Bipolar affective disorder is a chronic enduring mental illness characterised by periods of elation and depression in mood. The modern conceptualisation of this phenomenon as a distinct illness dates back to the 19th century when Falret first described the concept of folie circulaire. By the start of the 20th century, Kraepelin had coined the term manic depressive psychosis and unified the classification of affective disorders into manic depressive illnesses, which also included unipolar depressive episodes. The unipolar/bipolar distinction was formally introduced into diagnostic classifications in 1980 with the publication of DSM–III and has been carried forward into DSM–IV–TR. This gives a descriptive definition of bipolar disorder as the presence of discrete episodes of depression (indistinguishable from those of unipolar depression) and hypomania/ mania (American Psychiatric Association 2000).
Bipolar disorder encompasses a heterogeneous group of mood disorders, and longitudinal studies have highlighted the presence of a wide bipolar spectrum in clinical samples (Reference AngstAngst 1998). The spectrum includes hypomania, cyclothymia, mania without depression as well as bipolar I and II disorder, and continues to evolve. The inclusion of the ‘softer’ elements of this spectrum in diagnoses has led to an estimate of the rate of bipolar-spectrum illness in the general population as high as 8.3% (Reference Akiskal, Bourgeois and AngstAkiskal 2000). This article focuses on the DSM–IV–TR description of bipolar disorder as it is the most widely used in the literature.
Age at onset
The age at onset of bipolar disorder has implications for heritability, clinical course and treatment. The methods used to determine age at onset have included age at first treatment, age of first hospital admission or the first time diagnostic criteria are met. Early studies were predominantly of in-patient groups and almost all studies have looked at clinical samples, which, given the proportion of the bipolar disorder population who do not seek treatment, means that significant numbers are unrepresented. The extent to which this influences reported age at onset is unclear but it is certain that the bipolar II disorder and bipolar disorder not otherwise specified (NOS) groups will be underrepresented. Studies relying on retrospective self-report also have obvious sources of bias, not least the influence of a definite subsequent diagnosis. There is relatively little prospective data on the first incidence of bipolar disorder in either the general population or high-risk samples.
The problems of retrospective diagnosis are well illustrated by surveys from the National Depressive and Manic Depressive Association, which suggest that up to a third of individuals wait 10 years to receive an accurate diagnosis (Reference Lish, Dime-Meenan and WhybrowLish 1994). Children may experience even greater delays, although diagnosis in childhood remains controversial (Reference Goodwin, Anderson and ArangoGoodwin 2008). About 50% of adults diagnosed do not receive treatment for their first affective episode and women wait longer for diagnosis and maintenance treatment. First episodes are often depressive in nature and treated in primary care (Reference Chengappa, Kupfer and FrankChengappa 2003).
The Epidemiologic Catchment Area study found mean age at onset to be 21.2 years (Reference Weissman, Leaf and TischlerWeissman 1988), whereas the more recent Systematic Treatment Enhancement Program for Bipolar Disorder (STEP–BD) study reported a mean of 17.37 years (Reference Perlis, Miyahara and MarangellPerlis 2004). Women have an earlier age at onset than men, but receive the bipolar disorder diagnosis on average 4.4 years later than men and experience their first manic episode on average 5 years later than men (Reference Kennedy, Boydell and KalidindiKennedy 2005). There has been a considerable downward shift in the age at onset being reported over time. Reference Goodwin and JamisonGoodwin & Jamison (2007a) pooled data from 15 studies published after 1990 and found a weighted mean age at onset of 22.2 years (Table 1): pre-1990 studies report it as 6 years older. This shift seems unlikely to represent a true change in the disease. It will certainly reflect increased awareness of the milder forms of the illness and better reporting. Older cohorts excluded individuals with bipolar II disorder or misdiagnosed those with psychotic features as having schizophrenia. There is speculation that increased use of antidepressant and stimulant medication during the 1990s may have precipitated earlier onset in those already vulnerable, and increased use and availability of illicit substances and alcohol may have the same effect. In the absence of prospective studies or strong cross-sectional epidemiology, these assertions are hard to prove or disprove.
Genetics
Age at onset is a heritable trait. Early age at onset is associated with poor prognosis: increased rates of psychosis, higher rates of comorbid substance misuse and comorbid psychiatric disorders, increased suicide risk and greater neuropsychological dysfunction (Reference Leboyer, Henry and Paillere-MartinotLeboyer 2005). Poor outcomes in this group may simply relate to the cumulative impact of longer duration of illness rather than to a characteristic genotype. Nevertheless, the genetic link with age at onset appears robust – age at onset may distinguish clinical subtypes.
