sepsis is a syndrome of physiologic, pathologic and biochemical abnormalities, which is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection(Reference Fernando, Rochwerg and Seely1). Traditionally, the immune response after systemic inflammatory response syndrome (SIRS) was considered to shift towards hypoinflammation and immunosuppression called compensatory anti-inflammatory response syndrome (CARS) to balance the hypermetabolic situation(Reference Bone2). Currently, evidence suggests that SIRS and CARS are interdependent during the course of sepsis(Reference Gomez, Gonzalez and Londono3). Both pro- and anti-inflammatory cytokines can be detected simultaneously during sepsis, sepsis-associated acute inflammatory-metabolic stress and multiorgan dysfunction(Reference Fitrolaki, Dimitriou and Venihaki4). It is the dysregulation of the pro- and anti-inflammatory mediators during sepsis that leads to multiorgan failure(Reference Kumpf and Schumann5). T lymphocytes are crucial for effective immune responses against invading pathogens. sepsis-induced T lymphocyte dysregulation aggravates the inflammatory response and subsequent outcomes(Reference Cabrera-Perez, Condotta and Badovinac6).
T lymphocytes can be divided into two subsets: CD4+ and CD8+ T cells. CD4+ helper T (Th) cells have diverse effects on the innate immune system while the major function of CD8+ is to kill the infected cells(Reference Kasten, Tschop and Adediran7). Th cells are classified into Th1, Th2, Th17 and Th22 subsets, characterised by distinct cytokines and effector functions. Regulatory T (Treg) cells are a distinct CD4+ T cell subset that is implicated in suppressing excessive T cell responses and reduces pro-inflammatory mediator production(Reference Sakaguchi, Yamaguchi and Nomura8). Previous studies found decreased peripheral and splenic lymphocyte numbers and functions during sepsis(Reference Wesche, Lomas-Neira and Perl9, Reference Bandyopadhyay, De and Laudanski10). Restoration of the dysregulated T cell subsets is important in attenuating sepsis-induced inflammation and subsequent organ injury(Reference Cabrera-Perez, Condotta and Badovinac6).
Glutamine (GLN) is the most abundant free amino acid in the body. It is an important fuel source for rapid proliferating cells, including immune cells(Reference Andrews and Griffiths11). A number of studies demonstrated that GLN modulates inflammatory mediator expression and has benefits during catabolic and stressed conditions(122–Reference Hu, Pai and Yeh14). Previous study has demonstrated that dietary GLN suppressed Th1/Th17 and associated pro-inflammatory cytokine expression, thus elicited a more balanced Th/Treg polarisation in acute colitis mice(Reference Hsiung, Liu and Hou15). In a mouse model of lipopolysaccharide-induced lung injury, intragastric GLN administration reduced Th17 percentage and inflammatory cytokine levels in bronchoalveolar lavage fluid(Reference Hou, Pai and Liu16). Parenteral GLN administration after sepsis induction was found to reduce the percentage of IL-17-expressing CD4+ T cells and attenuated the dysregulated immune response in sepsis(Reference Hu, Hsiung and Pai17). However, an ex vivo study showed that high-dose GLN did not induce Th1/Th2/Th17 cytokine responses in peripheral blood mononuclear cells (PBMC) obtained from patients with severe sepsis(Reference Briassouli, Goukos and Daikos18). Also, GLN administration may repress heat shock protein 72 (HSP72) expression of PBMC after lipopolysaccharide exposure and enhance HSP72 proteins after heat shock induction in patients with sepsis(Reference Briassouli, Tzanoudaki and Goukos19). The influence of GLN on T cell regulation and subsequent outcome in sepsis remain inconsistent. Besides, previous researches showed that GLN had beneficial effects on immunomodulatory response during sepsis; GLN was provided shortly before or immediately after sepsis insult(Reference Kessel, Toubi and Pavlotzky12, Reference Oliveira, Oliveira and Santos13, Reference Hu, Hsiung and Pai17). Most study used a single dose of GLN injection to observe the changes of immune reaction within 24 h(144Reference Hu, Hsiung and Pai17, Reference Singleton, Serkova and Beckey20, Reference Hu, Yeh and Pai21). Studies investigating the effect of pretreated dietary GLN on T cell regulation at late septic phase are rare. Since the manifestations of remote organ dysfunction may occur several days after the initiation of sepsis, we designed the present study to evaluate GLN pretreatment on the impact of T cell polarisation and the subsequent kidney injury 3 d after sepsis.
