- ‘MUST’
malnutrition universal screening tool
Malnutrition: a public health problem?
Malnutrition (undernutrition) is a state of nutrition in which a deficiency or imbalance of energy, protein and other nutrients (including vitamins and minerals) causes measurable adverse effects on the structure and function of the body and clinical outcome that typically respond to nutritional treatment(Reference Elia1). Malnutrition impairs physical and psychological function and increases morbidity and mortality(Reference Stratton, Green and Elia2). Consequently, healthcare use (general practitioner visits, hospitalisations, hospital stay) is substantially greater in individuals who have, or are at risk of, malnutrition(Reference Stratton, Green and Elia2–Reference Elia, Stratton, Russell, Green and Pang4). The considerable costs of disease-related malnutrition, which are more than estimates for obesity (approximately £3·3–3·7×109/year(5)), highlight the scale of this condition and the need for it to be recognised as a public health problem. Malnutrition is a condition widely associated with disease, with a particularly high prevalence in hospital inpatients (42% of admissions to hospital are at risk of malnutrition)(Reference Stratton and Elia6), outpatients and in care homes(Reference Elia3, Reference Stratton, Hackston, Longmore, Dixon, Price, Stroud, King and Elia7, Reference Stratton and Elia8). However, recent data in older adults highlight the extent of malnutrition in the general population(Reference Elia and Stratton9) (Table 1).
Table 1. Prevalence of malnutrition in older adults in England(Reference Elia and Stratton9)
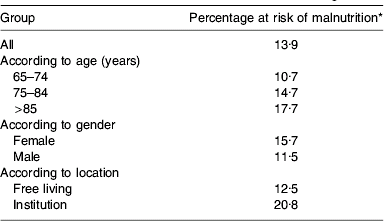
* Medium +high risk of malnutrition with Malnutrition Universal Screening Tool-type criteria (n 1155).
A secondary analysis of data from the National Diet and Nutrition Survey(Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke10) indicates that 13·9% of older adults (aged ≥65 years) are at risk of malnutrition in England(Reference Elia and Stratton9) (Fig. 1). This categorisation of malnutrition was made using similar criteria to that of the Malnutrition Universal Screening Tool (‘MUST’)(Reference Elia and Stratton9, 11). The prevalence of malnutrition increases with age and is greater in institutions than in free-living subjects (Table 1). This secondary analysis also suggests that the prevalence of malnutrition in older adults is similar in Scotland (14·4%) and Wales (11%), with an overall prevalence for Great Britain (England, Scotland and Wales) of 13·8% (7·3% medium risk of malnutrition and 6·5% high risk of malnutrition). When applied to the country as a whole (approximately 9 543 000 aged ≥65 years(12)) a very crude estimate suggests >1·31 million older adults are at risk of malnutrition. The total estimate is likely to be considerably higher when the sick, including those in hospitals and those who are <65 years, are included. Consequently, it is unsurprising that malnutrition costs the National Health Service ≥£7·3×109 per year, of which approximately £5·16×109 is for older adults alone(Reference Elia, Stratton, Russell, Green and Pang4). The main healthcare costs are those associated with provision of hospital care and long-term residential or nursing care. Additional costs that could not be included in this economic analysis are the cost of home visits by National Health Service workers, the costs of general practitioner and outpatient visits for those aged <65 years and the cost of private health care(Reference Elia, Stratton, Russell, Green and Pang4). Thus, it is likely that the costs of disease-related malnutrition are closer to £9×109 annually(Reference Elia, Stratton, Russell, Green and Pang4).

Fig. 1. Prevalence of malnutrition in and across England and in Great Britain (England, Scotland and Wales). (■), High risk; (□), medium risk. Regional comparison for England of south v. central v. north (χ2): **P=0·002 for trend.
Deficiencies of specific nutrients, including vitamins and minerals, should also be considered part of malnutrition. Indeed, the same national survey (National Diet and Nutrition Survey) shows the extent of a range of nutritional inadequacies in older adults(Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke10). In particular, low intakes (below the reference nutrient intake(13)) of some but not all micronutrients are evident in a substantial proportion of free-living and institutionalised older adults (Table 2). Clinical deficiencies of some micronutrients are also found, particularly in institutionalised older adults. Specifically, deficiency of folate (35%) and vitamin C (40%) are common(Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke10).