The distribution of age at onset based on the retrospective case-note recording of age at first diagnosis in a genetically homogenous Italian population confirmed three subgroups with mean ages of 18.1, 24.3 and 41 years (Reference Manchia, Lampus and ChillottiManchia 2008), very similar to the French sample studied by Reference Bellivier, Golmard and RietschelBellivier et al (2003). These subgroups aggregated within families, but clinical presentation varied widely across the subgroups further supporting the idea that age at onset is a potential clinical biomarker for bipolar disorder unrelated to observed phenotypic heterogeneity.
Is there a prodrome in bipolar disorder?
Bipolar disorder can only be diagnosed on the basis of clear-cut (hypo)manic symptoms. It remains tempting to suppose that a prodromal developmental state might exist for bipolar disorder, which might permit early intervention, as is currently in vogue for psychosis. A prodrome would imply a clinical state that would almost invariably predict the subsequent development of a full-blown syndrome.
Studies of offspring of people with bipolar disorder have found significant psychopathology, but such studies are difficult to do. A Canadian sample (Reference Duffy, Alda and CrawfordDuffy 2007) of ‘high-risk’ children (those with one parent who had a diagnosis of bipolar disorder) was followed for about 4 years between the ages of 16 and 20. Bipolar disorder of some kind was diagnosed in about 20% (in about two-thirds of these cases patients developed a bipolar diagnosis de novo). Comorbidity was common, but quite non-specific. Anxiety and sleep disorders were the most frequent.
The Amish Community Study (Reference Egeland, Shaw and EndicottEgeland 2003; Reference Shaw, Egeland and EndicottShaw 2005) found that during a 7-year follow-up, high-risk individuals experienced more episodic constellations of affective symptoms rather than a chronic gradation of emerging symptoms. The symptom profile included episodic mood lability, low energy, anxiety, hyperalertness, attention problems, school role impairment and excitability as well as the continuous presence of sensitivity, somatic complaints and stubborn behaviours. The authors observed a change in behaviour, with these features becoming more overtly manic. However, there were no early-onset cases in this cohort.
A retrospective study using a semi-structured interview found that first-episode mania was frequently preceded by a lengthy symptomatic period, which was insidious and subacute in nature, challenging the widely held view that mania develops precipitously (Reference Correll, Penzner and FredericksonCorrell 2007). Symptoms overlapped with descriptions of the prodromal phase of schizophrenia and lasted between 1.8 and 2.3 years depending on whether a first depressive episode was included. Given that the diagnosis of bipolar disorder relies on the presence of a manic or hypomanic episode, predictive clinical patterns within a prodrome could prove useful. However, their prospective value remains to be demonstrated. Life events play an important role in the onset of mood symptoms generally and some authors have suggested that they may serve to accelerate the prodromal symptoms (Reference Malkoff-Schwartz, Frank and AndersonMalkoff-Schwartz 1998). The problem is the likely sensitivity and specificity of any predictive measure, which remains to be established. For the moment, identifying a prodrome before the onset of bipolar disorder is aspirational and success by no means probable.
Time course and disease intensity
General statements about the duration, severity and frequency of mood episodes must be qualified by the origins and representativeness of the patient sample. As with age at onset averages, the findings may change as sampling frames move from clinic or in-patient samples with severe illness to larger populations. Capturing the full spectrum of bipolar disorder and the associated intensity of the disease burden remains a largely unmet objective.
Duration of affective episodes
In general, the duration of depressive episodes is greater than that of manic episodes. Mean duration of mania has been reported as being 6 weeks compared with 11 weeks for depression and 17 weeks for mixed states (Reference Coryell, Endicott and KellerCoryell 1990). People with agitated depression have longer episode duration than those with non-agitated depressive episodes (Reference Maj, Pirozzi and MaglianoMaj 2003). Mania has a relatively abrupt onset (notwithstanding the discussion of the prodrome above), whereas onset of a depressive episode can be more insidious; nevertheless, depression is still more abrupt in onset in bipolar disorder than in unipolar depressive disorders (Reference Keitner, Solomon and RyanKeitner 1996).
Frequency of episodes
The frequency of episodes has been a focus of bipolar disorder research since Kraepelin first observed a decline in periods of euthymia as the number of affective episodes increased. However, if the data are corrected for the fact that increased numbers of episodes are associated with shorter cycle lengths, the decrease in euthymic intervals is predominantly observed during the first three affective episodes, with unchanging euthymic intervals for further episodes (Reference Zis, Grof and WebsterZis 1980; Reference Kessing, Hansen and AndersenKessing 2004). Episode frequency appears to be correlated between relatives (Reference Fisfalen, Schulze and DePauloFisfalen 2005). It has also been suggested that antidepressant use may be associated with more frequent mood episodes (Reference Schneck, Miklowitz and MiyaharaSchneck 2008).