Materials and methods
Experimental animals
Five-week-old C57BL/6 male mice were purchased from the National Laboratory Animal Center (NLAC), Taipei, Taiwan. Mice were kept under a temperature- and humidity-controlled condition with a 12 h light–12 h dark cycle and were fed ad libitum in the present study. The study protocol was approved by the Institutional Animal Care and Use Committee of Taipei Medical University (LAC-2017-0038). The care of the laboratory animals was in full compliance with the updated Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Study protocols
After 1 week of acclimation, mice weighing 22–25 g were randomly assigned to one normal control (NC, n 8) group and two groups of sepsis which was induced by caecal ligation and puncture (CLP). CLP is a well-established animal model to mimic bowel perforation with polymicrobial infection in humans. Mice in the NC group and the positive control CLP group (CLP-C, n 8) received a common semi-purified diet (American Institute of Nutrition (AIN)-93G). The other sepsis group (CLP-G, n 8) was administrated a GLN-enriched diet that was AIN-93G-based composition, whereas some part of the casein was replaced by GLN, which provided 25 % of total amino acid nitrogen. This dose of GLN was reported to have an immunomodulatory effect in rodents(Reference Hong, Rounds and Helton22, Reference Wells, Kew and Yaqoob23). The two diets were isonitrogenous and similar in energy and nutrient distributions (Table 1). After the mice were fed the respective diets for 2 weeks, CLP was performed to induce sepsis. Briefly, mice were anesthetised with an intraperitoneal injection of Zoletil (25 mg/kg body weight) and Rumpon (10 mg/kg body weight), a 1 cm incision was made in the abdominal wall, and the cecum was exposed. Approximately, 50 % of the cecum was ligated just below the ileocaecal valve with 3-0 silk (Ethicon, Somerville). The distal cecum was then punctured in two places with a 22-gauge needle to allow a small amount of faecal material to extrude into the peritoneal cavity, after which it was replaced in the abdomen. The abdominal wound was closed in two layers. Post-operative pain was managed by treatment with 100 μl of 0·25 % bupivacaine at the incision site before skin closure. Mice were injected with 75 mg/kg body weight of antibiotic Ertapenem at 6 h and were killed at 72 h after surgery. Body weights were recorded daily during the experimental period. All mice were anesthetised and then euthanised by cardiac puncture. Blood samples were collected in tubes for cytometric analysis of the T lymphocyte subpopulation. Plasma was obtained by centrifuging the whole blood containing heparin. Spleen and kidney were harvested for further analysis.
Table 1. Composition of the experimental diets (g/kg)

* The mineral mixture contained the following (mg/g): calcium phosphate dibasic, 500; sodium chloride, 74; potassium sulfate, 52; potassium citrate monohydrate, 20; magnesium oxide, 24; manganese carbonate, 3·5; ferric citrate, 6; zinc carbonate, 1·6; curpric carbonate, 0·3; potassium iodate, 0·01; sodium selenite, 0·01; and chromium potassium sulfate, 0·55.
† The vitamin mixture contained the following (mg/g): thiamin hydrochloride, 0·6; riboflavin, 0·6; pyridoxine hydrochloride, 0·7; nicotinic acid, 3; calcium pantothenate, 1·6; D-biotin, 0·05; cyanocobalamin, 0·001; retinyl palmitate, 1·6; DL-α-tocopherol acetate, 20; cholecalciferol, 0·25; and menaquinone, 0·005.
Measurements of plasma biochemical parameters
Concentrations of IL-6, IL-10, keratinocyte-derived chemokine (KC), TNF-α, monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein 2 (MIP-2) in plasma samples were quantified using a MILLIPLEX MAP Mouse High Sensitivity T Cell Panel (Millipore). Creatinine (Cr) (Cayman) and blood urea N (BUN) (BioVision) were measured using commercial kits. Procedures followed the manufacturers’ instructions.
Distribution of T lymphocyte subpopulations in blood
To assess the distribution of circulating T cell populations, extracellular staining of CD45-PerCP (Biolegend), CD3-FITC (Biolegend), CD4-APC (eBioscience) and CD8-PE (Biolegend) was applied. The antibodies, mentioned earlier, were incubated with 50 μl aliquots of whole blood for 30 min at 4°C. Following lysis of erythrocytes, the remaining portions were washed twice with FACS buffer for further fluorescent detection.
Whole-blood aliquots of 100 μl were incubated with CD4-Pacific blue (Biolegend) to determine the phenotypes of helper T lymphocytes in the blood. Another 100 μl aliquot of whole blood was incubated with CD4-Pacific blue and CD25-APC (eBioscience) for 30 min to analyse the Treg cell percentage. Following rupture of erythrocytes, the remaining leucocytes were fixed and permeated for intracellular cytokine staining. The following antibodies were used for intracellular cytokine staining: IL-4-Alexa Fluor® 488 (Biolegend), interferon-γ-APC (BD Biosciences) and IL-17A-PE (Biolegend). For intracellular staining of forkhead box p3 (Foxp3), leucocytes were fixed and permeated with Foxp3 staining buffer (eBioscience) and Foxp3-PE (Biolegend). All fluorescent samples were analysed with a FACS Canto II flow cytometer (BD Biosciences). CD45-positive leucocytes were gated and then T-cell subsets were determined. As for phenotypes of Th cells, lymphocytes were gated according to their size and granularity using light scatter detectors (forward scatter (FSC)/side scatter (SSC)). CD4-positive lymphocytes were considered Th cells. The percentages of Th-associated cytokine-expressing cells among CD4+ lymphocytes were measured. Treg cells were presented as a percentage of CD25hiFoxp3+ cells among CD4+ lymphocytes.
