig. 2A). Although incompletely preserved, this Khatyspyt specimen has straight and thin first-order branches with an average divergence angle of 19.8° (Fig. 5.4, 5.5; Table 1). This Khatyspyt specimen shares more morphological similarities with C. gracilis specimens from the Shibantan Member than typical C. masoni material; therefore, it is more appropriate to reassign this specimen to C. gracilis. An incompletely preserved Charnia sp. specimen from the White Sea region (Fedonkin et al., Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007, fig. 232) may also be considered C. gracilis. This incomplete specimen possesses a near-parallel-sided petalodium and relatively straight first-order branches with an average divergence angle of 18.5° (Fig. 5.4; Table 1). An incomplete Charnia specimen from Siberia (Glaessner, Reference Glaessner, Moore, Robinson and Teichert1979, fig. 12.1), which was assigned to Glaessnerina sibirica (Sokolov, Reference Sokolov1973), is similar to the Shibantan C. gracilis specimens in its thin, straight, and parallel-sided first-order branches. Its inclined second-order branches are similar to those of the longest specimen in Shibantan limestone (Fig. 3.1). However, the poor preservation of the middle and left parts of its petalodium makes measurement of the divergence angle of the first-order branches difficult. Therefore, this specimen can be only provisionally placed in C. gracilis. A Charnia specimen reported by Nedin and Jenkins (Reference Nedin and Jenkins1998) from the Rawnsley Quartzite, South Australia, also resembles the Shibantan C. gracilis specimens in its straight, slender, and acutely divergent (17.5–26.1°) first-order branches, but its mean divergence angle (21.7°; n = 5) is slightly larger than those of the latter. In general, the Charnia specimens from the White Sea and Nama assemblages can be related to or even reassigned to C. gracilis, on the basis of the morphology of first-order branches, which are more acutely diverged, less curved, and apically thinner and straighter than those from the Avalon assemblage (e.g., Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, fig. 1F). However, C. gracilis is also somewhat similar to some specimens from Newfoundland in their parallel-sided outlines of the petalodium (e.g., Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007, fig. 4f–h; Liu et al., Reference Liu, Kenchington and Mitchell2015, fig. 2D; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, fig. 8A), despite the fact that their first-order branches are different (Fig. 5; Table 1).
Dunn et al. (Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019) studied Charnia masoni specimens from different localities and found that they are comparable in morphology but hard to accord with the morphological reconstructions of some other rangeomorphs such as Avalofractus (Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) and Rangea (Vickers-Rich et al., Reference Vickers-Rich2013), which possess an internal central stalk (also refer to Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021, fig. 5). Some researchers envisioned that an internal stalk may also be present in Charnia (Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009), but subsequent studies by other researchers found no evidence for a central stalk (Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019; see also Dunn et al., Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021, fig. 5). The Shibantan C. gracilis specimens, which are similar in overall morphology to Charnia fossils reported elsewhere, show no sign of a central stalk.
Discussion
The differences between Charnia gracilis n. sp. and Charnia masoni lie mainly in their first-order branches, as revealed by biometric analysis of Charnia specimens worldwide (Fig. 5). The Shibantan specimens of C. gracilis show notable differences from Charnia specimens elsewhere, with a straight blade-like shape, lower divergence angles, and greater length/width ratios for their first-order branches (Fig. 5.4, 5.5). These differences are unlikely to be artifacts of tectonic or taphonomic deformation. Tectonic shearing can be ruled out because associated holdfast structures are perfectly circular in shape (Fig. 3.2). Although taphonomic deformation did occur in some Shibantan fossils (e.g., Dickinsonia, Wang et al., Reference Wang, Chen, Pang, Zhou, Xiao, Wan and Yuan2021; see also slightly C-shaped fronds in Fig. 3.5), the tightly constrained first-order branches (Narbonne et al., Reference Narbonne, Laflamme, Greentree and Trusler2009) in C. gracilis left little room for their postmortem dislocation, rotation, or deformation. Postmortem compression or stretching of the petalodium, which would increase the length/width ratio of the petalodium, straighten the first-order branches, and decrease their divergence angles, also seemed unlikely to have happened in such a uniform fashion simultaneously.
