Background
Anorexia nervosa is a serious psychiatric condition with a standardised mortality ratio of 6.2 as reported in a recent Swedish registry study.Reference Papadopoulos, Ekbom, Brandt and Ekselius1 Diagnosis requires persistent deliberate energy intake restriction, intense fear of gaining weight and a disturbed self-perception of weight or shape.2 Although a small crude increase in the incidence of anorexia nervosa has been reported,Reference Steinhausen and Jensen3 there has been a decrease in mortality over the past two decades.Reference Papadopoulos, Ekbom, Brandt and Ekselius1 The condition affects most organ systems with 20.8% of patients becoming chronically illReference Steinhausen and Jensen3 and 80% of patients having cardiovascular complications, with a two-fold increased risk of death from cardiovascular causes.Reference Papadopoulos, Ekbom, Brandt and Ekselius1
Guidelines
The National Institute for Health and Care Excellence published revised guidelines in 2017 on eating disorders and advocate acute medical care for those with significant complications.4 The Management of Really Sick Patients with Anorexia Nervosa working group recommend admission to a specialist eating disorder unit for patients with severe anorexia nervosa, but medical admission and intensive care for treatments not available on a psychiatric ward.5 As medical and psychiatric wards may have different physical locations and intensity of medical support, correct identification of those patients that require medical admission is critical to ensure appropriate management.
Echocardiography has developed to the point where high resolution and highly portable cardiac imaging is now available at the bedside. Within the critical care setting cardiac ultrasound is now used widely for diagnosis, monitoring and assessment of therapeutic interventions.Reference Thomas and Colebourn6,Reference Colebourn, Colebourn and Newton7 Therefore, echocardiography may provide a rapid means to evaluate cardiac pathology and risk-stratify patients with anorexia nervosa. We undertook a systematic review and meta-analysis of the literature to determine the severity of cardiac structural and functional abnormalities identifiable with echocardiography in patients with anorexia nervosa.
Method
This protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42018088509) and can be accessed at https://www.crd.york.ac.uk/PROSPEROFILES/88509_PROTOCOL_20180317.pdf. It is reported according to the Preferred Reporting Items for Systematic Review and Meta-Analyses statement (Supplementary Appendix 1 available at https://doi.org/10.1192/bjp.2020.1). Ethical approval was not required because of the retrospective nature of the study. A systematic literature search of all patients with anorexia nervosa who had echocardiogram documented was conducted by a librarian (T.P.) on 19 April 2017, and then repeated on 16 January 2018. There was no essential requirement for a comparison group, but where included, healthy controls were incorporated into this review. The outcome was any echocardiographic abnormality. We searched the Medline, EMBASE, CENTRAL, Cochrane Database of Systematic Reviews and the World Health Organization International Clinical Trials Registry Platform with no date restrictions. We excluded animal studies, non-English citations and conference abstracts from EMBASE. The free-text search included ‘eating disorder’, ‘anorexia nervosa’, ‘bulimia nervosa’, ‘binge eating disorder’ and ‘echocardiography.’ The free-text search was expanded with MeSH terms to ensure full coverage and we deliberately kept the terms broad to maximise the number of citations detected (Supplementary Appendix 2). Of the full-text articles assessed for eligibility (n = 72), we hand-searched all the reference lists.
Exclusion criteria, validity assessment and data extraction
References identified were imported into Mendeley (Mendeley Desktop v1.17.13, MacOS, Mendeley Ltd, London, UK, https://www.mendeley.com/download-desktop/), a reference management software, for de-duplication. Two authors (J.S., C.C.) screened the references independently against the following exclusion criteria: studies not pertaining to anorexia nervosa, primary disease or condition not anorexia nervosa, known other primary disease of the heart, drug-induced heart disease or drug-induced anorexia nervosa, non-cardiac complications of anorexia nervosa, transoesophageal rather than transthoracic echocardiography, normal transthoracic echocardiography findings in anorexia nervosa, literature review and case report.
Papers were excluded on title first and then on abstract. Where there was disagreement between the authors, the paper was included for the next stage of full-text review.
Data extraction
We extracted in duplicate data pertaining to study details (study design, year, country), baseline characteristics of the study population (number of participants, mean age, body mass index (BMI)), assessment details (transthoracic echocardiographic data) and details pertinent to risk of bias analysis (control group BMI, quality of the echocardiographic study, description of follow-up). As a deviation from the published protocol, we sought the assistance of two additional authors: L.P. for the statistical analysis of the data and T.P. for their expertise. Where data was not locatable in the published results, we contacted the corresponding authors of studies.
Quantitative data extraction
For the assessment of continuous outcomes we used mean and s.d. For assessment of binary outcomes we extracted the number of participants with the condition divided by the total number of participants to derive a proportion. Two reviewers (J.S., C.C.) independently assessed the risk of bias at study level, using a modified Newcastle–Ottawa scaleReference Wells, Shea, O'Connell, Peterson, Welch and Losos8 (Supplementary Appendix 3).