Distribution of T lymphocyte subpopulations and activation of T lymphocytes in spleen
Spleens were minced and ground through a 40 μm cell strainer. Splenocytes were suspended in Roswell Park Memorial Institute (RPMI)-1640 medium and treated with erythrocyte lysis buffer (Biolegend) for 5 min. Using a buffer containing PBS and 0·5 % bovine serum albumin (BSA), splenocytes were washed twice and resuspended in the buffer. CD69 is an activation marker on T lymphocytes. Expression of CD69 on T lymphocytes was measured by flow cytometry. Aliquots (100 µl) of cell suspensions were incubated with CD45-PerCP, CD3-FITC, CD4-APC, CD8-PE and CD69-PE-Cy7 (Biolegend). CD45-positive leucocytes were gated. T-cell subsets were determined and activated T lymphocytes were evaluated by the expression of CD69 of T cell subpopulations.
CD4+ T cell isolation from spleens
To prepare single-cell suspensions, spleens were minced and ground through a 40 μm cell strainer and suspended in RPMI-1640 medium. Following treatment with erythrocyte lysis buffer (Biolegend) for 5 min, splenocytes were washed twice with a buffer containing PBS, pH 7·2, 0·5 % BSA and 2 mM EDTA, and resuspended in the buffer. Then, CD4+ T cells were separated from splenocytes using CD4+ T Cell Isolation Kit (Miltenyi Biotec).
RNA extraction and quantitative RT-PCR of renal tissues and CD4+ T cell of splenocytes
We applied Trizol reagent (Invitrogen) to extract total RNA from renal tissues and CD4+ T cells isolated from spleen. RNA (2·5 μg) was reverse transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific) with oligo (dT)18 primers, according to standard protocols. A real-time PCR was carried out in optical ninety-six-well plates on an ABI 7300 Real-Time PCR System (Applied Biosystems). Primers used in the present study are listed in online Supplementary Table S1. T-box expressed in T cells (T-bet), RAR-related orphan receptor γt (ROR-γt), GATA-3, Foxp3, Bim and Bcl-2 primers were used in gene analysis of CD4+ T cell isolated from splenocytes. Bim, Bcl-2, IL-1β, IL-6, TNF-α, MIP-2, KC, MCP-1, high mobility group box 1 (HMGB-1) and kidney injury molecule-1 (Kim-1) primers were used in the measurement of gene expression of renal tissues. The expression of each gene was assayed in a total volume of 25 μl containing 1× Maxima SYBR Green/ROX quantitative PCR Master Mix (Thermo Scientific), 200 nM of each primer and 50 ng of cDNA. Amplification was performed according to the thermocycling protocol recommended by the PCR system, with a final dissociation curve analysis. No-template controls and a melting curve analysis were used to confirm the specificity of the real-time PCR. The multiples of change of messenger (m)RNA were calculated by the equation 2−ΔΔCt (ΔCt indicates the difference of threshold cycles between the target gene and internal control (glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin) and ΔΔCt indicates the difference in ΔCt between the CLP and NC groups).
Statistical analysis
All data are shown as the means with their standard errors. All statistical analyses were performed with GraphPad Prism 5 software (GraphPad Software). Differences among the NC and the two sepsis groups were analysed by one-way ANOVA with Tukey’s post hoc test. Expression of genes was normalised by the NC group being considered as 1. The differences of the gene expression between the two sepsis groups were analysed by t test. A P value of <0·05 was considered significantly different.
Results
Body weights
There were no differences in the initial body weights among the three groups. The sepsis groups had lower body weights after CLP for 72 h. There were no differences in body weights between the CLP-C and CLP-G groups (data not shown).
Lymphocyte populations in blood
sepsis resulted in decreased percentages of T lymphocyte (CD3+ in gated CD45+ population) and CD4+ T cells (CD4+ in CD45+ CD3+ cells). The sepsis group with GLN (CLP-G) had higher percentages of T lymphocyte and CD4+ T cells than the sepsis control group (CLP-C) and had no difference from the NC group. Concerning the subpopulation of the CD4+ T cells, the percentages of Th1, Th2 and Th17 cells in the CLP-G group were significantly higher than the NC group. Also, the Th2- and Treg cell percentages were higher in the CLP-C group than the NC group at 72 h after CLP. Compared with the CLP-C group, the CLP-G group exhibited a lower Th2 and Treg cells. No differences in Th1 and Th17 cells were observed between the two sepsis groups (Fig. 1).