Table 2. Percentage of older adults in the UK with micronutrient intakes below the reference nutrient intakeFootnote *(Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke10)

* Reference nutrient intakes for men and women aged ≥50 years(13).
† No. of patients varies according to micronutrient and group (male and female).
A secondary analysis of the National Diet and Nutrition Survey involving those individuals at risk of malnutrition (a smaller subset with dietary intake data) again shows a substantial proportion of individuals with micronutrient intakes below the reference nutrient intake (Table 2). For some vitamins (including vitamins A, C, D and E) significantly poorer status has been highlighted in those at risk of malnutrition(Reference Elia and Stratton14) (Table 3). In hospitalised individuals poor intakes of micronutrients, as well as energy and protein, are commonly observed(Reference Stratton, Green and Elia2).
Table 3. Poorer vitamin status in the elderly at risk of malnutrition (secondary analysis of the UK National Diet and Nutrition Survey(Reference Elia and Stratton14))
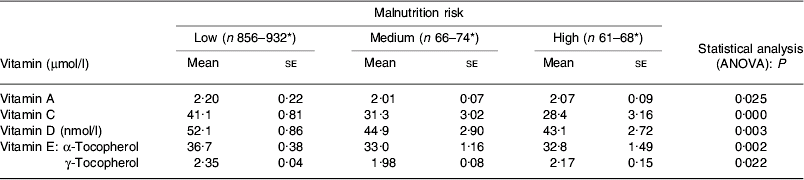
* No. of subjects varies according to vitamin measured.
A steadily-ageing population (estimates suggest the percentage aged ≥65 years will increase to 18 in 2015 and 23 in 2030(15)) means that the prevalence of malnutrition (both protein–energy deficiency and vitamin and mineral deficiencies) is likely to increase in coming years with a concomitant increase in associated clinical consequences and costs.
Malnutrition: a health inequality?
In addition to the scale of the problem of malnutrition, new data suggest that this condition is one of the many health inequalities that exists in England(Reference Stratton and Elia6, Reference Elia and Stratton9). Although malnutrition is not currently a priority area for many governments, health inequality is high on their agenda(16). Expert reports highlight the problems of health inequality, the adverse effects of deprivation on health and the important role of nutrition(16–18). Deprivation, including social, economic and environmental factors, may increase an individual's risk of developing nutritional problems such as malnutrition.
The National Diet and Nutrition Survey(Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke10) and the secondary analysis(Reference Elia and Stratton9) indicate geographical inequalities in the prevalence of protein–energy malnutrition and nutrient status across older adults in England. The results indicate a ‘north–south’ divide within England (see Fig. 1), raising issues of inequality. Malnutrition risk is found to be 73% higher in the northern region of England (the north, north-west, Yorkshire and Humberside) than in the southern (London, south-east and south-west) region and 58% higher than in the central (East Midlands, West Midlands and East Anglia) region (Fig. 1). When adjusted for age, gender and domicile, there is little change in the regional prevalence of malnutrition, which remains greater in the northern region of England than in the rest of England (OR 1·826 (95% CI 1·289, 2·587), P=0·001)(Reference Elia and Stratton9).
A north–south gradient in the status of some but not all micronutrients is also apparent. This analysis suggests that the status of vitamin C, vitamin D and a range of carotenoids and markers of vitamin K status (prothrombin time) and Se status (glutathione peroxidase activity) is significantly poorer in the north of England than in the south (controlled for age, gender and domicile)(Reference Elia and Stratton9). One specific example is vitamin C, a severe deficiency (<5 μmol/l) of which is significantly more common in the northern region (15%) than in the central (5·2%) and southern (2·1%) regions (P<0·001). Milder deficiency of vitamin C is much more common across England, but particularly in the northern region (33% v. 20% in the central region v. 10% in the southern region). Similar geographical differences in nutrient status across England are found in free-living and institutionalised older adults(Reference Elia and Stratton9).