Statistical methods
In our meta-analyses for continuous outcomes, we calculated the mean and standardised mean difference (SMD) and the corresponding 95% confidence interval. For dichotomous outcomes we calculated the proportion of participants with the condition and the corresponding 95% confidence interval. SMD calculations were pooled using the Der Simonian–Laird method with random-effects model, because of the heterogeneity of different measures included. Statistical heterogeneity was determined with the Q statistic and I 2 metric. Inconsistency for each outcome not attributable to chance was assessed visually with forest plots; I 2 < 25% reflected low inconsistency and I 2 > 75% reflected high inconsistency. Meta-analyses generated a SMD expressed in s.d. units and SMD of ±0.5 s.d. or greater was considered important. A random-effect meta-regression has been conducted for the left ventricular mass (LVM) parameter, including age, BMI and heart rate as potential confounder (see Supplementary Fig. 1). Unfortunately, most studies did not report on other potential confounders that could contribute to the echo parameters’ variance, such as length of anorexia diagnosis, biochemical alterations and endocrine problems. We did not conduct subgroup analyses because of the lack of consistent reporting across studies of anorexia nervosa behavioural subtypes (restrictive versus purging/binging behaviour). We did perform a sensitivity analysis by age to assess whether similar findings were evident in those aged 16 years and older. Publication bias assessment was conducted using the Fail-safe N analysis with the Rosenthal approach, and funnel plots were visually inspected and assessed with the Egger regression test (see Supplementary Table 1 and Supplementary Figs 2 and 3). To calculate differences between the means of each parameter in patients before and after weight restoration, we used the Mann–Whitney U test and considered statistical significance to be signalled by P values <0.05.
Data were analysed with the MAJOR package for meta-analysis by Kyle Hamilton (https://github.com/kylehamilton/MAJOR#major-meta-analysis-jamovi-r), developed for Jamovi version 0.9.1.10 for MacOS (The Jamovi Project (2019), Jamovi, https://www.jamovi.org).
Results
Eligibility criteria
The database search produced 332 publications in addition to five further references from bibliographic review, of which 229 were non-duplicates. Title and abstract screening excluded 170 papers. Fifty-five full-text articles were reviewed and there were thirty-two further exclusions as outlined in Fig. 1. The majority of the exclusions were owing to insufficient echocardiographic data (13 studies). Nine studies were excluded because of the outcome reported not being relevant to our review; for example, the focus being on other cardiac output measures. Twenty-three studies were included in the qualitative review, with study characteristics summarised in Table 1. Fourteen studies were included in the meta-analysis (469 patients).
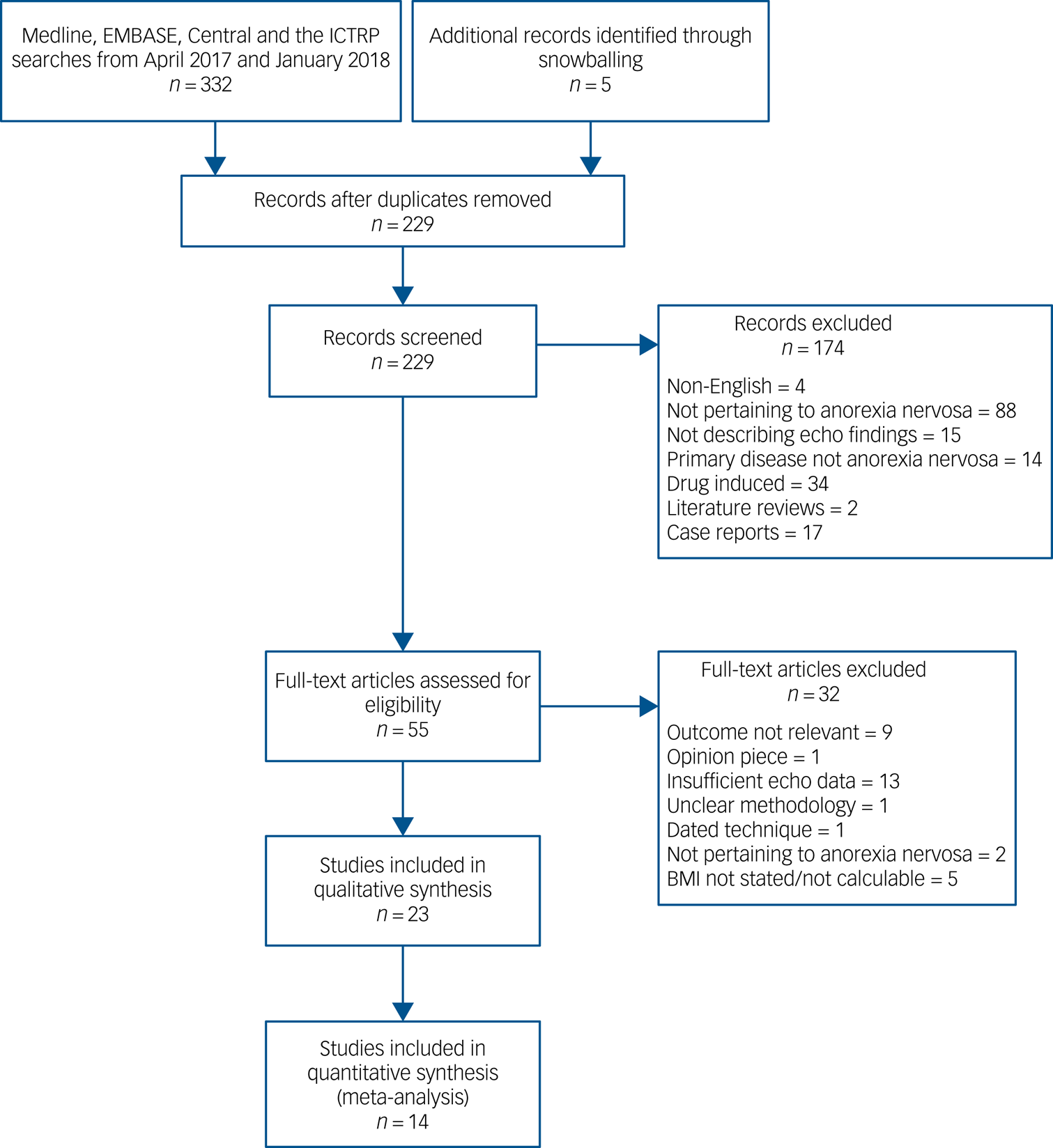
Fig. 1 Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram showing the number of articles screened at each stage.