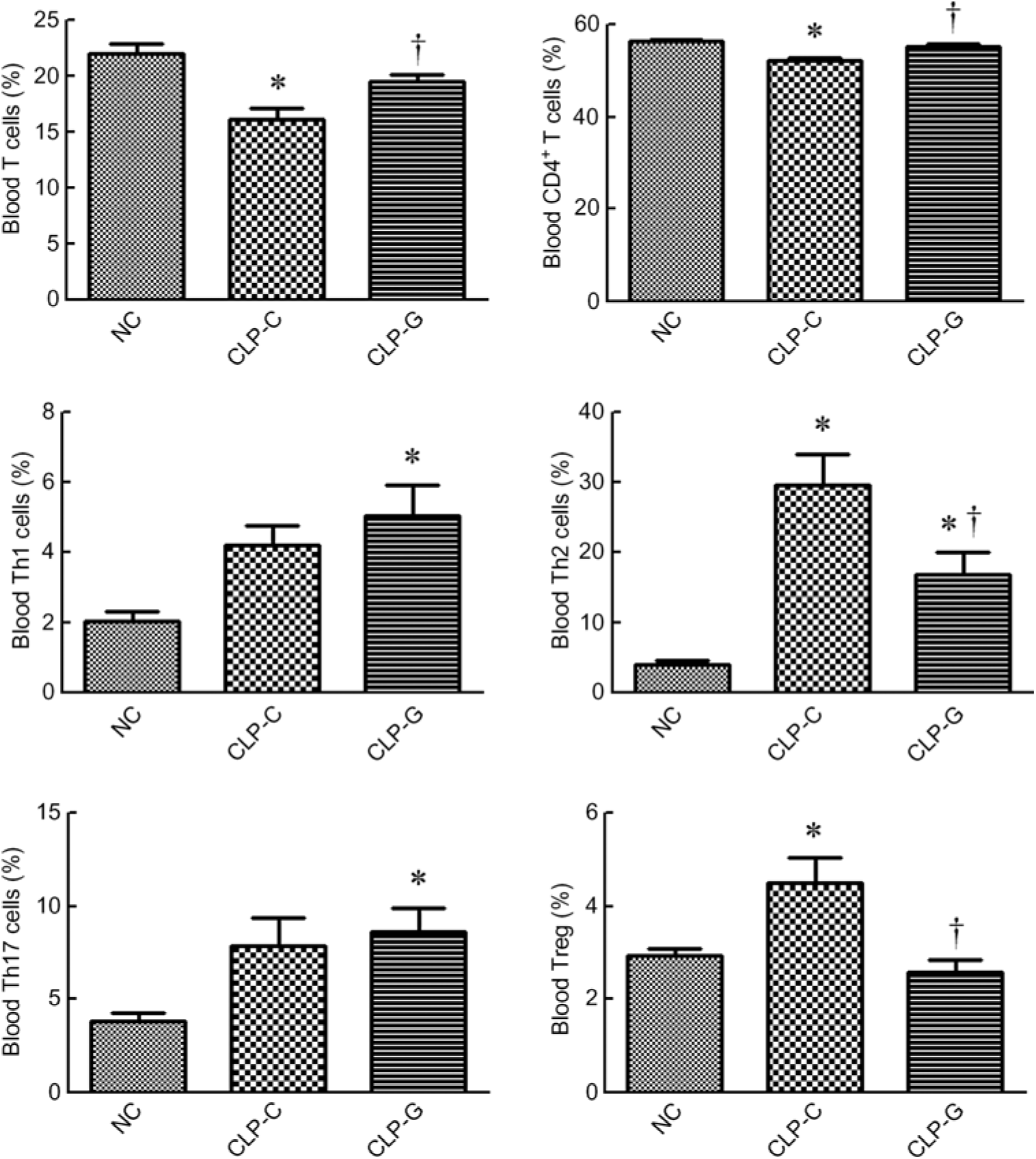
Fig. 1. Distribution of T lymphocytes and helper T (Th)-cell subsets in blood. Populations of T lymphocytes and Th cells were determined by CD3+ cells in CD45+ cells and CD4+ cells in CD45+CD3+ cells, respectively. As for phenotypes of Th cells, the percentages of Th1 (interferon-γ-expressing cells), Th2 (IL-4-expressing cells) and Th17 cells (IL-17A-expressing cells) among CD4+ lymphocytes were measured. Regulatory T (Treg) cells are presented as a percentage of CD25hiFoxp3+ cells among CD4+ lymphocytes. NC, normal control group; CLP-C, control group with caecal ligation and puncture (CLP) surgery; CLP-G, glutamine group with CLP operation. Values are means, with their standard errors represented by vertical bars (n 8 for each group). Differences in groups were analysed by one-way ANOVA with Tukey’s post hoc test. * Mean value was significantly different from that of the NC group (P < 0·05). † Mean value was significantly different from that of the CLP-C group (P < 0·05).
Plasma biochemical markers and inflammation-related chemokine concentrations
Plasma BUN and Cr levels in the septic groups did not differ from the NC group. There were no differences in plasma BUN and Cr levels between the two sepsis groups. sepsis resulted in elevation of plasma IL-6, KC and MCP-1 levels after CLP. Compared with the CLP-C group, the CLP-G group had significantly lower IL-6 and KC levels at 72 h post CLP. The MCP-1 levels were down-regulated in the CLP-G group, which was comparable with the NC group (Table 2).