Pattern of episodes
Many patients with bipolar disorder present with a depressive episode and about 40% receive a unipolar diagnosis (Reference Ghaemi, Sachs and ChiouGhaemi 1999). These individuals tend to develop a manic episode within 5 years of their first affective episode and have more depressive episodes than patients with true unipolar depression. The rate of switching from false unipolar depression to hypomania/mania is particularly high in younger cohorts, although these rates flatten out to about 1% per year after the age of 30 (Reference Goodwin and JamisonGoodwin 2007a). A 15-year follow-up of patients admitted to hospital for major depressive disorder revealed 46% had subsequently experienced a manic or hypomanic episode (Reference Goldberg, Harrow and WhitesideGoldberg 2001).
Polarity of onset may convey prognostic advantages, individuals with unipolar manic presentations experiencing the best prognosis. Polarity also has a familial pattern (Reference Kassem, Lopez and HedekerKassem 2006), although this is confined to onset of mania. There have been suggestions that women experience more depressive episodes than their male counterparts (although more women are admitted to hospital for mania), but the STEP–BD study found no gender difference with respect to past manic or depressive episodes (Reference Schneck, Miklowitz and MiyaharaSchneck 2008). Sequences of mood polarity remain relatively constant once established, with those who experience mania followed by depression then remission having the better outcome (Reference Turvey, Coryell and ArndtTurvey 1999). Poorer prognosis is predicted by the persistence of depressive episodes.
Bipolar II disorder
There has been relatively little research into bipolar II disorder and this group are frequently underrepresented or omitted from studies. Despite fewer hospital admissions, outcome does not appear to be any better than for bipolar I disorder because it is largely dominated by the intensity of the depressive pole of the illness. Bipolar I and II disorder represent illnesses defined by the arbitrary distinction between mania, hypomania and manic symptoms, an apparent continuum of mood elevation. Nevertheless, bipolar II disorder as an individual diagnosis is stable enough to warrant separate classification (Reference Judd, Akiskal and SchettlerJudd 2003). Individuals with bipolar II disorder have similar characteristics at age at onset and first episode to those with bipolar I disorder. Just 7.5% converted from bipolar II disorder to bipolar I disorder in a 10-year study, although drop-out rates were about 15% (Reference Coryell, Keller and EndicottCoryell 1989).
Bipolar II disorder is associated with a high lifetime prevalence of anxiety disorders and tends to follow a chronic course, with significantly more minor and major depressive episodes and shorter periods of inter-episode recovery. Overall, individuals with bipolar II disorder have more affective episodes than those with bipolar I disorder (Reference Vieta, Colom and GastoVieta 1997).
Bipolar disorder NOS
This includes disorders that have bipolar features but do not meet criteria for any specific disorder. It is a DSM–IV category that includes any of the following:
-
• recurrent subthreshold hypomania in the presence of intercurrent major depression;
-
• recurrent (at least two episodes) hypomania in the absence of recurrent major depression with or without subthreshold major depression;
-
• recurrent subthreshold hypomania in the absence of intercurrent major depression with or without subthreshold major depression.
The number of required symptoms for sub-threshold hypomania is confined to two criterion B symptoms (from the DSM–IV requirement of three, or four if the mood is only irritable). It seems likely that the incidence of bipolar disorder NOS is underrepresented in clinical samples. It is often used to described children believed to have bipolar disorder and has been agreed as a working diagnosis to facilitate research on this broader phenotype.
A number of alternative classification systems for bipolar disorder have been devised to accommodate the NOS and spectrum diagnoses. However, none have yet been formally recognised and it is unclear as to whether better defined criteria will be included within DSM–V. Much of the focus has been on redefining the threshold at which hypomanic symptoms should become significant enough to change a diagnosis of unipolar depressive disorder to bipolar disorder. The current cut-off point is 4 days and is used arbitrarily to distinguish bipolar II disorder from bipolar disorder NOS (or unipolar depression if the mood elevation is not recurrent). Forty per cent of adults with hypomania have episodes that last between 1 and 3 days (Reference Wicki and AngstWicki 1991) and paediatric samples rarely meet the 4-day criteria. New definitions for bipolar disorder NOS have been developed in light of these observations. A minimum duration of 1–3 days for hypomania in children has been suggested (Reference Leibenluft, Charney and TowbinLeibenluft 2003).