BMI, body mass index; ICTRP, International Clinical Trials Registry Platform.
Table 1 Characteristics of the included studies
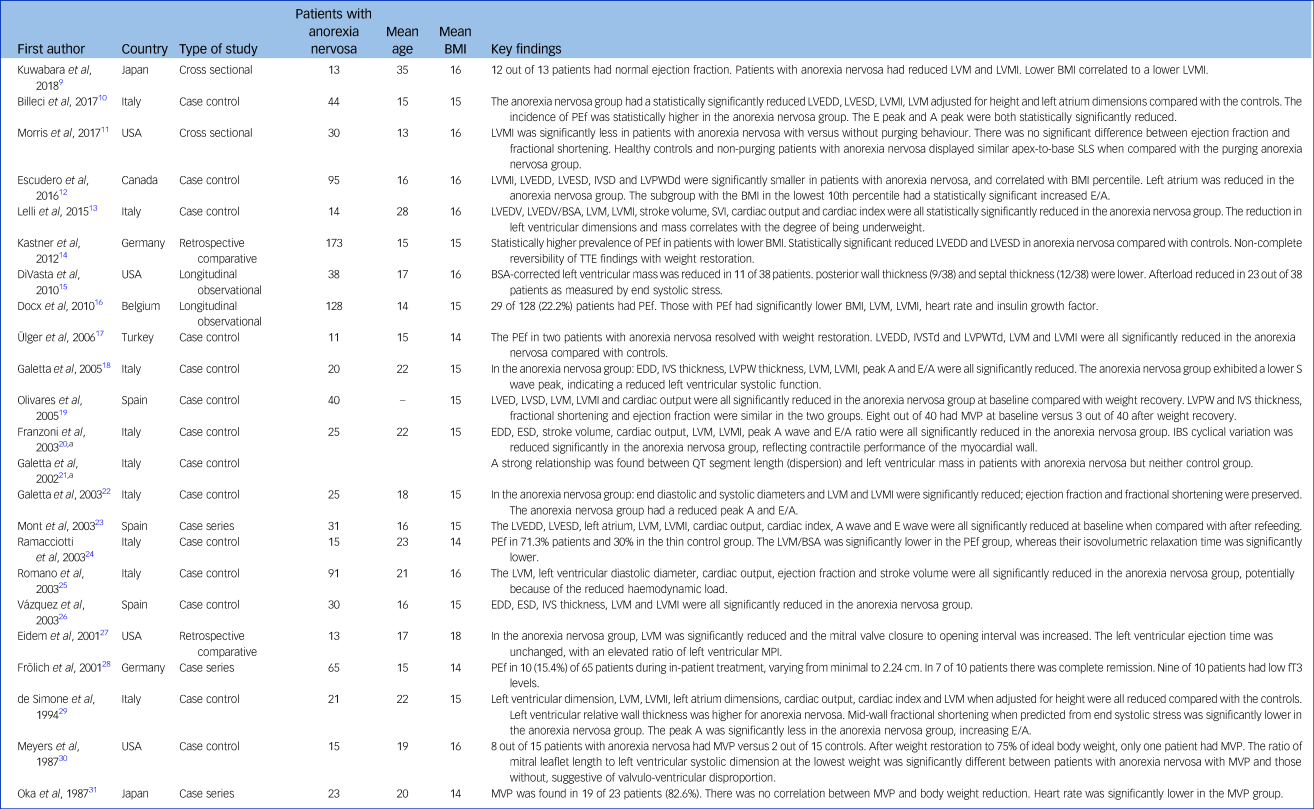
BMI, body mass index; LVM, left ventricular mass; LVMI, left ventricular mass index; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; PEf, pericardial effusion; SLS, segmental longitudinal strain; IVSD, interventricular septal diameter; LVPWDd, left ventricular posterior wall diameter in diastole; LVEDV, left ventricular end diastolic volume; LVEDV/BSA, left ventricular end diastolic volume/body surface area; SVI, stroke volume index; TTE, transthoracic echocardiogram; BSA, body surface area; IVSTd, interventricular septal diameter diastole; LVPWTd, left ventricular posterior wall thickness diastole; EDD, end diastolic diameter; IVS, interventricular septum; LVPW, left ventricular posterior wall; MVP, mitral valve prolapse; ESD, end systolic diameter; IBS, integrated backscatter; LVM/BSA, left ventricular mass/body surface area; MPI, myocardial performance index; fT3, free triiodothyronine.
a. Same data-set.
Electronic communication
A summary with the results of the systematic review and meta-analysis were communicated to authors of the 23 included studies.
Description of study selection
There were fourteen (61%) case-control studies, four (17%) case series, three (13%) retrospective comparative studies and two (9%) longitudinal observational studies. Two studies, which did not report duplicate publication, from the same centre with the same authorship had identical echocardiographic and Doppler measures and therefore only one set of data was included (Table 1).
The number of patients in each study varied from 11 to 173, including a total of 960 patients with anorexia nervosa. The mean age of studied patients was 17 years (range 13–45 years). Overall, the mean BMI was 15.2 kg/m2. BMI was classified (by mean) as ‘mild’ (BMI ≥ 17 kg/m2) in one study, ‘moderate’ (BMI 16–16.99 kg/m2) in two studies, ‘severe’ (BMI 15–15.99 kg/m2) in fourteen studies and ‘extreme’ (BMI ≤ 15 kg/m2) in five studies, using the DSM-5 criteria.2
Study quality assessment
Among the 23 included studies, 3 were at high risk of bias (13%), 11 were at moderate risk (48%) and 9 were at low risk (39%) (Supplementary Appendix 3)
Echocardiographic measures
We identified five echocardiographic themes and one clinical theme that could be examined from the included studies: LVM and size, left ventricular systolic function, diastolic function, valvular disorders, pericardial effusions, and the clinical theme of follow-up with weight restoration. We therefore describe our results below using these categories.