Table 2. Plasma levels of biochemical markers and inflammation-associated chemokines 72 h after caecal ligation and puncture (CLP)‡
(Mean values with their standard errors; n 8 per group)
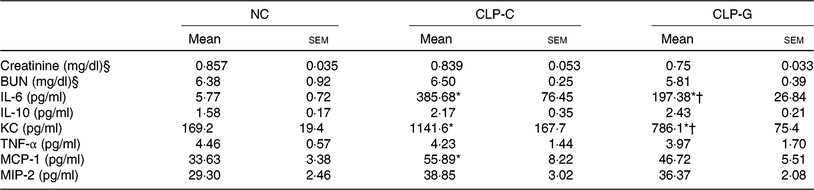
NC, normal control group; CLP-C, control group with CLP surgery; CLP-G, glutamine group with CLP operation; BUN, blood urea N; KC, keratinocyte-derived chemokine; MCP-1, monocyte chemoattractant protein-1; MIP-2, macrophage inflammatory protein 2.
* Mean value was significantly different from that of the NC group (P < 0·05).
† Mean value was significantly different from that of the CLP-C group (P < 0·05).
‡ Differences among groups were analysed by one-way ANOVA with Bonferroni's post hoc test.
§ To convert creatinine in mg/dl to μmol/l, multiply by 88·4. To convert BUN in mg/dl to mmol/l, multiply by 0·357.
Distribution of T lymphocyte subpopulations and activation of T lymphocytes in spleen
The percentages of T lymphocyte and CD4+ T cells were lower, whereas CD8+ T cell population (CD8+ in CD45+CD3+ cells) was higher in the CLP-C group than those of the NC group. There was no difference in T lymphocyte and CD8+ T cell percentages between the NC and the CLP-G groups. When assessing the differences between the two sepsis groups, proportions of CD4+ and CD69-expressing CD4+ T cells in the CLP-G groupwere significantly higher than the CLP-C group. In contrast, thepercentages of CD8+ and CD69-expressing CD8+ Tcellswere lower in the CLP-G group than the CLP-C group (Fig. 2).
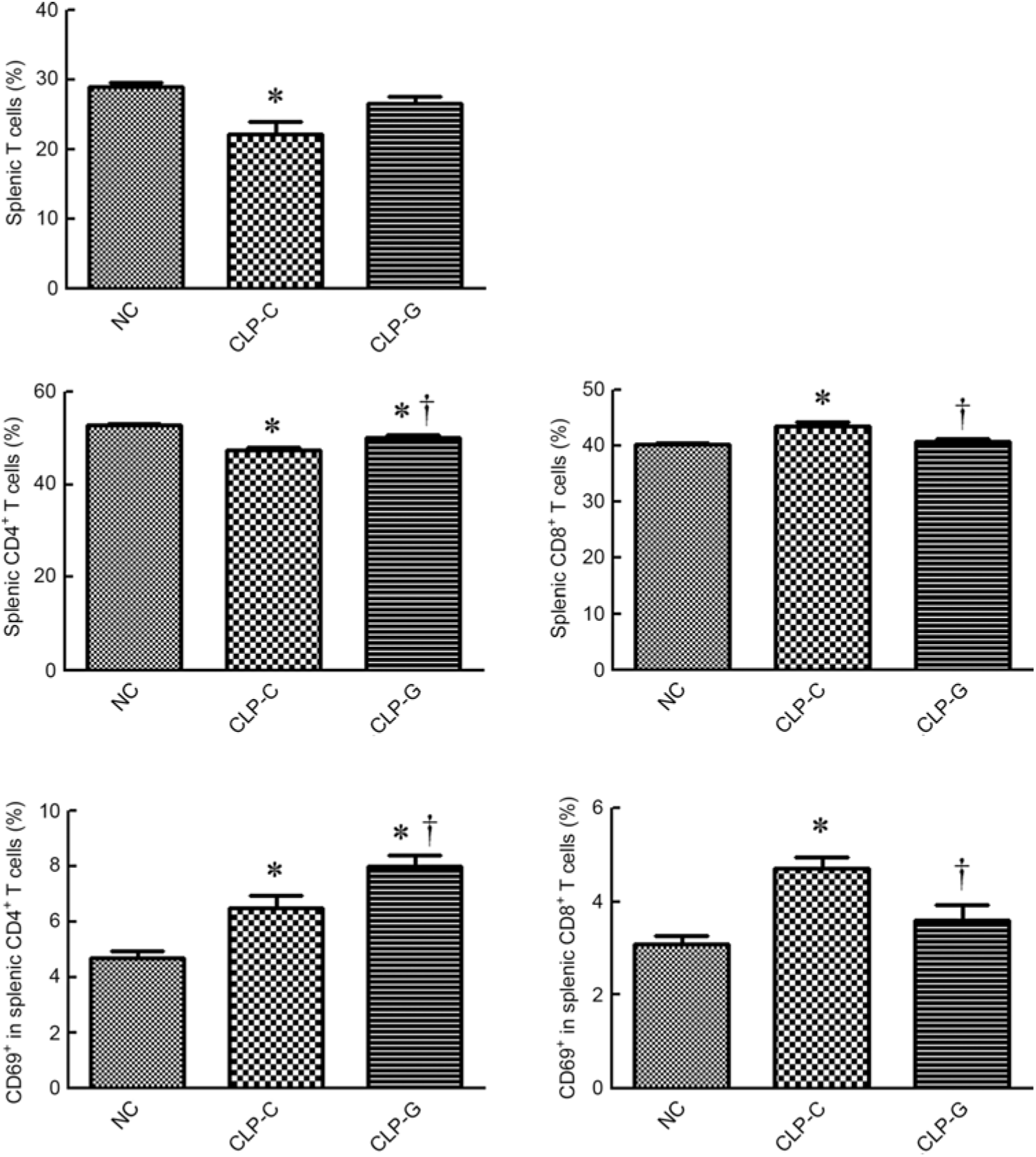
Fig. 2. Distribution of T-cell subpopulations and activation of CD4+, CD8+ T cells in spleen. T cells were determined by CD3+ cells in CD45+ cells using a flow cytometer. CD4+ and CD8+ T cells were respectively identified by CD4- and CD8-expressing cells among CD45+ CD3+ cells. Activated T cells were determined by the expression of CD69 on CD4+ or CD8+ T cell subsets. NC, normal control group; CLP-C, control group with caecal ligation and puncture (CLP) surgery; CLP-G, glutamine group with CLP operation. Values are means, with their standard errors represented by vertical bars (n 8 for each group). * Mean value was significantly different from that of the NC group (P < 0·05). † Mean value was significantly different from that of the CLP-C group (P < 0·05).
Bcl-2 and Bim expression in CD4+ T cells and CD4 transcription factors gene expression in the spleen
There was no difference in pro-apoptotic Bim gene expression between the two sepsis groups. However, the anti-apoptotic Bcl-2 gene expression and the Bcl-2:Bim ratio were higher in the CLP-G group than the CLP-C group. Messenger RNA levels of transcription factors for Th cells (T-bet, GATA-3, ROR-γt) and Treg cells (Foxp3) were significantly higher in the CLP-G group than those of the CLP-C group (Fig. 3).