In addition to the shape of first-order branches of Charnia gracilis specimens, it is unlikely that their lower divergence angles result from alignment by strong currents. Some Shibantan Charnia specimens do show evidence of alignment (Fig. 3.5), perhaps by water currents, although there is no sedimentary evidence for strong water currents in the fossil-bearing horizons (Chen et al., Reference Chen, Zhou, Xiao, Wang, Guan, Hua and Yuan2014; Duda et al., Reference Duda, Zhu and Reitner2016). It is conceivable that the lower divergence angles of C. gracilis specimens may be a biostratinomic artifact related to deformation by strong currents, but this interpretation contradicts the observation that the Shibantan C. masoni specimen, which has a twisted stem and a wrinkled holdfast that may be caused by water currents (Fig. 2.1), has larger divergence angles than the Shibantan C. gracilis specimens (Figs. 2.4–2.8, 3.1). Some C. masoni specimens from Newfoundland (Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019, figs. 7, 8) exhibit a slender frond with parallel-sided margins, similar to the Shibantan C. gracilis specimens, and they possess a long connecting region, which has been interpreted as an artifact caused by twisting upon felling (Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019) that may also be affected by water currents. However, the divergence angles of these Newfoundland specimens are larger than 20°, and their first-order branches are sigmoidal in shape (Fig. 5.5), both of which are different from C. gracilis specimens.
The slender, straight, and blade-like shape, and the lower divergence angle, of first-order branches of Charnia gracilis are therefore considered a species-level taxonomic distinction. The more or less straight first-order branches of the Shibantan C. gracilis specimens lead to slightly jagged lateral margins and a sharp V-shaped apex of the petalodium (Fig. 2.4). The distal end of the first-order branches of Shibantan C. gracilis specimens is not distinctly curved (Fig. 2.4–2.8), in contrast to Charnia specimens elsewhere, indicating that the lateral margins of the petalodium in C. gracilis may have been somewhat unfurled and not morphologically influenced by water currents when alive. A petalodium with furled lateral margins would be better streamlined to reduce the drag of water currents, whereas one with unfurled lateral margins would fully expose the surface area of the frond to water currents, thus enhancing the feeding efficiency. However, considering that both C. gracilis and C. masoni are present in the Shibantan Member, and that there are relatively few specimens of either taxon, it is difficult to distinguish whether the Shibantan specimens are furled. It is also uncertain whether furling is a persistent and taxonomically informative character, an ecophenotypic behavior related to feeding strategies and water current intensity, or a biostratinomic feature.