These geographical differences in the prevalence of malnutrition (protein–energy status using ‘MUST’ and nutrient status) persist after controlling for socio-economic factors (such as income, benefits, living alone, education)(Reference Elia and Stratton9). Health may be a contributory factor, as poorer self-rated health and a higher proportion of individuals with false teeth and swallowing problems etc. appear more common in the north than in other regions. However, as with other inequalities, the causes are likely to be complex and multifactorial. Indeed, greater prosperity and improvements in health in the UK and other developed countries do not appear to have decreased inequalities in income, mortality or the outcome of diseases. This analysis suggests that poor nutrient status can now be added to a cluster of other geographical inequalities in England that need attention(Reference Elia and Stratton9).
Malnutrition, inequality and outcome in the clinical setting
As indicated earlier, in the general population geographical inequality, including deprivation, is associated with malnutrition, including poorer nutrient status(Reference Elia and Stratton9, Reference Armstrong, Dorosty and Reilly19), and with poorer outcome (e.g. increased mortality)(18, Reference Acheson20, Reference Shaw, Davey Smith and Dorling21). New data suggest that within a smaller geographical locality in England similar associations and inter-relationships exist in patients admitted to hospital(Reference Stratton and Elia6).
A recent study of 1000 individuals admitted to hospital shows that 42% were at risk of malnutrition (medium and high risk assessed using ‘MUST’)(Reference Stratton and Elia6). Malnutrition risk (assessed using ‘MUST’) was found to be associated with a doubling of mortality and a 50% increase (5 d) in length of hospital stay (see Fig. 2). Furthermore, patients with medium and high risk of malnutrition were admitted from areas with significantly greater deprivation when compared with patients at low risk of malnutrition (P=0·019; Fig. 3(a)). Deprivation was assessed using the Index of Multiple Deprivation 2000(22), which includes measures (domains) of ‘income’, ‘employment’, ‘health deprivation and disability’, ‘education, skills and training’, ‘housing’ and ‘access to services’(Reference Stratton and Elia6, 22). The index is not specific to individuals but to geographical areas termed wards. There are 8414 wards in England, which are ranked in order of deprivation from 1 (most-deprived ward) to 8414 (least-deprived ward). In the study an individual's postcode was used to determine the geographical ward they were admitted from and its associated deprivation rank. The indices of deprivation were analysed as ranks and as quartiles (from the least deprived to the most deprived). Specifically, this study shows that the prevalence of malnutrition risk increases significantly with each quartile of deprivation rank (increasing deprivation; predicted OR 1·14 (95% CI 1·02, 1·28), binary logistic regression model, adjusted for age and gender), and Fig. 3(b) shows the greater prevalence of malnutrition in the most-deprived quartile compared with the least-deprived quartile (OR 1·59 (95% CI 1·11, 2·28)). In particular, greater ‘health deprivation and disability’, ‘income’ deprivation and ‘employment’ deprivation were found in those with increased malnutrition risk. This study also suggests an association between deprivation and increased in-hospital mortality, although little effect of deprivation on length of hospital stay was found. While other studies have suggested a relationship between deprivation and poorer outcome in some patient groups in hospital(Reference Hutchings, Raine, Brady, Wildman and Rowan23, Reference Leigh, Seagroatt, Goldacre and McCulloch24), these studies have not considered nutrition. Interestingly, in this study the effects of deprivation on mortality were found to be independent of malnutrition (using binary logistic regression analysis). Similarly, the adverse effect of malnutrition on in-hospital mortality was found to be independent of deprivation(Reference Stratton and Elia6). Thus, this study in hospital patients highlights that malnutrition and deprivation are interrelated yet both have independent adverse associations with patients' outcome. Evidence clearly suggests that intervening with nutritional treatments in hospital (Table 4), particularly in the acutely-ill and older patient with malnutrition risk improves outcome (e.g. can reduce mortality)(Reference Stratton and Elia8). Further exploration is required to investigate how the consequences of deprivation on outcome can be addressed and its potential impact on the effectiveness of nutritional treatments.