Fig. 3. Expression of genes related to apoptosis and transcription factors in CD4+ T cells from spleen. ![]() , Control group with caecal ligation and puncture (CLP) surgery (CLP-C);
, Control group with caecal ligation and puncture (CLP) surgery (CLP-C); ![]() , glutamine group with CLP operation (CLP-G); T-bet, T-box expressed in T cells; ROR-γt, RAR-related orphan receptor γt; Foxp3, forkhead box p3. Quantification of mRNA changes was analysed by a real-time PCR and was calculated by the comparative CT (2−ΔΔCt) method. The mRNA expression levels in the normal control group were used as a calibrator. Values are means, with their standard errors represented by vertical bars (n 8 for each group). Differences between the CLP-C and CLP-G groups were analysed by t test. * Mean value was significantly different from that of the CLP-C group (P < 0·05).
, glutamine group with CLP operation (CLP-G); T-bet, T-box expressed in T cells; ROR-γt, RAR-related orphan receptor γt; Foxp3, forkhead box p3. Quantification of mRNA changes was analysed by a real-time PCR and was calculated by the comparative CT (2−ΔΔCt) method. The mRNA expression levels in the normal control group were used as a calibrator. Values are means, with their standard errors represented by vertical bars (n 8 for each group). Differences between the CLP-C and CLP-G groups were analysed by t test. * Mean value was significantly different from that of the CLP-C group (P < 0·05).
Gene expression levels in the kidneys
Expression levels of the anti-apoptotic Bcl-2 gene were higher, whereas mRNA expressions of MIP-2, KC and Kim-1 were lower in the CLP-G than those of the CLP-C group. There were no differences in gene expression of Bim, HMGB-1, MCP-1, IL-1β, IL-6 and TNF-α between the two sepsis groups (Fig. 4).

Fig. 4. Expression of genes related to apoptosis, inflammatory mediators and tissue injuries in the kidney. ![]() , Control group with caecal ligation and puncture (CLP) surgery (CLP-C);
, Control group with caecal ligation and puncture (CLP) surgery (CLP-C); ![]() , glutamine group with CLP operation (CLP-G); MIP-2, macrophage inflammatory protein 2; KC, keratinocyte-derived chemokine; MCP-1, monocyte chemoattractant protein-1; HMGB-1, high mobility group box 1; Kim-1, kidney injury molecule-1. Quantification of mRNA changes was analysed by a real-time PCR and was calculated by the comparative CT (2−ΔΔCt) method. The mRNA expression levels in the normal control group were used as a calibrator. Values are means, with their standard errors represented by vertical bars (n 8 for each group). Differences between the CLP-C and CLP-G groups were analysed by t test. * Mean value was significantly different from that of the CLP-C group (P < 0·05).