Previous studies proposed that the growth of Charnia was achieved by the insertion of new first-order branches (e.g., Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Antcliffe and Brasier, Reference Antcliffe and Brasier2008; Laflamme and Narbonne, Reference Laflamme and Narbonne2008), but recent studies hypothesized that Charnia grew by the insertion of new first-order branches followed by their subsequent inflation (Wilby et al., Reference Wilby, Kenchington and Wilby2015; Dunn et al., Reference Dunn, Liu and Donoghue2018). Two small frondose fossils (Fig. 3.4, 3.5) from the Shibantan limestone are recognized as juvenile specimens of C. gracilis. Although poorly preserved, it seems that these juvenile specimens contain fewer first-order branches (~12; Fig. 3.4, 3.5) than the largest specimen in our collection (>26; Fig. 3.1), supporting insertion of new first-order branches as a key growth mechanism. However, the number of second-order branches (~12–20) in each first-order branch does not seem to change much among specimens of different sizes (Fig. 4.1), whereas the shape of the second-order branches varies from near rectangular in the small- and medium-sized specimens (e.g., Fig. 2.3, 2.4), to axially rhomboidal in large-sized specimens (e.g., Fig. 3.1, 3.2); their size also increases greatly (Fig. 4.2). Considering that first-order branches increase in number and size during growth (Fig. 5.6, 5.7) whereas second-order branches increase mainly in size rather than number in every first-order branch (Fig. 4), it seems that the inflation of first-order branches was achieved mainly by inflation of second-order branches rather than insertion of new second-order branches. These observations support the hypothesis that growth of Charnia was accomplished by the insertion and subsequent inflation of first-order branches (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Laflamme and Narbonne, Reference Laflamme and Narbonne2008; Wilby et al., Reference Wilby, Kenchington and Wilby2015; Dunn et al., Reference Dunn, Liu and Donoghue2018), which grew largely by inflation rather than insertion of second-order branches. Each first-order branch is basally initiated from the third to sixth second-order branches of the subtending first-order branch in the Shibantan specimens (e.g., Fig. 2.3), similar to those described by Dunn et al. (Reference Dunn, Liu, Grazhdankin, Vixseboxse, Flannery-Sutherland, Green, Harris, Wilby and Donoghue2021). The smaller