Fig. 2. Malnutrition (assessed using the Malnutrition Universal Screening Tool(Reference Elia3, 11)) increases mortality (a) and length of hospital stay (b). (a) For the medium+high-risk group, OR 2·07 (95% CI 1·03, 4·14; binary logistic regression adjusted for age, gender and deprivation (index of multiple deprivation(22); IMD) quartile). (b) For those with medium+high risk of malnutrition the length of stay was higher than that for the low-risk group: ***P<0·0005 (Cox regression model adjusted for mortality, age, gender and deprivation (IMD) quartile).

Fig. 3. (a) Relationship between malnutrition risk (assessed using the Malnutrition Universal Screening Tool (‘MUST’)(Reference Elia3, 11)) and deprivation (assessed using the index of multiple deprivation (IMD) 2000(22)). The most-deprived ward (a ward is a geographic area; for details, see p. 525) in England ranks 1 and the least-deprived ward ranks 8414 (median rank of patient group (n 1000) 3890 (range 601–8375)). The deprivation rank for the medium+high-risk group was significantly different from that for the low-risk group (P=0·019). (b) Prevalence of malnutrition (assessed using ‘MUST’) in patients from the most-deprived and least-deprived areas (deprivation assessed using the IMD 2000; the IMD rank for the least-deprived quartile was 7319 (range 6355–8375) and that for the most-deprived quartile was 1282 (range 601–2251)). There was a greater prevalence of malnutrition in the most-deprived quartile compared with the least-deprived quartile OR 1·59 (95% CI 1·11, 2·28; binary logistic regression adjusted for age and gender).
Implications for the management of malnutrition
The issues discussed earlier have a number of implications for the management of malnutrition, particularly as this condition is largely treatable.
First, routine screening should be implemented for high-risk groups or areas (e.g. older adults, areas of deprivation) and within the healthcare system, including primary and secondary care. Use of a simple validated evidence-based tool to screen for malnutrition is recommended by many national agencies, such as the British Association for Parenteral and Enteral Nutrition, in conjunction with the British Dietetic Association, the Royal College of Nursing and the Registered Nursing Homes Association(Reference Elia3), the Royal College of Physicians(25) and the National Institute for Health and Clinical Excellence(26). One example is ‘MUST’(Reference Elia3, 11, Reference Todorovic, Russell, Stratton, Ward and Elia27), which is suitable for use for public health and in clinical settings and can also be used to detect obesity. Consideration should also be given to micronutrient status and any deficiencies should be corrected.
Second, if malnutrition or other nutritional problems are identified, then the underlying cause(s) should be identified and treated or corrected wherever possible. It is likely that in many cases disease, trauma (accidents, surgery) and/or related symptoms (e.g. nausea, dysphagia, dyspnoea) or disabilities (e.g. arthritis of the hands limiting food preparation and ingestion) will be the cause. Diagnosis and management, where possible, is important and may involve pharmacological intervention and input from the multidisciplinary team (doctors, occupational therapists, physiotherapists, dietitians, speech and language therapists etc.). However, as highlighted earlier, deprivation and other socio-economic factors must also be considered and issues of food insecurity(Reference Bukhari, Margetts and Jackson28) tackled.