, glutamine group with CLP operation (CLP-G); MIP-2, macrophage inflammatory protein 2; KC, keratinocyte-derived chemokine; MCP-1, monocyte chemoattractant protein-1; HMGB-1, high mobility group box 1; Kim-1, kidney injury molecule-1. Quantification of mRNA changes was analysed by a real-time PCR and was calculated by the comparative CT (2−ΔΔCt) method. The mRNA expression levels in the normal control group were used as a calibrator. Values are means, with their standard errors represented by vertical bars (n 8 for each group). Differences between the CLP-C and CLP-G groups were analysed by t test. * Mean value was significantly different from that of the CLP-C group (P < 0·05).
Discussion
In the present study, dietary GLN was administered for 2 weeks prior to induction of sepsis and was continued till the 3rd day after CLP. This model mimics the preventive use of oral GLN in the nutrition regimen to the abdominal surgical patients during the period of hospital stay who may at risk of sepsis after surgery (such as pancreatoduodenectomy, gastrectomy, colectomy, etc.). Antibiotic was used in the sepsis mice after the operation of CLP, because antibiotic therapy is the standard protocol for the treatment of patients with polymicrobial sepsis. Our study design would be more clinically relevant for studying sepsis-induced remote organ injury, which usually occurs at late phase after sepsis induction and frequently deteriorates despite appropriate antibiotic therapy(Reference Lelubre and Vincent24). T-bet, GATA-3 and ROR-γt are transcription factors that respectively participate in the differentiation of Th1, Th2 and Th17 cells(Reference Chi25). Since naïve T cells are activated in the lymph node or spleen and differentiate into different effector subsets, percentages of T cell activation and gene expression of transcription factors were analysed to evaluate the polarisation of Th subsets and Treg cells in splenic CD4+ T cells. The findings of the present study demonstrated that preventive use of GLN reverses sepsis-induced T lymphocyte and CD4+ T cell decrement and promote the activation of CD4+ T cell subsets, which may attenuate immune dysregulation and remote kidney injury at late phase of sepsis.
A previous study found that naïve T cell activation and their responses were adversely affected during sepsis(Reference Cabrera-Perez, Condotta and Badovinac6). A significant decline of CD4+ T cell numbers in spleen and peripheral blood at 72 h post CLP were reported(Reference Shubin, Monaghan and Heffernan26, Reference Chang, Hou and Pai27). Consistent with the studies mentioned earlier, we also found that the percentages of circulating and splenic T lymphocytes and CD4+ T cells decreased. On the other hand, our findings showed that the IL-4-producing CD4+ T cells and Treg were up-regulated in the CLP-C (positive control) group. IL-4 is a cytokine produced by Th2, whereas interferon-γ is produced by Th1 lymphocytes. Th1 cytokines enhance cellular immunity and Th2 enhance humoral immunity(Reference DiPiro28). Th1 and Th2 response are counter-regulatory. Previous studies have indicated that a shift from Th1 towards Th2 type response occurs during sepsis. Consequently, marked suppression of cellular immunity makes the host more susceptible to infection and increased mortality(Reference Ferguson, Galley and Webster29). Foxp3 is a transcription factor which is responsible for the development and function of Treg cells(Reference Sakaguchi, Yamaguchi and Nomura8). Since the work of Treg cell counteracts the activation of effector Th cell and restrains excessive inflammation, overexpression of Treg may result in immune suppression at late phase of sepsis. Excessive Treg expression was found to increase mortality in the animal model of sepsis(Reference Cabrera-Perez, Condotta and Badovinac6). Although one clinical study showed progressive decline of CD14/HLA-DR expression on circulating macrophages but not the number of Treg cells in patients with severe sepsis as compared with healthy control subjects(Reference Papadopoulos, Pistiki and Theodorakopoulou30), another study showed increased frequency of Treg cells in septic patients(Reference Leng, Liu and Liu31). In the present study, we found that the inflammatory mediators, including IL-6, KC and MCP-1, increased in CLP-C groups, indicating systemic inflammation and T cell dysregulation occurs during polymicrobial sepsis.