LVM and structure
LVM
LVM measurements are based on estimating the difference between the epicardial and endocardial left ventricular volumes, and multiplying by the standardised myocardial density. In 11 studies, patients with anorexia nervosa had reduced LVM when compared with normal-weight controls, and this was statistically significant (mean difference 32.2 g, 95% CI 23.4–40.0 g; SMD 1.82, 95% CI 1.32–2.31; P < 0.001; see Fig. 2).Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17–Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference Vázquez, Olivares, Fleta, Lacambra and González26,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 This finding was consistent in four of these studies where the anorexia nervosa group was compared with a thin (normal-low BMI) control group (SMD 2.53, 95% CI 1.48–3.59; see Supplementary Fig. 1),Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 and when adults were studied alone (SMD 1.90, 95% CI 1.01–2.80).
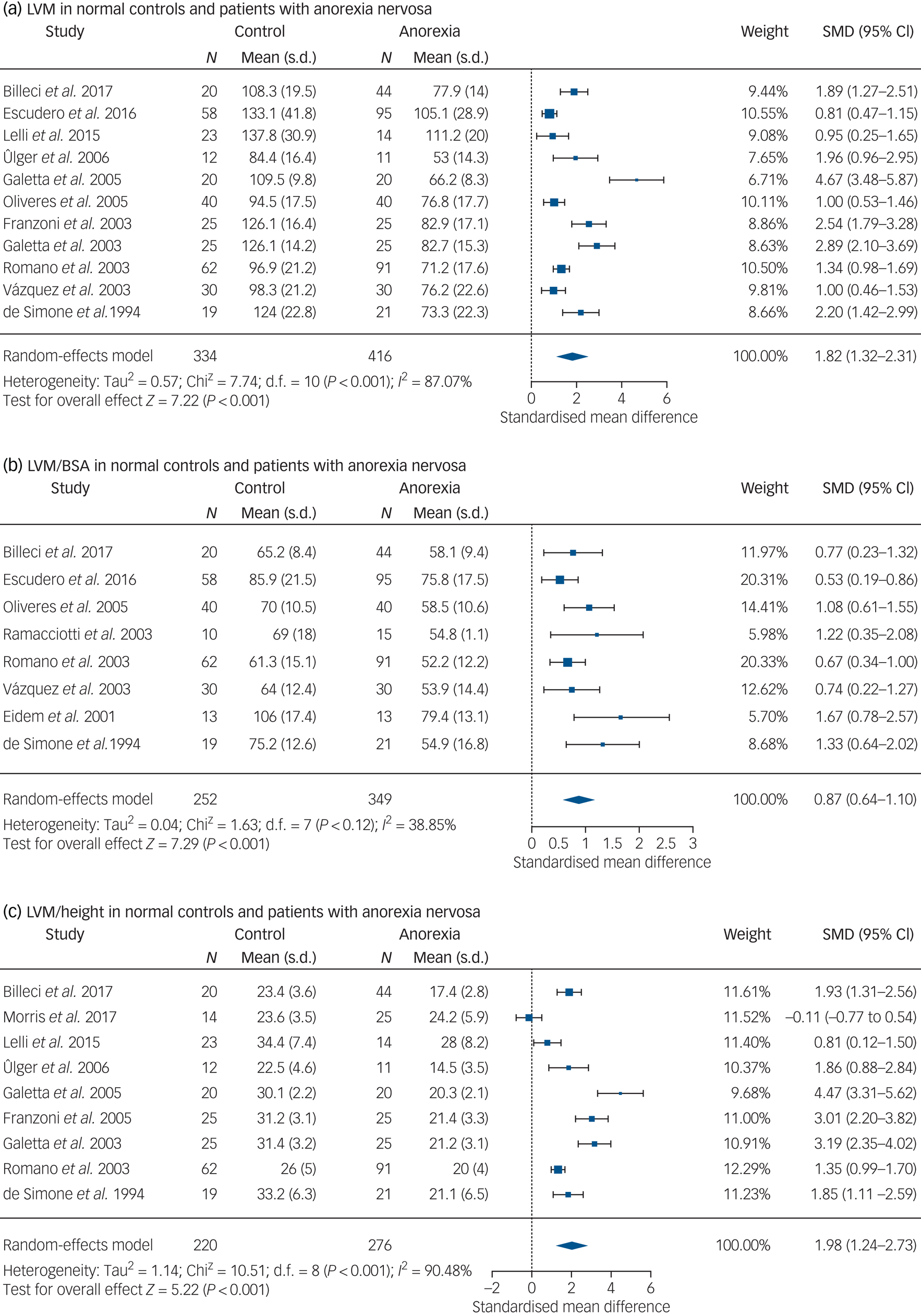
Fig. 2 (a) LVM in normal controls and patients with anorexia nervosa. (b) LVM/BSA in normal controls and patients with anorexia nervosa. (c) LVM/height in normal controls and patients with anorexia nervosa. LVM, left ventricular mass; LVM/BSA, left ventricular mass standardised for body surface area.
SMD, standardised mean difference.
Because of the lack of consistent and exhaustive reporting of possible confounders, we assessed age, BMI and heart rate as possible covariates in two models to explain the variance in the difference of LVM between anorexia nervosa and controls, respectively (Supplementary Fig. 1 of meta-regression scatterplots). In both models, these factors only partially explained the variance in LVM (Supplementary Fig. 4).