Third, as part of the screening process, a plan for the nutritional management of malnutrition should be in place. There are a range of nutritional interventions for malnutrition that can be used. However, considering the prevalence of energy, protein and micronutrient deficiencies highlighted earlier, it is likely that strategies that consider a range of nutrients (and not just energy) will be more effective. Certainly, specially-formulated oral nutritional supplements (that contain energy, protein and a range of micronutrients) have been shown to improve nutritional intakes, body weight, function and clinical outcome(Reference Stratton, Green and Elia2, Reference Stratton and Elia8, Reference Milne, Avenell and Potter29–Reference Stratton and Elia31) (Table 4). Recent evidence suggests that the use of liquid multinutrient supplements may reduce hospital re-admissions(Reference Gariballa, Forster, Walters and Powers32) and reduce costs in some patient groups(Reference Elia, Stratton, Russell, Green and Pang4, Reference Elia and Stratton33). Currently-available information suggests that oral nutritional supplements used in addition to the diet are more effective than using dietary strategies alone, including food snacks(Reference Baldwin, Parsons and Logan34–Reference Stratton, Bowyer and Elia39). If resources permit, many patients would benefit from input from a specialist in nutrition, such as a dietitian. However, as resources can be limited, dietetic input is often reserved for those requiring specialist advice or artificial nutrition (enteral-tube feeding, parenteral nutrition). Indeed, for some patients oral strategies are insufficient and additional artificial forms of nutritional support are required, often for weeks or even years. Indeed, in the UK there are approximately 27 000 individuals receiving tube feeding at home per year, often as the only source of nutrition(Reference Jones, Stratton, Holden, Russell and Micklewright40). These patients are typically elderly (>60% are aged ≥60 years), most (60%) live at home and have high levels of disability(Reference Jones, Stratton, Holden, Russell and Micklewright40). Consequently, these patients often have a multitude of problems that require nursing and social support, as well as dietary support, which need to be considered but are often overlooked.
Table 4. Summary of evidenceFootnote * and recommendations for the use of oral nutritional supplements in some specific patient groupsFootnote †

COPD, chronic obstructive pulmonary disease; ONS, oral nutritional supplements; RCT, randomised controlled trials; MUST, Malnutrition Universal Screening Tool.
* Evidence from randomised controlled trials comparing ONS with routine care.
† More information is given on individual RCT in the groups described, in other conditions (e.g. liver and gastrointestinal disease, renal disease, oncology, diabetes) or general evidence for ONS(Reference Stratton, Green and Elia2,8,29,51–Reference Stratton, Bircher, Fouque, Stenvinkel, de Mutsert, Engfer and Elia53). Relevant systematic reviews and meta-analyses are summarised(Reference Stratton and Elia8).
‡ Use of a liquid carbohydrate supplement in patients undergoing gastrointestinal surgery given pre-operatively up until 2 h before anaesthesia may reduce post-operative insulin resistance and improve well-being and reduce hospital stay(Reference Thorell, Nygren and Ljungqvist54, Reference Nygren, Thorell and Ljungqvist55).
Fourth, equity of access to screening and to nutritional services and treatments (as well as other treatments and services) is an important issue. There is little data to suggest whether there is inequity of access to nutritional screening (a process that is not currently widely adopted) across the country. Similarly, it is uncertain whether the availability of nutritional treatments such as oral nutritional supplements or access to nutritional services, including a dietitian, is similar across the country, and further investigation is warranted. However, the British Artificial Nutrition Survey has highlighted wide variation in the use of enteral-tube feeding and parenteral nutrition across the UK as a whole and also within smaller geographical regions within the UK(Reference Jones, Stratton, Holden, Russell and Micklewright40). Table 5 indicates the differences in the prevalence of home enteral tube feeding within the south-west region of England, which ranges from eighty-two to 632 patients/million of the population.
Table 5. Variation in the use of home enteral tube feeding (HETF) within the south and west region of England (British Artificial Nutrition Survey data(Reference Stratton, Bowyer and Elia39))

* Value was significantly different from the average expected for the population: P<0·05.
† No. of patients on HETF.
‡ No. of patients on HETF per million of the regional population.
Summary
In summary, malnutrition is just one of the many health inequalities affecting millions of individuals in the UK that needs to be more effectively identified and managed. In addition to marked geographical differences in the prevalence of malnutrition across England and an inter-relationship between malnutrition, poor outcome and deprivation, there are the considerable costs to the National Health Service to consider. As malnutrition is a largely treatable condition, prompt identification and effective management are imperative, with equity of access to nutritional services and treatments for malnutrition assured. As malnutrition is a public health problem, it needs to become a priority for governments and healthcare planners as well as for healthcare and social-care professionals, carers and patients themselves.










