The world's population is ageing rapidly, with 21 % of the population (264 million people) estimated to be 60 years or older in 2009. This estimate is projected to increase to 33 % (416 million) by the year 2050(1). The impact of age-related cognitive decline (ARCD) is a major societal health concern, with up to 50 % of adults aged 64 years and over reporting difficulties with their memory(Reference Reid and MacLullich2). In addition to normal cognitive decline with ageing, over 20 million people worldwide are also living with dementia, an estimate that is predicted to reach 80 million by the year 2040(Reference Ferri, Prince and Brayne3). Alzheimer's disease (AD) is the most common form of dementia, affecting about 10 % of the population over the age of 65 years in the USA(Reference Mishra and Palanivelu4). In response to the reality of an ageing population, there has been increased research focus, in recent years, on the development of effective dietary interventions that may be preventative against the onset of dementia and help to ameliorate age-related declines in cognitive ability.
Age-related deficits in cognitive abilities have been consistently reported across a range of cognitive domains, including processing speed, attention, episodic memory, spatial ability and executive function(Reference Zelinski and Burnight5–Reference Verhaeghen and Cerella13). Longitudinal and cross-sectional brain imaging studies have revealed that ARCD is correlated most strongly with cortical volume decreases in the frontostriatal system(Reference Bugg, Zook and DeLosh14–Reference West18), with decreases in prefrontal cortex volume estimated to occur at a rate of approximately 5 %/decade after the age of 20 years(Reference Hedden and Gabrieli16, Reference Kramer, Fabiani, Colcombe, Birren and Schaie17). Age-related reductions in grey matter are due to a number of factors in addition to neuronal loss, including shrinkage of neurons, reduction of synaptic spines and lowered numbers of synapses. In contrast, age-related reductions in white matter may be attributed in part to large reductions in the length of myelinated axons, by as much as 50 %(Reference Fjell and Walhovd19). Current understanding of ARCD points to multiple aetiological factors in the brain. These include depletion of endogenous antioxidants(Reference Gracy, Talent and Kong20), elevation in NO(Reference Calabrese, Mancuso and Calvani21) and homocysteine (HCy) levels(Reference Haan, Miller and Aiello22), chronic inflammation(Reference Zipp and Aktas23), glutamatergic excitotoxicity(Reference Hynd, Scott and Dodd24), accumulation of redox metals(Reference Rogers and Lahiri25), mitochondrial dysregulation(Reference Kidd26) and Ca dysregulation(Reference Thibault, Porter and Chen27), as well as abnormal insulin levels and/or responsiveness(Reference Craft and Watson28).
In the case of AD, additional pathological changes occur in the brain, comprising the formation of extracellular senile plaques from β-amyloid (Aβ) proteins and intracellular neurofibrillary tangles from τ proteins, resulting in widespread damage to neural structures and profound impairment to cognitive abilities(Reference Kidd29–Reference Hardy and Selkoe31). The current Food and Drug Administration-approved pharmaceutical treatments for dementia are the cholinesterase inhibitors tacrine, donepezil, rivastigmine and galantamine, as well as the N-methyl-d-aspartate receptor antagonist memantine(Reference Wang, Wang and Wei32, Reference May, Lit and Xue33). Despite the recent UK National Institute for Clinical Excellence recommendation to reverse its previous decision and allow these drugs for mild as well as moderate AD, the cholinesterase inhibitors are not always well tolerated, with all four having adverse effects related to cholinergic hyperactivity including nausea, vomiting, diarrhoea, fatigue, muscle cramps and dizziness. There are also few data to support the notion of cholinesterase inhibitors providing any more than symptomatic relief by increasing dwindling levels of acetylcholine as the disease progresses(Reference Jones34). Furthermore, few studies have been conducted that assess the efficacy of cholinesterase inhibitors for longer than 1 year duration or answer the question as to whether they can significantly delay the progression from mild cognitive impairment to AD(Reference Raina, Santaguida and Ismaila35).