first-order branches at the apical end of the petalodium (e.g., Fig. 3.4) suggest that new first-order branches were generated distally, in contrast to the long-considered analogs of sea pens (Antcliffe and Brasier, Reference Antcliffe and Brasier2007). The presence of a stem in the larger C. masoni specimen (Fig. 2.1) and the absence of a stem in the juvenile specimens of C. gracilis implies that the stem may have been absent in younger stages but emerged later in the adult stage of the Charnia frond. Therefore, there may have been another generative zone at the proximal end of the Charnia frond where the stem was generated, in addition to the apical growth zone where new first-order branches were inserted (Dunn et al., Reference Dunn, Liu and Donoghue2018).
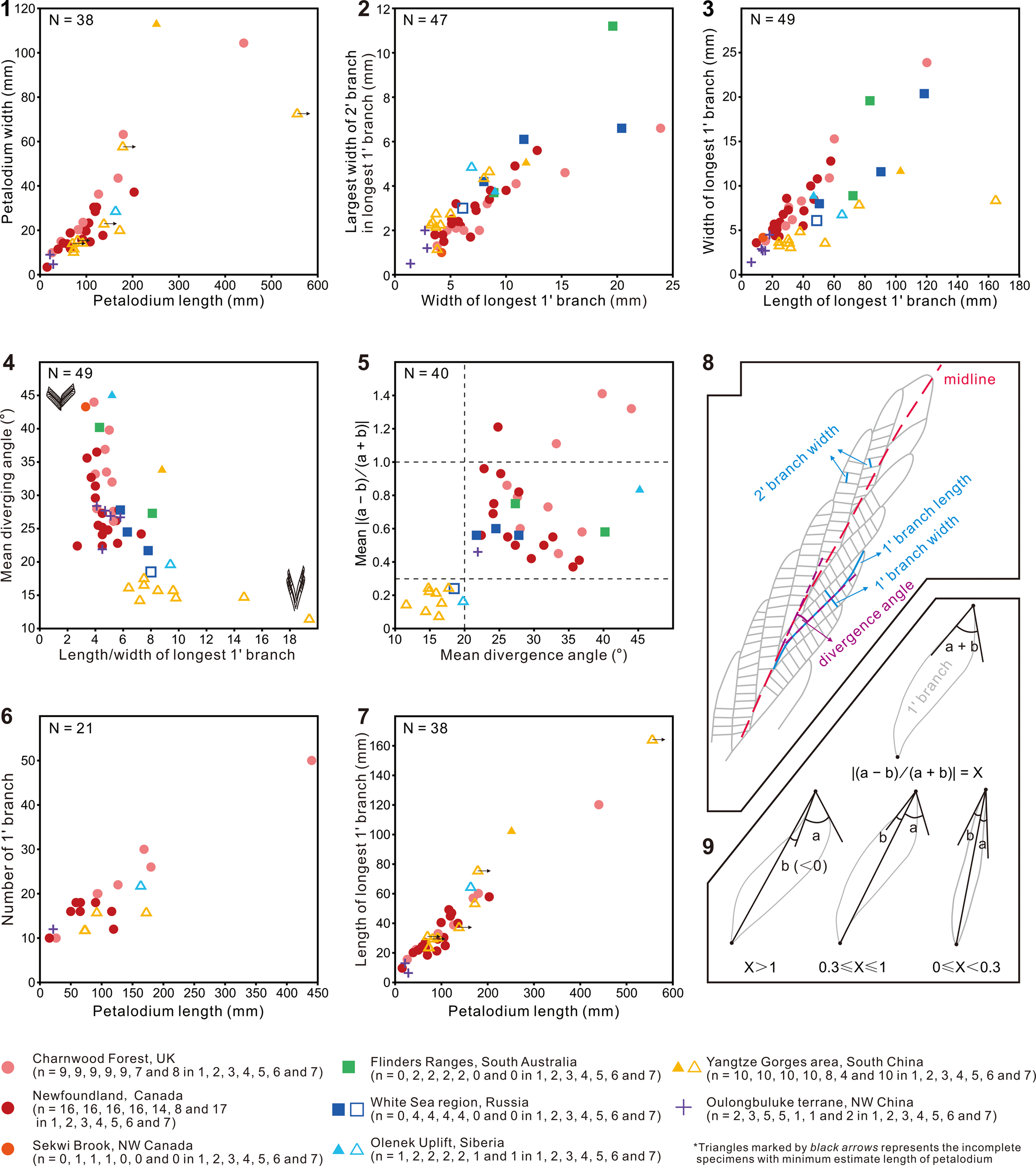
Figure 5. Biometric plots of Charnia from Shibantan limestone and other localities. (1) Cross-plot of petalodium length versus width. (2) Cross-plot of width of the longest first-order branch (1'branch) versus width of the widest second-order branch (2' branch) in the longest first-order branch. (3) Cross-plot of length versus width of the longest first-order branch. (4) Cross-plot of the length/width (L/W) ratio of the longest first-order branch versus mean divergence angle of first-order branches. Sketches at the lower right and upper left in (4) show first-order branches of two representative endmembers. (5) Cross-plot of mean divergence angle versus mean value of X = |(a − b)/(a + b)|; “a” and “b” represent the included angles between the diagonal line and borderlines at the distal end of first-order branches. (6) Cross-plot of petalodium length versus countable number of first-order branches. (7) Cross-plot of petalodium length versus length of the longest first-order branch. (8) Schematic diagram depicting how measurements for first- and second-order branches were made. (9) Schematic diagram depicting how measurements for X = |(a − b)/(a + b)| were made to distinguish the sigmoidal or straight shape of the first-order branches; note that in the lower left case, angle “b” < 0 and angle “a” > 0 (X > 1), whereas in the two other lower cases, both angle “b” and angle “a” are ≥0 (0 ≤ X ≤ 1). Localities are represented by symbols with different colors and shapes. Triangles with an attached arrow represent specimens with incomplete petalodium length in (1) and (7). Dots represent the Avalon assemblage, squares represent the White Sea assemblage, and triangles and crosses represent younger assemblages, possibly belonging to the Nama assemblage. Hollow symbols represent measurements of C. gracilis new species, whereas filled symbols represent measurements of other Charnia species.