Rapid cycling bipolar disorder
The concept of rapid cycling bipolar disorder was introduced by Dunner & Fieve in 1974 (Reference Dunner and FieveDunner 1974). Despite its somewhat arbitrary definition of four or more affective episodes per year, it is an important marker of outcome. Prevalence is estimated at 12–24% and has been correlated with earlier age at onset, comorbid substance misuse and greater severity of depressive episodes (Reference Cruz, Vieta and ComesCruz 2008). Notable geographic differences in incidence occur, the highest rates being observed in Norway (28.6%) and the lowest in Portugal (5.6%) (Reference Cruz, Vieta and ComesCruz 2008). The EMBLEM study (Reference Cruz, Vieta and ComesCruz 2008) found a predominance of females with bipolar I disorder (the study did not examine those with bipolar II disorder). Of the individuals entering the STEP–BD trial, 32% met criteria for rapid cycling in the previous year (Reference Schneck, Miklowitz and MiyaharaSchneck 2008) but no correlation was found with bipolar disorder subtype, female gender and rapid cycling. The definition of episode onset and ending obviously influences any measure of cycle frequency and is a source of variation within and between studies.
Paediatric bipolar disorder
There has been a five- to sixfold increase in the reported incidence of paediatric bipolar disorder in recent years, especially in the USA (Reference Blader and CarlsonBlader 2007), and a massive 40-fold increase in the number of out-patient visits made by patients with the diagnosis (Reference Moreno, Laje and BlancoMoreno 2007). It is unclear to what extent these reported increases relate to misdiagnosis of other psychiatric conditions or greater identification, but it seems unlikely that the majority of these individuals will have their diagnoses confirmed on transition to adulthood. A comparable increase in diagnosis in adults has not been observed.
The age at which diagnosis can be made remains as contentious as the criteria used to define bipolar disorder in this group. Some argue for the use of narrow definitions, which provide more certainty regarding progression of the disorder through to adulthood. Others argue in favour of a broader phenotype which allows for early intervention, preventing, in principle, some of the more negative consequences of the disorder.
There is a relative paucity of data regarding paediatric bipolar disorder and it is uncertain to what extent the major phenotypes remain stable throughout the life course. Studies suggest that between 70 and 100% of children experience remission, but recurrence has been reported in 80% at 4-year follow-up (Reference Geller, Tillman and CraneyGeller 2004; Reference Birmaher and AxelsonBirmaher 2006a). A follow-up study of a group of adolescents admitted to hospital following their first manic or mixed episode found that 85% experienced syndromic remission at 1 year, but 52% of these individuals experienced a relapse within 1 year. Only 20% achieved syndromic, symptomatic and functional improvement at 1 year (Reference DelBello, Hanseman and AdlerDelBello 2007). Subsyndromal mood symptoms are common and individuals are 10% less likely to recover with every subsequent year of illness (Reference Birmaher, Axelson and StroberBirmaher 2006b).
Treatment response
A full discussion of treatment is beyond the scope of this review. It has received a full re-assessment in a British Association for Psychopharmacology guideline (Reference GoodwinGoodwin 2009). The prevention of long-term relapse is the major challenge in bipolar disorder and delays in the initiation of treatment lead to greater morbidity, functional impairment and a negative impact on future treatment response. The previously accepted notion that patients with bipolar disorder recover fully and remain well has long been disregarded. Treatment has centred on lithium and anticonvulsant therapies but antipsychotics have always been used (particularly atypical antipsychotics nowadays) in all phases of the illness. Overall, between 30 and 50% of individuals respond to lithium or an anticonvulsant when in the manic phase. The response rate to atypical antipsychotics is similar.
In acute bipolar depression, response rates to lithium and anticonvulsants are about 30% (Reference Post, Uhde and Roy-ByrnePost 1986; Reference Davis, Bartolucci and PettyDavis 2005), with the exception of lamotrigine, for which response rates are about 50%. Quetiapine appears to have superior efficacy in acute bipolar depression and has been found to lead to remission in over 50% of patients (Reference Cookson, Keck and KetterCookson 2007).
The prescription of antidepressants in bipolar depression has been recently challenged by the finding, under naturalistic conditions, that they are no more efficacious than mood stabilisers alone (Reference Sachs, Nierenberg and CalabreseSachs 2007).
Evidence base
One of the major difficulties in the treatment of bipolar disorder is the gap between the evidence base, which is focused on monotherapies, and clinical practice where complex regimens are commonplace. Just 5–10% of patients are estimated to be on monotherapy, while nearly 50% are on three or more agents (Reference Lim, Tunis and EdellLim 2001). It should be noted that these data are from the USA, where prescribing practice differs from that in the UK. A further challenge is that few well-tolerated treatments with efficacy in all phases of illness are available. Non-adherence to medication is relatively common, with only half of patients reporting good adherence (Reference Colom, Vieta and Martinez-AranColom 2000). Those with comorbid personality disorders and more hospital admissions are more likely to stop medication.
Rapid cycling
Data regarding treatment of rapid cycling bipolar disorder and mixed affective states are far less robust, although the notion that people with rapid cycling bipolar disorder respond poorly to lithium may relate in part to increased use of antidepressants. The literature appears to support the ongoing administration of an antimanic agent for maintenance treatment, which may in part account for the predominance of depressive episodes in bipolar disorder.