There was a statistically significant difference for LVM standardised for body surface area (LVM/BSA) between patients with anorexia nervosa and the control group (mean difference 11.8 g/m2, 95% CI 8.69–14.93 g/m2; SMD 0.87, 95% CI 0.64–1.10; P < 0.001; see Fig. 2).Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Ramacciotti, Coli, Biadi and Dell'Osso24–Reference Eidem, Cetta, Webb, Graham and Jay27,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 Similar findings were seen when adults were studied alone (SMD 0.84, 95% CI 0.53–1.15). When the LVM was standardised for height, the anorexia nervosa group had a reduced LVM/height ratio, which was statistically significant when compared with both healthy controls with a BMI >20 kg/m2 (mean difference 7.42 g/m, 95% CI 4.65–10.2 g/m; SMD 1.98, 95% CI 1.24–2.73; P < 0.001; see Fig. 2)Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 and thin controls with a BMI of 18 kg/m2 (SMD 2.07, 95% CI 0.76–3.39, P = 0.002; see Supplementary Fig. 2).Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29
Fifteen studiesReference Kuwabara, Niwa, Yamada and Ohta9,Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17–Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22–Reference Vázquez, Olivares, Fleta, Lacambra and González26,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 describe a reduction in LVM in patients with anorexia nervosa with correlating factors, including BMI (r 2 = 0.34–0.74, P < 0.004),Reference Kuwabara, Niwa, Yamada and Ohta9,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Vázquez, Olivares, Fleta, Lacambra and González26 heart rate reduction (r 2 = 0.55, P < 0.006)Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17 and free-thyroxine reduction (r 2 = 0.32, P = 0.001).Reference Wells, Shea, O'Connell, Peterson, Welch and Losos8
Left ventricular dimensions
Left ventricular dimensions were quantified in the included studies, using M-Mode assessment of the left ventricular internal diameters. Eleven out of twelve studies describing left ventricular dimensions found a difference in either the systolic, diastolic or wall dimensions in the anorexic population compared with the normal range.Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17–Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference Vázquez, Olivares, Fleta, Lacambra and González26,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 Eleven studies describe a reduction in end diastolic left ventricular cavity dimensionsReference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Kastner, Salbach-Andrae, Renneberg, Pfeiffer, Lehmkuhl and Schmitz14,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17–Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference Vázquez, Olivares, Fleta, Lacambra and González26,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 ranging from 3.69 to 4.13 cm. This represents 4–16.1% absolute reduction compared with controls. Five studies demonstrated a significant reduction in wall thickness: one study affecting the interventricular septum onlyReference Vázquez, Olivares, Fleta, Lacambra and González26 (mean BMI 15.3 kg/m2, 0.61 ± 0.11 cm v. 0.69 ± 0.1 cm in the control group, P = 0.006) and four studies affecting both the interventricular septum and LVPW (mean BMI 15.2 kg/m2).Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18
Left ventricular systolic function
Fractional shortening represents the change in left ventricular mid-cavity size with left ventricular ejection. Ejection fraction represents the fraction of blood ejected from the left ventricle during each cardiac cycle. Stroke volume is the volume of blood that forms the ejection fraction per cardiac cycle, and cardiac output is stroke volume multiplied by heart rate. Data describing the effect of anorexia nervosa on cardiac output were available for seven studies.Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference Vázquez, Olivares, Fleta, Lacambra and González26,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 There was a statistically significant reduction in cardiac output for the anorexia nervosa group compared with healthy controls (mean difference 1.43 l/min, 95% CI 1.03–1.83 l/min; SMD 1.92, 95% CI 1.38–2.45; P < 0.001; see Fig. 3), which was also evident in the adult subgroup (SMD 1.84, 95% CI 1.26–2.42).

Fig. 3 (a) Cardiac output in normal controls and patients with anorexia nervosa. (b) Diastolic function (E/A) in normal controls and patients anorexia nervosa. (c) Proportion of patients with anorexia nervosa and pericardial effusion.
SMD, standardised mean difference.
There were six studies examining the effect of anorexia nervosa on stroke volume. In all six studies the stroke volume was significantly reduced,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Cupisti, Belliti, Prattichizzo and Rolla21,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 but in only two studies was the stroke volume index (adjusted for height) significantly reduced compared with the control group.Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29
Based on ejection fraction and fractional shortening eight studies did not report left ventricular function outside the normal range.Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11,Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Mont, Castro and Herreros23,Reference Vázquez, Olivares, Fleta, Lacambra and González26 In two studies the ejection fraction was significantly reduced compared with the control group.Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25