Although Charnia is widely considered to have been an epibenthic organism, its posture on the seafloor has been debated. Some researchers consider it a reclining organism on the basis of the inference that most Charnia specimens preserve only one side of the frond, assuming that the two sides might be different (Grazhdankin, Reference Grazhdankin2004; McIlroy et al., Reference McIlroy, Dufour, Taylor and Nicholls2021). However, the twisted stem in a Shibantan C. masoni specimen (Fig. 2.1) implies the influence of water currents that rotated a standing frond, consistent with an erect living lifestyle (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Narbonne et al., Reference Narbonne, Laflamme, Trusler, Dalrymple and Greentree2014; Wilby et al., Reference Wilby, Kenchington and Wilby2015; Droser et al., Reference Droser, Tarhan and Gehling2017). The presence of a holdfast in some Charnia specimens (Fig. 2.1, 2.7) is also consistent with an erect lifestyle, although holdfasts are rarely preserved or observed. Some Charnia specimens from other localities preserve a small and bulbous holdfast (Laflamme et al., Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Wilby et al., Reference Wilby, Kenchington and Wilby2015; Dunn et al., Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2019), which has been taken as evidence that the holdfast was buried below the sediment–water interface (Burzynski and Narbonne, Reference Burzynski and Narbonne2015). One Shibantan C. gracilis specimen preserves a faint holdfast (Fig. 2.7), and another C. masoni specimen bears a distinctly larger holdfast with filiform texture (Fig. 2.1, 2.2; Table 1). It is possible that the larger holdfast illustrated in Figure 2.1 represents an uprooted specimen that was pulled out of the sediment by water currents; this interpretation is also consistent with the twisted stem in this specimen, which may have been caused by rotation of the frond relative to the holdfast because of water currents. Overall, the evidence available seems to suggest that Charnia stood rather than lay on the seafloor.
The occurrence of Charnia in the Shibantan biota expands the paleogeographic distribution of this taxon and represents one of the youngest examples of this genus. The Shibantan Member preserves taxa that were thought to be characteristic of the Nama assemblage (e.g., Cloudina; S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020) and White Sea assemblage (e.g., Dickinsonia; Wang et al., Reference Wang, Chen, Pang, Zhou, Xiao, Wan and Yuan2021). Previous researchers regarded the Shibantan biota as an example of the Nama assemblage (Boag et al., Reference Boag, Darroch and Laflamme2016; Muscente et al., Reference Muscente2019; Wu et al., Reference Wu, Chen, Pang, Wang, Wan, Zhou and Yuan2021), an example of the White Sea assemblage (Laflamme et al., Reference Laflamme, Gehling and Droser2018), or a transition between these two assemblages (S. Xiao et al., Reference Xiao, Chen, Pang, Zhou and Yuan2020). Regardless, a recent radiometric date of 543.4 ± 3.5 Ma from the overlying Baimatuo Member (Huang et al., Reference Huang, Chen, Ding, Zhou and Zhang2020) indicates that the Shibantan biota preserves one of the youngest occurrences of Charnia, roughly comparable in age to two other terminal Ediacaran occurrences, in the Khatyspyt Formation in Siberia (~553–544 Ma; Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008; Rogov et al., Reference Rogov, Karlova, Marusin, Kochnev, Nagovitsin and Grazhdankin2015) and in the Zhoujieshan Formation in Qaidam (~550–539 Ma; Pang et al., Reference Pang, Wu, Sun, Ouyang, Yuan, Shen, Lang, Wang, Chen and Zhou2021). Meanwhile, the oldest occurrences of Charnia come from the Drook Formation in Newfoundland, Canada (~574–560 Ma; Narbonne and Gehling, Reference Narbonne and Gehling2003; Matthews et al., Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2021) and the Bradgate Formation in Charnwood Forest, UK (~562–557 Ma; Ford, Reference Ford1958; Noble et al., Reference Noble, Condon, Carney, Wilby, Pharaoh and Ford2015); the genus is also present in the White Sea assemblage (Martin et al., Reference Martin, Grazhdankin, Bowring, Evans, Fedonkin and Kirschvink2000; Gehling and Droser, Reference Gehling and Droser2013). In terms of paleogeographic