In consideration of the diverse aetiology of ARCD and dementia, together with the limitations of current pharmaceutical treatments, attention has turned to consideration of dietary constituents for their potential to simultaneously target multiple mechanisms and play a prophylactic role(Reference Van der Schyf, Gal and Geldenhuys36). Epidemiological and prospective cohort studies have identified a number of dietary constituents that are associated with a reduction in ARCD and a lowered risk of dementia. The Mediterranean diet, with a high intake of MUFA from olive oil, high consumption of fish and whole-grain cereals and a moderate intake of red wine, has been found to be protective against ARCD in a number of studies(Reference Panza, Solfrizzi and Colacicco37, Reference Solfrizzi, Panza and Capurso38). Other dietary constituents that have been found to be protective against ARCD are antioxidants, including vitamins E and C, fruit and vegetables and the B vitamins (B6, B12 and folate)(Reference Smith and Blumenthal39). Until recently, the efficacy of dairy products in ameliorating ARCD has received comparatively less research focus. However, a growing body of epidemiological research now links dairy consumption to a number of beneficial health outcomes, including lowered risk of the metabolic syndrome, heart disease and stroke(Reference Weaver40). In light of the considerable inter-relationship between CVD, the metabolic syndrome and brain function(Reference Keller41), it is timely to assess the impact that dairy constituents have on neurocognitive health. The objective of the present study is to review the evidence linking different forms of dairy products with amelioration of ARCD, when used regularly as part of the diet over the lifespan. The review also presents current theories regarding likely mechanisms of action by which the main constituents of dairy products may have an impact on neurocognitive health.
Dairy products and general health
A number of epidemiological studies have provided evidence to suggest that consumption of dairy products, in particular low-fat dairy products, may be associated with a number of beneficial health outcomes. These benefits include a decrease in systolic blood pressure(Reference van Meijl and Mensink42–Reference Engberink, Geleijnse and De Jong45), a decrease in incidence of type 2 diabetes and insulin resistance(Reference Ünal, Akalin and Akbulut46–Reference Elwood, Pickering and Givens48), as well as a decreased risk of stroke and heart disease(Reference Elwood, Givens and Beswick47–Reference Van Der Pols, Gunnell and Williams49). Low-fat dairy product intake has also been found to be associated with decreased levels of inflammatory markers(Reference Esmaillzadeh and Azadbakht50, Reference van Meijl and Mensink51) as well as with a reduced risk of colorectal cancer(Reference Elwood, Givens and Beswick47, Reference Huncharek, Muscat and Kupelnick52, Reference Pufulete53). In a 65-year follow-up study of the Boyd Orr cohort, a childhood diet that was high in dairy products was not found to be associated with a greater risk of heart disease or stroke, and Ca intake during childhood was found to be inversely associated with stroke mortality(Reference Van Der Pols, Gunnell and Williams49). A prospective Japanese study has also provided evidence to suggest that milk intake is inversely associated with the risk of developing vascular dementia(Reference Yamada, Kasagi and Sasaki54). Conversely, some studies have suggested the possibility of an increased risk of prostrate cancer associated with high dairy product consumption, although the evidence is inconsistent(Reference Huncharek, Muscat and Kupelnick52, Reference Huncharek, Muscat and Kupelnick55). While much of this evidence has been obtained from epidemiological and prospective cohort studies rather than from randomised controlled clinical trials, it is nonetheless encouraging evidence in support of low-fat dairy products being of positive health benefit.