Children
The particular problem with efforts to identify childhood cases is that treatment has been by extrapolation of data from treatment studies in adults. The use of medicines, often in combination, in very young children carries uncertain risks/ benefits.
Long-term outcome
Long-term outcome in bipolar disorder has been studied in a number of cohorts, all of which support the notion that bipolar disorder is a lifelong illness. Persistence of depressive symptoms during follow-up appears to predict poor outcome, but early episodes of mania do not appear to be relevant (Reference Coryell, Turvey and EndicottCoryell 1998). A 40-year follow-up of the Zurich Cohort found 16% had recovered (recovery defined as no episode for the past 5 years), but over 50% were still experiencing recurrent episodes (Reference AngstAngst 1980).
Remission and subsyndromal symptoms
Remission is a key goal in bipolar disorder but there is no consensus as to how it should be defined or measured. There is increasing data to suggest that significant inter-episode impairment exists even in remitted states, and that remission is often not sustained. Reference Judd, Akiskal and SchettlerJudd et al (2005) reported that individuals were subsyndromal 15% of the time and had minor symptoms for a further 20% of the time. Depressive symptoms caused most impairment, whereas subsyndromal hypomanic symptoms appeared to enhance functioning in bipolar II disorder (Reference Judd, Akiskal and SchettlerJudd 2005).
A subsequent prospective study (Reference Paykel, Abbott and MorrissPaykel 2006) found subsyndromal symptoms to be present for twice as long as full-blown symptoms and were three times more likely to be depressive than manic. Risk factors for subsyndromal symptoms were similar to those for major episodes, relating to greater severity and previous history. Baseline functional impairment has also been observed to play a role in predicting depressive (but not manic) symptom levels at 12 months. If the last episode was depressive in nature, more impairment in daily function due to residual depressive symptoms has been observed.
Deficits have been found in various domains of cognitive functioning in euthymic bipolar disorder including executive function, attention/ concentration, visuospatial organisation, learning and memory (Reference Clark and GoodwinClark 2004; Reference Malhi, Ivanovski and Hadzi-PavlovicMalhi 2007). Cognitive deficits have also been found to be correlated with lower self-reports of quality of life.
Comorbid conditions
Axis I disorders
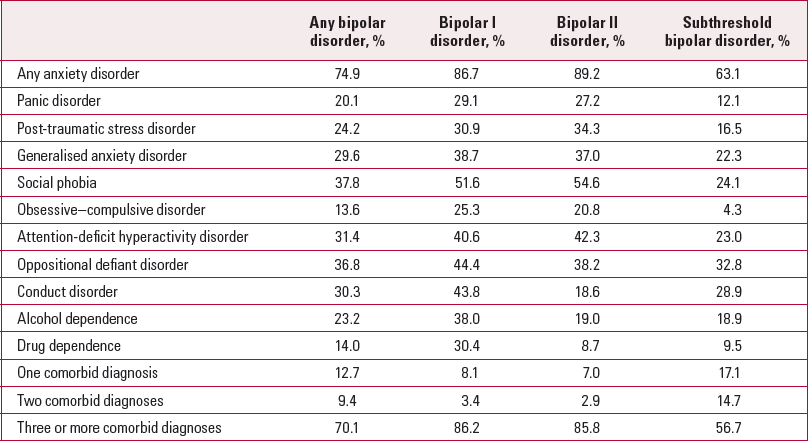
Sixty-five per cent of patients with bipolar disorder have at least one comorbid psychiatric disorder, with a significant number having two or more (Reference McElroy, Altshuler and SuppesMcElroy 2001) (Table 2). Recent studies suggest that this could be even higher when subthreshold bipolar disorder is included (Reference Merikangas, Akiskal and AngstMerikangas 2007).
It is of note that many of the Axis I comorbidities are more commonly found in people with bipolar II disorder, suggesting that more chronic forms of comorbidity are associated with the more chronic form of bipolar disorder. The role of anxiety in mediating the processes that underlie bipolar disorder have been neglected and merit investigation.
Alcohol misuse
A lifetime history of alcohol misuse is one of the more common comorbidities occurring in about 46% of people with bipolar I disorder. It has been suggested that alcohol may help alleviate early symptoms of mania. Individuals with bipolar disorder are also more likely to drink when in a depressed state than individuals with unipolar depression (Reference Sharma, Mazmanian and PersadSharma 1995). The presence of a mixed affective state is also associated with higher alcohol intake compared with non-mixed states. If alcohol misuse pre-dates the onset of bipolar disorder, outcome appears to be better. This group have later illness onset, perhaps suggesting that alcohol misuse causes the emergence of more benign forms of the illness. For those whose alcohol misuse increases following illness onset, prognosis is much worse in terms of both psychopathology and overall outcome.