Beyond ejection fraction, fractional shortening, stroke volume and cardiac output, other techniques for assessing the left ventricular were described. There were five studies that described impaired systolic function in patients with anorexia nervosa,Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Eidem, Cetta, Webb, Graham and Jay27,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 including a subgroup of patients with anorexia nervosa with purging behaviour with significantly decreased apical segmental longitudinal strain (the rate at which the myocardium develops contractile power) values.Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11 Franzoni et al found that cyclic variation in myocardial integrated backscatter was significantly lower in the anorexia group than in the control group when adjusted for body weight, reflecting poor contractile performance of the myocardial wall.Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20 de Simone et al found the percentage predicted m-shortening (mid-wall fractional shortening as a percentage of that predicted from end systolic stress) and percentage predicted e-shortening (endocardial fractional shortening as a percentage of predicted) were significantly reduced in the anorexia nervosa group.Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29
Diastolic function
Diastolic function is measured in the apical four chamber view, using pulsed wave Doppler at the tips of the mitral valve leaflets. This determines peak early filling (E wave) and late diastolic filling (A wave). The E/A ratio is the ratio of these velocities and is normally between 1 and 2. A ratio of greater than two suggests restrictive filling owing to stiffening of the left ventricular wall. In the pooled data of six studies, there was a significant increase in the E/A ratio in the anorexia nervosa group compared with the control group (mean difference 0.61, 95% CI 0.93–0.30; SMD −1.10, 95% CI −1.67 to −0.54; P < 0.001; see Fig. 3).Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference Eidem, Cetta, Webb, Graham and Jay27,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 This finding was also seen when compared with thin controls, in data from four pooled studies (SMD −1.79, 95% CI −2.14 to −1.45; P < 0.001; see Fig. 3).Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29
Four studies reported a normal transmitral E wave velocity and a reduced transmitral A wave velocity (ranging from 33.7 to 43 cm/s, representing an absolute reduction of 23–26%) and an increased E/A ratio (1.96 to 2.8, representing an absolute increase of 22 to 32%) (mean BMI 15.2 kg/m2).Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18,Reference Franzoni, Galetta, Cupisti, Rolla, Santoro and Pentimone20,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 One study reported this finding in the subgroup analysis of the patients with less than 10th percentile of BMI (mean BMI 15 kg/m2).Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12 Multivariable linear regression analysis showed that heart rate was the only independent predictor of E/A ratio.Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18
Valvular disorders
Mitral valve prolapse (MVP) is the most consistently described valvular abnormality, with the two oldest studies reporting primarily on the prevalence of MVP in the anorexia nervosa population.Reference Meyers, Starke, Pearson, Wilken and Ferrell30,Reference Oka, Ito, Matsumoto, Suematsu and Ogata31 To make the diagnosis of MVP one study used M-mode,Reference Meyers, Starke, Pearson, Wilken and Ferrell30 and one study looked at mitral leaflet movement into the left atrium beyond the functional mitral annulus.Reference Oka, Ito, Matsumoto, Suematsu and Ogata31 The studies include a total of 38 patients with anorexia nervosa and report a prevalence of MVP ranging from 53.3 to 82.6%. Only one study used case controls and they report a prevalence of 13% in the control group.Reference Meyers, Starke, Pearson, Wilken and Ferrell30 The study with the highest reported prevalence describes both double- and single-leaflet MVP in 19 out of the 23 patients with anorexia nervosa studied.Reference Oka, Ito, Matsumoto, Suematsu and Ogata31
Four further studies describe the presence of MVP in their populations as a secondary observation without describing how it was measured. The reported prevalence of MVP amongst this second group of studies ranged from 9 to 21%.Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12,Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29
Pericardial effusions
Pericardial effusions are classified according to depth: trace <0.5 cm, small 0.5–1.0 cm, moderate 1.0–2.0 cm and large >2.0 cm. The overall proportion of patients with anorexia nervosa with pericardial effusion in 10 studies was 25% (P < 0.01; 95% CI 0.17–0.34; I 2 = 80%; Supplementary Fig. 3).Reference Kuwabara, Niwa, Yamada and Ohta9–Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Ramacciotti, Coli, Biadi and Dell'Osso24 The effusions were all silent, with the majority being mild to moderate in size. In six out of seven case controlled studies healthy controls were not diagnosed with pericardial effusion.Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10–Reference Kastner, Salbach-Andrae, Renneberg, Pfeiffer, Lehmkuhl and Schmitz14,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17 Only one studyReference Ramacciotti, Coli, Biadi and Dell'Osso24 demonstrated three cases (30%) of pericardial effusion in healthy controls. One study found the LVM/BSA to be significantly lower in the group of patients with anorexia nervosa and a pericardial effusion (54.8 ± 11 v. 68 ± 18 g/m2, P < 0.001).Reference Ramacciotti, Coli, Biadi and Dell'Osso24
Follow-up after weight restoration
There was a significant increase in left ventricular end diastolic diameter and a significant decrease in the proportion of patients with anorexia nervosa with pericardial effusion following weight restoration. The remaining data demonstrates trends in improvement with weight restoration: an increase in the LVM, LVM index, LVM/height, cardiac output; and a reduction in the E/A ratio and the proportion of patients with an MVP (see Table 2).
Table 2 Effect of weight restoration on echocardiography findings
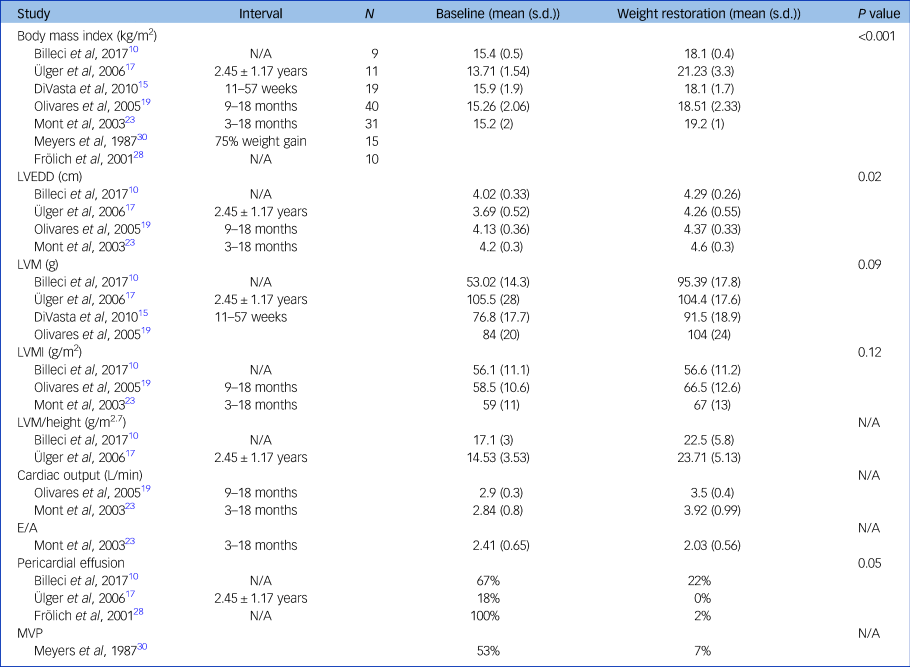
Differences between baseline and weight restoration calculated with the Mann–Whitney U test. LVEDD, left ventricular end diastolic diameter; LVM, left ventricular mass; LVMI, left ventricular mass index; MVP, mitral valve prolapse.