Concerns over fatty acid content in dairy products
There is now a general consensus that dietary intake of SFA represents a significant risk factor for CVD, due to elevation of serum LDL-cholesterol(Reference Zock56). Dairy products have been found to be the primary source of dietary SFA(Reference Henderson, Gregory and Irving57), with an estimated 40 % of all SFA obtained through milk and dairy products in the UK(Reference Hulshof, Van Erp-Baart and Anttolainen58); and for this reason, there has been concern that the consumption of dairy products may increase the risk of CVD(Reference Berner59). However, an important distinction needs to be made between milk and other forms of dairy products. The proportion of SFA and other fatty acids in cheese and butter is substantially higher than that found in milk(Reference Aro, Antoine and Pizzoferrato60). Table 1 lists the fatty acid composition of milk, butter and cheese from a selection of European countries. Here, it can be seen that, while the proportions of fatty acids are roughly equivalent between the three forms of dairy product, the overall total fat content (g/100 g) of butter and cheese is much higher. The epidemiological evidence suggests that it is the ratio of high-fat:low-fat dairy products that is associated with a greater risk of CHD, not dairy consumption per se (Reference Hu, Stampfer and Manson61). Nevertheless, in consideration of the widespread consumption of bovine milk, investigations have begun into developing techniques to reduce the SFA content and increase the cis-MUFA content of milk(Reference Givens62).
Table 1 Fatty acid composition of milk, butter and cheese across European countries*†
(Mean values and percentages of methyl esters)
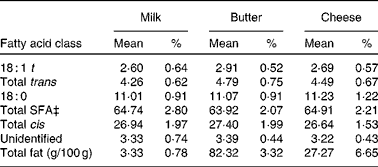
* Countries included are Belgium, Denmark, Finland, France, Germany, Greece, Iceland, Italy, The Netherlands, Norway, Portugal, Spain, Sweden and the UK.
† Adapted from Aro et al. (Reference Aro, Antoine and Pizzoferrato60).
‡ Excluding less than eight-carbon fatty acids.
It is also important to note that certain fatty acids found in milk may not represent as much of a risk factor for CVD as originally thought. There is evidence to suggest that lauric acid (12 : 0) and myristic acid (14 : 0) increase the levels of protective HDL-cholesterol, in addition to LDL-cholesterol, thereby helping to reduce the ratio of total cholesterol:HDL-cholesterol(Reference Givens63, Reference Mensink, Zock and Kester64). However, palmitic acid (16 : 0) has been found to have a less favourable effect on ratios of total cholesterol to HDL-cholesterol(Reference Givens62). In addition to SFA, dairy products also contain a number of other fatty acids. cis-MUFA is a fatty acid which has been found to be beneficial in reducing the risk of CVD(Reference Hu, Stampfer and Manson65), and dairy products have been found to supply up to 30 % of dietary intake of cis-MUFA in some European countries(Reference Hulshof, Van Erp-Baart and Anttolainen58). Trans-fatty acids, which are generally associated with a substantially increased CVD risk(Reference Willett, Stampfer and Manson66), are also found in dairy products. It has been estimated that, on average, dairy products contribute 38 % of total trans-fatty acids across European countries(Reference Hulshof, Van Erp-Baart and Anttolainen58). It is worth noting that the isomeric forms of trans-fatty acids found in dairy products do not appear to be as detrimental to cardiovascular health as the trans-fatty acid isomers that are derived from industrial hydrogenation of vegetable oils(Reference Chardigny, Destaillats and Malpuech-Brugère67).
Dairy and the metabolic syndrome
The cluster of disorders including dyslipidaemia (high fasting TAG levels combined with low HDL-cholesterol levels), hypertension, obesity and glucose intolerance has become grouped together under the umbrella term metabolic syndrome(Reference Grundy, Brewer and Cleeman68). A diet with a low glycaemic index that is high in whole-grain cereal and has a high unsaturated fatty acids:SFA ratio has been found to be associated with a lower prevalence of the metabolic syndrome(Reference Pereira, Jacobs and Van Horn69–Reference Nelson, Schmidt and Kelley71). Due to the fatty acid content of milk fat, it was originally suspected that milk consumption would have a negative impact on the metabolic syndrome; however, several studies have subsequently revealed that consumption of milk and other dairy products is inversely correlated with the metabolic syndrome(Reference Pereira, Jacobs and Van Horn69, Reference Pfeuffer and Schrezenmeir72, Reference Van Meijl, Vrolix and Mensink73). Various constituents of dairy products that have a beneficial effect on the metabolic syndrome have been identified; for example, whey protein has been found to be insulinotropic(Reference Nilsson, Stenberg and Frid74), milk peptides and Ca have been found to reduce blood pressure(Reference Nakamura, Yamamoto and Sakai75, Reference McCarron and Reusser76) and milk peptides and Ca may also help reduce plasma cholesterol(Reference Nagaoka, Futamura and Miwa77). Ca has been found to have a particularly beneficial effect on serum lipid profiles, with evidence from a number of studies demonstrating that Ca increases HDL-cholesterol while decreasing both total cholesterol and LDL-cholesterol(Reference Van Meijl, Vrolix and Mensink73). A possible mechanism by which Ca may affect lipoprotein metabolism is through the inhibition of fat absorption in the small intestine(Reference Denke, Fox and Schulte78).