Cannabis use
Cannabis use increases the time spent in an affective state, is associated with rapid cycling, and while the majority of cannabis use remits during hospital admission, it is rapidly reinstated following discharge (Reference Strakowski, DelBello and FleckStrakowski 2007).
Anxiety disorders
Comorbid anxiety disorders are common and there is a greater association with bipolar II disorder than bipolar I disorder (Reference McElroy, Altshuler and SuppesMcElroy 2001). Patients with bipolar disorder with high anxiety scores have higher rates of suicide, alcohol misuse and cyclothymia, and show a poorer response to lithium (Reference Young, Cooke and RobbYoung 1993).
Lifetime prevalence of obsessive–compulsive disorder is reported as 21%, although observed to be episodic and often related to mood (Reference Strakowski, Sax and McElroyStrakowski 1998).
Attention-deficit hyperactivity disorder
In the STEP–BD cohort, 9.5% had both bipolar disorder and lifetime attention-deficit hyperactivity disorder (ADHD). Rates for comorbid ADHD were higher in men than in women (14.7% v. 5.8%) and onset of the mood disorder in those with ADHD was approximately 5 years earlier than in those without. Those with ADHD also had a greater number of other comorbid psychiatric diagnoses, longer duration of relapses and were more frequently depressed (Reference Nierenberg, Miyahara and SpencerNierenberg 2005).
Eating disorders
Up to 27% of patients with bipolar disorder also have an eating disorder (Reference Ramacciotti, Paoli and MarcacciRamacciotti 2005), although most studies suggest it is nearer 6–9%, with 3–7% having bulimia nervosa and a further 9–18% having binge eating disorder (Reference MacQueen, Marriott and BeginMacQueen 2003; Reference Ramacciotti, Paoli and MarcacciRamacciotti 2005).
Axis II disorders
Prevalence of Axis II disorders in bipolar disorder is reported as being as high as 89% (Reference Turley, Bates and EdwardsTurley 1992), although more conservative estimates are between 25 and 50% (Reference Ucok, Karaveli and KundakciUcok 1998; Reference Kay, Altshuler and VenturaKay 1999; Reference Vieta, Colom and Martinez-AranVieta 2000; Reference George, Miklowitz and RichardsGeorge 2003). The majority of studies in this area establish the presence or otherwise of Axis II disorders in individuals who have ongoing affective symptoms. The only study to look at prevalence in euthymia revealed Axis II disorders to be present in 42.5%, with Cluster B personality disorders being most common (Reference Rosso, Albert and BogettoRosso 2008). No difference in prevalence was found between bipolar I disorder and bipolar II disorder. Although this study was limited to individuals who were able to achieve and maintain euthymia (thus excluding all of those with persistent affective symptoms), the prevalence of Axis II disorders is in keeping with earlier studies. Axis II disorders are highly relevant as they alter the course of bipolar disorder, convey a worse prognosis and are associated with higher rates of substance misuse.
Physical illness
Bipolar disorder is associated with a number of physical illnesses. Although there has been some recent evidence to suggest that this association may not be as clear as previously thought, longer-term data are awaited (Reference Dutta, Boydell and KennedyDutta 2007). Longer prospective studies have reported elevated mortality rates, especially from vascular causes.
Blood pressure and glucose regulation
Even when metabolic syndrome is controlled for, individuals with bipolar disorder are more likely to smoke and have hypertension (Reference Johannessen, Strudsholm and FoldagerJohannessen 2006).
Disturbances in glucose regulation within psychiatric populations have been long observed, and bipolar disorder is no exception: 9% of in-patients were found to have Type II diabetes mellitus (Reference Cassidy, Ahearn and CarrollCassidy 1999). This group had a more severe course and required more hospital admissions.
Obesity
Obesity is an important comorbidity relevant to metabolic disease, with reports that up to 35% of individuals with bipolar I disorder are obese independent of medication status or the duration over which medication had been taken. Obesity is associated with a greater number of lifetime depressive and manic episodes, more severe and difficult-to-treat index affective episodes, and a greater likelihood of developing an affective recurrence, particularly a depressive recurrence (Reference Fagiolini, Kupfer and HouckFagiolini 2003).
Thyroid disease
Thyroid disease is more commonly found in bipolar disorder populations, especially in women, and this finding is present in populations which have not received lithium therapy (Reference Valle, Ayuso-Gutierrez and AbrilValle 1999). In bipolar II disorder, rates are estimated to be as high as 9%.