distribution, Charnia has been reported from almost all major Ediacara-type fossil localities ranging from low to high paleolatitudes (Fig. 6; see also Boddy et al., Reference Boddy, Mitchell, Merdith and Liu2022). In terms of paleoenvironmental distribution, Charnia specimens have been reported from siliciclastic sediments in deep marine basins (Hofmann et al., Reference Hofmann, O'Brien and King2008; Liu et al., Reference Liu, Kenchington and Mitchell2015) to sandstones in shallow shelf environments, including lagoon/delta front and lower shoreface (or sheet-flow sands) (Gehling and Droser, Reference Gehling and Droser2013; McMahon et al., Reference McMahon, Liu, Tindal and Kleinhans2020) to carbonate shelves (Grazhdankin et al., Reference Grazhdankin, Balthasar, Nagovitsin and Kochnev2008; this paper). Thus, Charnia is an Ediacara-type macrofossil genus with a remarkably long stratigraphic range and broad paleogeographic range (Fig. 6). This implies that the sessile Charnia must have had some sort of dispersal strategies, presumably through planktonic larvae (Darroch et al., Reference Darroch, Laflamme and Clapham2013) or waterborne asexual propagules (Mitchell et al., Reference Mitchell, Kenchington, Liu, Matthews and Butterfield2015; Mitchell and Kenchington, Reference Mitchell and Kenchington2018; see also Liu and Dunn, Reference Liu and Dunn2020). In addition, a juvenile specimen of Charnia is preserved together with a Helminthoidichnites-like trace fossil (Fig. 3.3), indicating that Charnia could survive in an environment occupied by bilaterian trace makers. The co-occurrence of Helminthoidichnites-like trace fossils and Ediacara-type body fossils has also been observed in other Ediacaran successions (e.g., Gehling and Droser, Reference Gehling and Droser2018). Such co-occurrences can provide key insights into the ecological interactions between trace-making animals and Ediacara-type soft-bodied macro-organisms and may help to test the biotic replacement hypothesis that bilaterian bioturbation may have led to the demise of sessile frondose taxa in the terminal Ediacaran (e.g., Seilacher, Reference Seilacher1989, Reference Seilacher1992; Laflamme et al., Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Darroch et al., Reference Darroch2015).

Figure 6. Paleogeographic distribution of Charnia (marked by yellow dots) worldwide. The paleogeographic map (~550 Ma) is based on Zhao et al. (Reference Zhao, Wang, Huang, Dong, Li, Zhang and Yu2018) and Pang et al. (Reference Pang, Wu, Sun, Ouyang, Yuan, Shen, Lang, Wang, Chen and Zhou2021). C-Qil = Central Qilian; N-Qt = North Qiangtang; S-Qt-Ls = South Qiangtang and Lhasa; IC = Indochina.
Conclusion
A systematic description of Charnia masoni and Charnia gracilis n. sp. from the Shibantan biota (ca. 550–543 Ma) is presented in this paper. The Shibantan C. gracilis specimens show differences in the overall morphology, length/width ratio, and divergence angle of first-order branches from the type species, C. masoni. Nevertheless, morphological aspects of their first and second-order branches, their tightly constrained and one-sided first-order branches, and their zig-zag central suture indicate that they belong to the genus Charnia. The Shibantan Charnia specimens also present features that seem to support an insertion–inflation growth model and an erect sessile epibenthic lifestyle. Charnia masoni and C. gracilis from the Shibantan limestone represent one of the youngest occurrences of the genus Charnia and extend the paleogeographic, paleoenvironmental, and stratigraphic distributions of this genus. Charnia seems to be an evolutionarily resilient genus that persisted for ~30 Myr and witnessed the rise and fall of the Ediacara-type macro-organisms.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 42130207, 42272005, and 41921002), the Chinese Academy of Sciences (CAS) (grants XDB26000000 and QYZDJ-SSW-DQC009), the National Key Research and Development Program of China (grant 2022YFF0802700), the Youth Innovation Promotion Association of the CAS (grant 2021307), and the State Key Laboratory of Palaeobiology and Stratigraphy (20201102). We thank Associate Editor J. Schiffbauer, G. Narbonne, A. Liu, and M. Laflamme for constructive reviews.
Declaration of competing interests
The authors declare none.