There is increasing evidence of a link between the metabolic syndrome and both cognitive decline and dementia, particularly in individuals with high levels of inflammation(Reference Yaffe79, Reference Yaffe, Kanaya and Lindquist80). Epidemiological evidence links many of the components of the metabolic syndrome to increased ARCD and dementia, including hypertension(Reference Launer, Masaki and Petrovitch81, Reference Qiu, Winblad and Fratiglioni82), diabetes(Reference Gregg, Yaffe and Cauley83, Reference Yaffe, Blackwell and Kanaya84), dyslipidaemia(Reference Moroney, Tang and Berglund85, Reference Yaffe, Barrett-Connor and Lin86) and obesity(Reference Whitmer, Sidney and Selby87). There are a number of mechanisms that may potentially link the metabolic syndrome to cognitive decline. These mechanisms include micro- and macrovascular disease(Reference Launer88), inflammation and atherosclerosis(Reference Yaffe, Lindquist and Penninx89), as well as the secretion of inflammatory factors from adipose tissue(Reference Fewlass, Noboa and Pi-Sunyer90, Reference Benoit, Clegg and Seeley91). However, perhaps the most important mechanism linking the metabolic syndrome to cognitive decline is that of glucose regulation(Reference Craft and Watson28, Reference Luchsinger, Tang and Shea92–Reference Yaffe, Blackwell and Whitmer94).
Glucose regulation and brain function
In healthy, non-diabetic subjects, blood glucose levels peak at approximately 1 h after the start of a meal and then return to baseline levels within 2–3 h(95, Reference Polonsky, Given and Hirsch96). Within 10 min of food intake, there is a large release of endogenous insulin known as the first-phase insulin response. However, for individuals with increased insulin resistance or type 2 diabetes, the first-phase insulin response is either absent or significantly reduced, resulting in chronically elevated glucose levels throughout the day(Reference Pfeifer, Halter and Porte97). As a result of prolonged hyperglycaemia, insulin levels also remain high, and with continued elevation of insulin levels, insulin resistance develops(Reference Del Prato, Leonetti and Simonson98).
Those with good glucose tolerance, that is those whose blood glucose returns to baseline quickly following a meal, have been shown to demonstrate better memory than those whose blood glucose levels remain elevated for longer periods of time(Reference Craft, Murphy and Wemstrom99). Also in healthy young adults, poor gluco-regulation, and thus a greater increase in blood glucose after a glucose drink, has been associated with poorer performance on tests of memory(Reference Owens and Benton100, Reference Donohoe and Benton101), vigilance(Reference Donohoe and Benton101, Reference Benton, Owens and Parker102), planning(Reference Donohoe and Benton101) and dichotic listening(Reference Parker and Benton103). Preclinical evidence suggests that feeding young rats with high levels of saturated fats leads to impaired glucose tolerance and reduced insulin sensitivity(Reference Clandinin, Cheema and Field104–Reference Storlien, Higgins and Thomas106), resulting in memory impairment(Reference Greenwood and Winocur107, Reference Greenwood and Winocur108). One experiment(Reference Greenwood and Winocur109) examined the impact of gluco-regulation on a variable interval-delayed alternation task, a task that is associated with hippocampal function. Rats fed high-fat diets produced performance deficits, suggesting that impaired glucose regulation moderated hippocampal-dependent memory. However, these deficits were reversed by a 100 mg/kg glucose injection(Reference Greenwood and Winocur109). Interestingly, there was no effect of glucose in animals that received a normal diet, suggesting that glucose was only effective in cases where there was a pre-existing deficit in glucose regulation.