Migraine
Migraine headaches were more likely to occur in women with bipolar II disorder. Males with bipolar disorder who have migraines are more likely to have an earlier onset of illness and higher prevalence of anxiety disorders, whereas females with bipolar disorder who have migraines have higher rates of comorbid medical problems (Reference McIntyre, Konarski and WilkinsMcIntyre 2006).
Pregnancy
Pregnancy is an important risk factor for relapse in the course of bipolar disorder, which has been reported to be as high as 50% (Reference Jones and CraddockJones 2005). In Denmark, very large record linkage studies have confirmed that the risk of admission to psychiatric care is raised almost threefold for women in general in the period 10–19 days postpartum; however, 27% of women with a bipolar diagnosis are admitted to psychiatric care in the first postpartum year (Reference Munk-Olsen, Laursen and MendelsonMunk-Olsen 2009).
Reference Viguera, Whitfield and BaldessariniViguera et al (2007) conducted a small prospective observational cohort study of pregnant women with bipolar disorder. They found that the risk of recurrence during pregnancy was 71%. The majority of these episodes were depressive in nature and nearly half had occurred within the first trimester. Recurrence was more likely if a woman had discontinued medication, had bipolar II disorder and had previously been treated with mood stabilisers.
These results emphasise the need to balance the risks posed by ongoing treatment to the fetus with those of a recurrence of an affective episode. The former are well recognised, while the latter are perhaps regrettably less so (Reference GoodwinGoodwin 2009).
Mortality
People with bipolar disorder have a worse prognosis than the average population with respect to both suicide and medical causes of death. The Zurich Cohort reported a 61% higher risk of death (standardised mortality ratio, SMR = 1.6), with the greatest risk being that of suicide (SMR = 12.28). The SMR for cardiovascular mortality was 1.6 and for accidental death it was 1.9. However, a follow-up study of individuals admitted to hospital for affective disorders found that those with bipolar depression were less likely to die by suicide than those with unipolar depression, and that those who had received long-term psychotropic medication were less likely to take their own life than those who had not (Reference Angst, Stassen and ClaytonAngst 2002).
Suicide rates are high in bipolar disorder: SMR = 15 in males and 22.4 in females, and suicide tends to occur early in the course of the illness (Reference Osby, Brandt and CorreiaOsby 2001). However, analysis is limited because suicide is a rare event, and sampling of first-episode cases or those admitted to hospital combined with the short follow-up time of most studies makes extrapolation of data over the lifespan difficult. Attempted suicide is common, with 35% reporting a history of suicide attempt on entry to the STEP–BD trial (Reference Kogan, Otto and BauerKogan 2004). In patients with bipolar I disorder, reported mean time to suicide from first presentation was 8.1 years, with 7% of men and less than 1% of women having died by suicide at the end of a 19-year study period (Reference Dutta, Boydell and KennedyDutta 2007). Overall, suicide mortality had a 12-fold increase for men and a fourfold increase for women; however, no increase in cardiovascular or cerebrovascular mortality was observed, unlike previous reports.
These results were broadly similar to those from studies in Denmark (Reference Høyer, Mortensen and OlesenHøyer 2000) and Sweden (Reference Osby, Brandt and CorreiaOsby 2001), although the study by Reference Dutta, Boydell and KennedyDutta et al (2007) had lower SMRs, which the authors relate to the wider cohort and longer follow-up of their study. They also postulate that cardio/cerebrovascular causes of death were greater in the bipolar disorder group because follow-up studies were of patients who had been admitted to hospital, arguing that patients with a comorbid physical illness were more likely to be admitted to hospital and were therefore overrepresented in these samples.
Conclusions
It has been argued before that bipolar disorder is the real heartland of psychiatry (Reference Goodwin and GeddesGoodwin 2007b) – it presents a less controversial diagnosis, and the evolution of treatment appears to require a more consistent application of the biomedical model than in schizophrenia. This article illustrates the major challenges in understanding the onset and development of the disorder which, in turn, provide the obvious locus for early intervention and treatment. The course of the disorder and the intensity of illness episodes and comorbidity imply a major burden of disease, reflected by objective measures of disability (Key points). Treatment innovation in relation to both pharmacological and non-pharmacological modalities is a major contemporary target for the application of neuroscience.
KEY POINTS Bipolar disorder: key messages
-
• Age at onset is often 15–20 years
-
• 40% of individuals are initially diagnosed with unipolar depression
-
• Bipolar I disorder remains a relatively rare, frequently psychotic disorder: significant inter-episode cognitive impairment may exist in the absence of an affective episode
-
• Bipolar II disorder is a stable diagnosis, now made more frequently and associated with a chronic course in which depression is usually the predominant polarity
-
• Bipolar-spectrum diagnoses rightly reflect the prevalence of mild elated states but carry uncertain implications for treatment
-
• Long treatment delays are common
-
• Childbirth is associated with high rates of relapse
-
• Treatment strategies for bipolar I disorder have evolved rapidly in recent years
MCQs
Select the single best option for each question stem
-
1 Age at onset of bipolar disorder:
-
a has little prognostic relevance
-
b is not a heritable trait
-
c has been observed to be higher in more recent studies
-
d is higher in women than men
-
e has implications for clinical course.