Four studies describe improvements in left ventricular dimensions, left ventricular mass and size with weight restoration.Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Mont, Castro and Herreros23 LVM index and left ventricular end diastolic diameter significantly increased in four studies following refeeding,Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10,Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Mont, Castro and Herreros23 with a mean increase of 13.5%Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Mont, Castro and Herreros23 and 10.3%, respectively.Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Mont, Castro and Herreros23 Two studies describe a significant increase in left ventricular end systolic diameter of between 5 and 9%.Reference Kastner, Salbach-Andrae, Renneberg, Pfeiffer, Lehmkuhl and Schmitz14,Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19 Cardiac output improved by 21–38% with weight recovery in two studies.Reference Olivares, Vázquez, Fleta, Moreno, Pérez-González and Bueno19,Reference Mont, Castro and Herreros23
In two studies demonstrating evidence of MVP, this improved with weight gain.Reference Ramacciotti, Coli, Biadi and Dell'Osso24,Reference Meyers, Starke, Pearson, Wilken and Ferrell30 Oka et al reported a pre-treatment prevalence of MVP at 83%, and after 2–24 months of weight restoration the prevalence reduced to 45%.Reference Oka, Ito, Matsumoto, Suematsu and Ogata31 Meyers et al found that after achieving more than 75% of ideal body weight, the ratio of leaflet length to end systolic dimension (longer length reflecting increased prolapse) in patients with anorexia nervosa changed from 0.86 ± 0.14 to 0.7 ± 0.11 (P < 0.02).Reference Meyers, Starke, Pearson, Wilken and Ferrell30
Five studies including 354 patients describe resolution of pericardial fluid in 62%,Reference Docx, Gewillig, Simons, Vandenberghe, Weyler and Ramet16 66%,Reference Billeci, Brunori, Scardigli, Curzio, Calderoni and Maestro10 70%,Reference Frölich, von Gontard, Lehmkuhl, Pfeiffer and Lehmkuhl28 88%Reference Kastner, Salbach-Andrae, Renneberg, Pfeiffer, Lehmkuhl and Schmitz14 and 100%Reference Ülger, Gürses, Ozyurek, Arikan, Levent and Aydoğdu17 of patients with weight restoration.
Discussion
This review demonstrates anorexia nervosa has significant effects on the structure and the function of the heart, which are easily identifiable by echocardiography. Our key findings are that anorexia nervosa is associated with a reduction in LVM, a reduction in cardiac output, diastolic dysfunction, MVP and pericardial effusions, the resolution of which seemingly occurs with weight restoration.
The reduction in LVM is an observation that persists even when the control group is thin. Malnutrition and immobility may cause cardiac atrophy as it does in skeletal muscle.Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15,Reference Galetta, Franzoni, Prattichizzo, Rolla, Santoro and Pentimone22,Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25 Other postulated mechanisms for the reduction in muscle mass seen in this condition are reduced preload causing left ventricular remodeling;Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11,Reference de Simone, Scalfi, Galderisi, Celentano, Di Biase and Tammaro29 and reduction in afterload, which acts as a stimulus for the downregulation of left ventricular mass to subnormal levels.Reference Romano, Chinali, Pasanisi, Greco, Celentano and Rocco25,Reference St John Sutton, Plappert, Crosby, Douglas, Mullen and Reichek32 The cellular and molecular components of this myocyte volume loss are imperfectly understood. This phenomenon is seen in other animals, such as the Burmese python, which infrequently feeds and can increase its heart mass by 40% following a large meal. This significant hypertrophy, not hyperplasia, is an adaptive response to the increased metabolic rate, with increased triglycerides, free fatty acids and activation of P13 K/Akt/mTOR signalling pathways.Reference Riquelme, Magida, Harrison, Wall, Marr and Secor33 Although age, BMI and heart rate explain some of the differences in LVM between anorexia nervosa and controls, it is only partially explained, and therefore other features of anorexia nervosa are likely to be contributing, such as the length of diagnosis, or associated metabolic and endocrine abnormalities. Heart rate might be a consequence of the cardiac abnormalities seen in patients with anorexia nervosa rather than a biological contributor to these morphological changes.
Our second key finding is that anorexia nervosa is associated with a reduction in cardiac output. Abnormalities in left ventricular systolic function may be related to the changes in structural properties of the myocardium,Reference DiVasta, Walls, Feldman, Quach, Woods and Gordon15 reduced contractility owing to atrophy,Reference Moodie34 ventricular remodelling and altered regional function,Reference Morris, Prasad, Asaro, Guzman, Sanders and Hauck11 nutritional deficiencies (magnesium, phosphorus, thiamine or selenium)Reference Birmingham and Gritzner35 and reduced adherence.Reference Galetta, Franzoni, Cupisti, Morelli, Santoro and Pentimone18
There is a statistically significant increased E/A ratio in patients with anorexia nervosa compared with normal-weight and thin controls, and comparison with thin controls demonstrates homogeneity. There is evidence that left atrial dysfunction and impaired left ventricular filling occurs with severe weight loss.Reference Escudero, Potts, Lam, De Souza, Mugford and Sandor12 Myocardial fibrosis has been demonstrated histologically and on magnetic resonance imaging in patients with anorexia nervosa,Reference Oflaz, Yucel, Oz, Sahin, Ozturk and Yaci36 and may contribute to restrictive physiology.