In the elderly, there is a natural decline of gluco-regulation. Messier et al. (Reference Messier, Tsiakas and Gagnon110) compared the cognitive performance of older participants, aged 55–84 years, with better glucose regulation with those with poorer glucose regulation (as measured by the increase in blood glucose from a fasting to peak level following a glucose tolerance test). It was observed that older participants with poorer glucose regulation were impaired in several tests, including verbal memory and arithmetic tasks(Reference Messier, Tsiakas and Gagnon110). Some studies have also detected cognitive effects associated with impaired glucose regulation in younger participants, although the impairment is less noticeable in comparison with older participants(Reference Awad, Gagnon and Desrochers111, Reference Messier, Desrochers and Gagnon112). It has been found that those with poorer glucose regulation performed worse on several verbal declarative measures, including immediate and delayed paragraph recall, verbal-free recall and order reconstruction tasks(Reference Awad, Gagnon and Desrochers111). Donohoe & Benton(Reference Donohoe and Benton113) performed a glucose tolerance test on undergraduate students and measured blood glucose levels every 30 min following ingestion of a glucose drink. They observed that between 2 and 3 h after the ingestion of 50 g glucose, there was a dip in blood glucose below fasting levels that was followed shortly by a return to fasting levels. They found that the quicker the return to fasting levels, the better the performance on cognitive tests of memory consolidation and retrieval. Other measures of glucose levels did not correlate with memory performance except that faster reaction time was found to be associated with higher baseline blood glucose during the test performance.
A number of other gluco-regulatory indices have been previously evaluated for their relationship with cognitive performance in both younger and older participants. These include fasting blood glucose levels, peak glucose levels, recovery and evoked glucose to baseline levels and incremental area under the curve. Overall evidence suggests that gluco-regulation may exert direct effects on cognitive function so that those with poor gluco-regulation demonstrate mild cognitive deficits in comparison with those with good gluco-regulation(Reference Craft, Murphy and Wemstrom99, Reference Donohoe and Benton101, Reference Awad, Gagnon and Desrochers111, Reference Donohoe and Benton113–Reference Vanhanen, Koivisto and Kuusisto119). However, there is evidence to suggest that a long-term deficit in gluco-regulation may also result in chronic cognitive impairments(Reference Lamport, Lawton and Mansfield120) and be a contributing factor in the development of ARCD and dementia(Reference Craft and Watson28). A number of epidemiological studies have provided evidence to suggest that type 2 diabetes is a significant risk factor for developing dementia(Reference Gregg, Yaffe and Cauley83, Reference Leibson, Rocca and Hanson121–Reference Ott, Stolk and Van Harskamp123).
Whey protein, obesity and glucose regulation
In bovine milk, whey protein consists of α-lactalbumin, β-lactoglobulin, IgG, serum albumin, IgA and trace amounts of lactoferrin(Reference Korhonen and Pihlanto124). In terms of protein in the diet, it has been previously observed that free consumption of a high-protein diet increases the rate of fat loss in obese subjects compared with those on low-protein diets(Reference Skov, Toubro and Rønn125). It is believed that weight loss by increased density of protein in the diet is due to increased satiety leading to reduced total energy intake coupled with greater total energy expenditure (due to increased thermogenesis from protein digestion)(Reference Crovetti, Porrini and Santangelo126). However, these studies have used mixed protein meals, with more recent research suggesting that the type of protein may be of particular importance for improving body composition. Current evidence suggests that ‘complete’ proteins that contain all essential amino acids show larger increases in energy expenditure following consumption in comparison with lower-quality proteins(Reference Westerterp-Plantenga, Nieuwenhuizen and Tomé127). Whey protein is not only more complete than other forms of protein such as egg albumin or protein derived from red meat, but also it may offer additional benefits. It is high in branched-chain amino acids, in particular leucine, which means whey is particularly beneficial for preventing muscle-wasting during weight-loss programmes(Reference Zemel128). In terms of fat reduction, whey protein concentrate has been shown to reduce body weight in rats(Reference Belobrajdic, McIntosh and Owens129, Reference Badger, Ronis and Hakkak130), whereas increased dietary red meat has been demonstrated to increase body weight in rats(Reference Belobrajdic, McIntosh and Owens129). In a clinical trial with elderly women, a 15 d whey supplement was also found to significantly lower body weight(Reference Hays, Kim and Wells131).