Dietary GLN supplementation before sepsis exerts several favorable effects that were not found in the sepsis group without GLN. First, GLN pretreatment maintained the percentage of T lymphocyte and CD4+ T cell population in blood, increased Th1/Th2 ratio and decreased Treg percentage. These findings suggest that a more balanced T cell polarisation was found at late phase of sepsis when pretreated with GLN. An experiment with human PBMC from septic patients revealed that a high dose of GLN could not alter the Th1:Th2(Reference Briassouli, Goukos and Daikos18). Since the study mentioned earlier is an ex vivo experiment, the study design may not reflect the actual physiological situation in the body. Second, consistent with the circulating T cell distribution, GLN administration reversed sepsis-induced T lymphocyte and CD4+ T cell decline in the spleen. Bcl-2 is an anti-apoptotic molecule, whereas Bim is a pro-apoptotic one. A previous study found that Bcl-2 promotes T cell survival by antagonising Bim expression(Reference Wojciechowski, Tripathi and Bourdeau32). The balance between Bcl-2 and Bim plays a critical role in regulating the homeostasis of T cells. Since the Bcl-2:Bim ratio of the splenic CD4+ cells was higher in the CLP-G than the CLP-C, GLN supplementation maintained spleen CD4+ T cell population after sepsis may possibly result from preventing the apoptosis of the T cells. Previous study also found that GLN enhanced γδ T cell activation and prevented γδ T cell apoptosis in sepsis(Reference Hu, Yeh and Pai21). Third, pretreatment with GLN exerted a greater extent of splenic CD4+ T cells activation after sepsis. CD69 is a surface antigen expressed by T cells. CD69 expression can be induced after stimulation and persist for at least 3 d to promote T cell activation and proliferation(Reference Ziegler, Ramsdell and Alderson33). This may partly explain the up-regulated and maintained Th- and Treg-associated transcription factors gene expression observed in the CLP-G group. This finding may also indicate that immunosuppression was not observed in the sepsis group pretreated with GLN. Fourth, GLN pretreatment attenuate kidney injury during sepsis. In the present study, conventional biochemical markers of renal function, including BUN and Cr, were analysed and the levels did not rise after CLP. Although these indicators are frequently used, they are influenced by many non-renal events and considered non-specific for detection of renal injury(Reference Star34, Reference Bellomo, Kellum and Ronco35). Kim-1 is a transmembrane protein expressed on tubule epithelial cells. It is considered as a novel biomarker for acute renal injury(Reference Huo, Zhang and Nie36). In the present study, we found that Kim-1 expression in the GLN sepsis group was down-regulated. Also, the gene expression of inflammatory mediators MIP-2 and KC decreased while anti-apoptotic Bcl-2 gene increased significantly when the sepsis group was pretreated with GLN. MIP-2 is a major CXC chemokine involved in the migration of neutrophil to sites of inflammation(Reference Matzer, Baumann and Lukacs37). KC is a CXC chemokine that play an important role in mediating neutrophil recruitment(Reference Sawant, Poluri and Dutta38). These findings indicated that prophylactic administration of GLN prevented apoptosis and alleviated inflammation that may consequently attenuate kidney injury at late phase of sepsis.
A multicenter clinical trial reported that GLN supplementation had no benefits in clinical outcome and even harmful when given to critical patients with multiorgan failure(Reference Heyland, Muscedere and Wischmeyer39). Previous study also found that early immune-enhancing nutrition supplemented with GLN, arginine and antioxidants may modulate cytokine production, but had no impact on outcome in children with septic shock(Reference Briassoulis, Filippou and Kanariou40). Furthermore, a review article reported that GLN supplementation does not reduce mortality or late-onset sepsis in early life and is not recommended for immune-competent patients(Reference Briassouli and Briassoulis41). Since impairment of GLN utilisation occurs in organ dysfunction, the outcomes mentioned earlier may not apply to different septic situation. A systemic review reported that GLN supplementation reduces mortality and hospital stay in septic patients following resolution of multiorgan failure(Reference Wischmeyer, Dhaliwal and McCall42). GLN is a precursor for the endogenous antioxidant glutathione (GSH). Previous studies demonstrated that GLN promoted T cell proliferation and decreased T cell apoptosis possibly through GLN-GSH axis and the redox capacity(Reference Rohde, MacLean and Klarlund Pedersen43Reference Chang, Yang and Chuang45). GLN pretreatment may provide sufficient fuel source to fulfil metabolic needs and regulate the T cell homeostasis that may consequently result in a more balanced T cell subsets and alleviate inflammation after sepsis. However, the mechanisms responsible for the effects of GLN on regulating T cell polarisation requires further investigation.
In summary, the present study demonstrated that GLN administration before sepsis maintained the percentage of T lymphocyte and CD4+ T cell population in blood, activated and prevented apoptosis of splenic CD4+ T cell and elicited a more balanced systemic CD4+ T cell polarisation. Also, antecedent administration of GLN attenuated inflammation and kidney injury at late phase of sepsis. These findings imply that GLN use may have beneficial effects in patients of abdominal infection risk.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519000990
Acknowledgements
The present study was supported by the Metabolic and Mini-Invasive Surgery Foundation, Taipei, Taiwan.
Y.-C. H and M.-T. L. contributed to the concept and designed the study. Y.-C. H, J.-M. W. and K.-Y. C. did most of the data analysis. P.-D. C and C.-S. L. did part of the analyses. S.-L. Y and M.-T. L. prepared the manuscript. All authors read and approved the final submitted manuscript.
No conflict of interest is declared for the present study.