MVP is common in the anorexia nervosa group, with valvulo-ventricular disproportionReference Meyers, Starke and Pearson37 being a proposed cause. Loss of left ventricular mass causes distortion of left ventricular structure and the atrioventricular tissues, and therefore dysfunction of the valve. Some studies failed to demonstrate full resolution of MVP with weight restoration,Reference Oka, Ito, Matsumoto, Suematsu and Ogata31,Reference Meyers, Starke and Pearson37,Reference Goldberg, Comerci and Feldman38 suggesting that high baseline prolapse is a trait-related marker reflecting a population at high risk of eating or anxiety disorder.Reference Johnson, Humphries, Shirley, Mazzoleni and Noonan39
Twenty-five per cent of patients with anorexia nervosa have a pericardial effusion that is clinically silent, unrelated to hypoproteinaemiaReference Ramacciotti, Coli, Biadi and Dell'Osso24,Reference Kircher, Park, Cheezum, Hulten, Kunz and Haigney40 and mostly resolves with weight restoration. We are aware of two case reports of patients with anorexia nervosa requiring pericardiocentesis.Reference Kircher, Park, Cheezum, Hulten, Kunz and Haigney40,Reference Polli, Blengino, Moro, Zappulli, Scacchi and Cavagnini41 Although our data did not demonstrate it, risk factors include a low BMI.Reference Docx, Gewillig, Simons, Vandenberghe, Weyler and Ramet16 Proposed mechanisms include myocardial wasting and the loss of pericardial fat,Reference Docx, Gewillig, Simons, Vandenberghe, Weyler and Ramet16 causing pericardial layer separation.Reference Ramacciotti, Coli, Biadi and Dell'Osso24
Our results strongly suggest that resolution of cardiac structural changes occur with weight restoration. However notably, re-feeding can be marked by the onset of new severe cardiac complications owing to the abrupt increase in preload, metabolic requirements and electrolyte abnormalities.Reference Ramacciotti, Coli, Biadi and Dell'Osso24 This is the most dangerous time in the management of patients with severe anorexia nervosa and cardiac abnormalities,Reference Birmingham and Gritzner35 with four case reports describing Takotsubo's cardiomyopathyReference Shimizu, Ogura, Wasa, Hirose, Shimazu and Nagasaka42–Reference Abed, Judeh, Abed, Kim, Arabelo and Gurunathan45 owing to refractory hypoglycaemia, a feature of the terminal stages of starvation.Reference Shimizu, Ogura, Wasa, Hirose, Shimazu and Nagasaka42–Reference Abed, Judeh, Abed, Kim, Arabelo and Gurunathan45
One previous systematic review looking at cardiovascular complications in anorexia nervosa has been undertaken.Reference Sachs, Harnke, Mehler and Krantz46 However this review does not meta-analyse the data and is much broader, including repolarisation and conduction abnormalities, haemodynamic changes and peripheral vascular abnormalities. In addition, there have been five key studies since its publication.Reference Kuwabara, Niwa, Yamada and Ohta9–Reference Lelli, Rotella, Castellini, Benni, Lo Sauro and Barletta13
Our results demonstrate strong messages regarding the destruction of normal cardiac structure in anorexia nervosa and include data from 960 patients. Since admission to secondary care with this disease is uncommon, this data-set represents a valuable snapshot of a condition where cardiac abnormalities are consistently seen. We aim to disseminate these findings to patients and families by incorporating them into guidelines and by publicising the results with organisations, which will facilitate dissemination.
Limitations
The current literature comes from high-income countries, limiting the external validity.47 Although availability of echocardiography has traditionally varied between countries, the decreasing cost and greater portability means access to echocardiography for patients with anorexia nervosa will be significantly easier in the future, and therefore will provide a readily available tool to optimise cardiac care for these patients. The studies were heterogenous and many were of small sample size. We did not age restrict the studies since early work has informed current practice. However, the studies span a 25-year period, during which time significant advances have been made in echocardiography. We combined data-sets from children and adults, but it is plausible that a paediatric heart may respond differently to malnutrition compared with an adult heart, which may also be exposed to other cardiovascular risk factors that accumulate with age. Our sensitivity analysis did not demonstrate significant differences when the adult group was studied separately, but further studies with larger samples are likely to be of interest. Other limitations include the retrospective nature of some studies, inconsistent study designs, moderate to high risk of bias, the frequent lack of reporting of the anorexia nervosa subtypes, the differences in illness severity and the different methods of measurements (Supplementary Appendix 4).
In conclusion, patients with anorexia nervosa are generally young and have reversible and potentially catastrophic cardiac changes. To avoid and manage the medical complications in this cohort of patients our findings suggest due diligence should be paid to cardiac involvement. Given the increased availability of echocardiography in the acute and critical care setting, systematic assessment of cardiac changes associated with anorexia nervosa is likely to produce useful data. Longer-term cardiac follow-up of these patients would also be of value to understand the effects of loss of myocyte mass on later health. In tandem with this data collection the authors suggest that inclusion of cardiac structural assessment with agreed triggers should be considered in the next consensus statement.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2020.1.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Acknowledgements
We thank Drs Dimitri Kontogeorgis and Angela Tewari for their advice with the review and Dr George Hadjipavlou for his input with the statistics.
Data availability
The data-set is available from the corresponding author.
Author contributions
J.S. and C.C. designed the study. T.P. conducted the searches. J.S. and C.C. acquired, analysed and interpreted the data. J.S., C.C., L.P., T.P. and P.L. drafted the manuscript. J.S. and L.P. performed the statistical analysis. C.C. and P.L. supervised the study. All authors had full access to the data and can take responsibility for its integrity and accuracy of the analysis. J.S. is the guarantor. The manuscript guarantor (J.S.) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.








eLetters
No eLetters have been published for this article.