-
-
2 Individuals with bipolar disorder:
-
a rarely receive a diagnosis of unipolar depression
-
b have longer episodes of mania than depression
-
c commonly have psychiatric comorbidities
-
d have fewer depressive episodes than those with unipolar depression
-
e show poorer prognosis if they have predominantly manic episodes.
-
-
3 When compared with bipolar I disorder, bipolar II disorder:
-
a is associated with better inter-episode functioning
-
b is similar and frequently develops into bipolar I disorder
-
c is associated with fewer affective episodes overall
-
d has a less chronic course
-
e has a significantly higher age at onset.
-
-
4 Regarding the treatment of bipolar disorder:
-
a delays in initiating treatment are rare
-
b the vast majority of patients respond to lithium or an anticonvulsant treatment when in a manic phase
-
c quetiapine leads to remission in over 50% of patients in the depressive phase
-
d there are a number of well-tolerated treatments that are effective in all phases of the illness
-
e the majority of patients are maintained on monotherapies.
-
-
5 Common comorbid conditions include:
-
a anxiety disorders in 5% of patients
-
b rheumatoid arthritis
-
c thyroid disease
-
d tension headache
-
e unipolar depression.
-
MCQ answers

| 1 | e | 2 | c | 3 | a | 4 | c | 5 | c |
TABLE 1 Age at onset of bipolar disorder in post-1990 studies
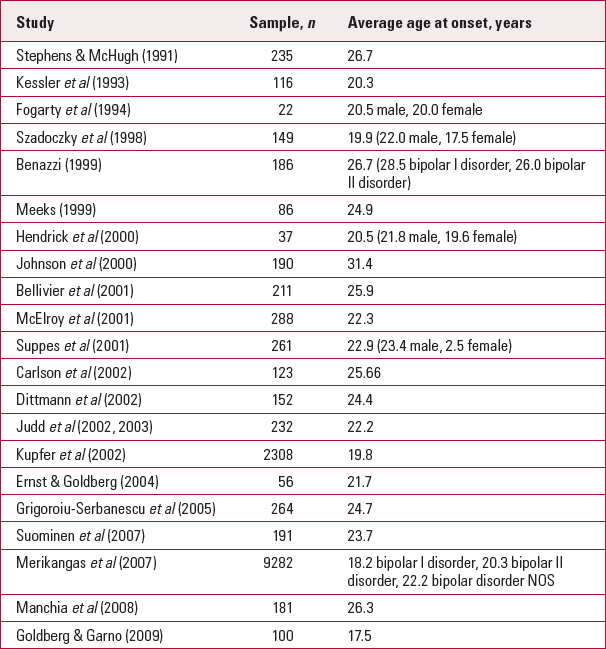
TABLE 2 Lifetime comorbidity of DSM–IV/CIDI bipolar disorder with other DSM–IV/CIDI disorders
| Any bipolar disorder, % | Bipolar I disorder, % | Bipolar II disorder, % | Subthreshold bipolar disorder, % | |
|---|---|---|---|---|
| Any anxiety disorder | 74.9 | 86.7 | 89.2 | 63.1 |
| Panic disorder | 20.1 | 29.1 | 27.2 | 12.1 |
| Post-traumatic stress disorder | 24.2 | 30.9 | 34.3 | 16.5 |
| Generalised anxiety disorder | 29.6 | 38.7 | 37.0 | 22.3 |
| Social phobia | 37.8 | 51.6 | 54.6 | 24.1 |
| Obsessive–compulsive disorder | 13.6 | 25.3 | 20.8 | 4.3 |
| Attention-deficit hyperactivity disorder | 31.4 | 40.6 | 42.3 | 23.0 |
| Oppositional defiant disorder | 36.8 | 44.4 | 38.2 | 32.8 |
| Conduct disorder | 30.3 | 43.8 | 18.6 | 28.9 |
| Alcohol dependence | 23.2 | 38.0 | 19.0 | 18.9 |
| Drug dependence | 14.0 | 30.4 | 8.7 | 9.5 |
| One comorbid diagnosis | 12.7 | 8.1 | 7.0 | 17.1 |
| Two comorbid diagnoses | 9.4 | 3.4 | 2.9 | 14.7 |
| Three or more comorbid diagnoses | 70.1 | 86.2 | 85.8 | 56.7 |





eLetters
No eLetters have been published for this article.