Obesity has been found to be a significant factor in the development of insulin resistance, due to chronic systemic inflammation in adipose tissue(Reference O'Rourke132). Furthermore, in elderly subjects, there is also a natural decline of gluco-regulation(Reference Messier, Tsiakas and Gagnon110). Therefore, any dietary intervention which assists in the maintenance of healthy body weight may also assist in maintaining efficient glucose regulation, which, as mentioned previously, results in better cognitive function both acutely and chronically(Reference Owens and Benton100, Reference Donohoe and Benton101, Reference Lamport, Lawton and Mansfield120). In a recent study by Belobrajdic et al. (Reference Belobrajdic, McIntosh and Owens133), insulin-resistant rats fed a high-whey-protein diet for 6 weeks have been found to have significantly reduced energy intake and body fat. In comparison with rats receiving protein in the form of red meat, rats receiving the high-whey-protein diet have also been found to have significantly lower weight gain and significantly increased insulin sensitivity. The authors concluded that the improved insulin sensitivity was due to a reduction of visceral fat.
Abnormal insulin levels and dementia
Early-stage AD has been found to be associated with high insulin concentrations in response to glucose challenge (hyperinsulinaemia) in combination with reduced insulin-mediated glucose uptake (insulin resistance) in a number of individuals(Reference Craft, Newcomer and Kanne134). In the central nervous system (CNS), insulin has the effect of promoting the release of intracellular Aβ(Reference Gasparini, Gouras and Wang135), with aggregation of Aβ known to be a central feature of AD pathophysiology(Reference Hardy and Selkoe31). In chronic peripheral hyperinsulinaemia, insulin crosses the blood–brain barrier and promotes an increase in the release of Aβ into extracellular compartments(Reference Craft and Watson28). Due to high plasma levels of insulin, there will also be increased concentrations of peripheral Aβ that will result in obstruction to the clearance of Aβ from the brain(Reference Craft and Watson28). Furthermore, increased levels of insulin in the CNS also inhibit the degradation of Aβ. This is because insulin-degrading enzyme plays an important role in clearing intracellular Aβ(Reference Kurochkin and Goto136, Reference Qiu, Walsh and Ye137), and when there are high levels of insulin in the brain, it must compete with insulin as a target(Reference Craft and Watson28).
However, with sustained high levels of insulin in the brain, there is an eventual down-regulation of insulin transport into the brain and inhibition of brain synthesis of insulin(Reference Schwartz, Figlewicz and Kahn138, Reference Gerozissis, Orosco and Rouch139). With a lowered level of insulin in the brain, there is a reduction in the release of Aβ from intracellular compartments as well as a reduction in insulin-degrading enzyme, also bringing about reduced clearance of Aβ from the brain(Reference Craft and Watson28). Furthermore, a reduction in ACh and cerebral blood flow has also been found to be associated with low concentrations of insulin in the CNS(Reference Hoyer140). Thus, both increased and decreased insulin concentrations in the brain contribute to the pathogenesis of AD. In addition to Aβ aggregation, there is also evidence to suggest that low brain concentrations of insulin may lead to an increase in tau hyperphosphorylation(Reference Hong and Lee141), while high peripheral insulin levels have been found to exacerbate central inflammation(Reference Sabayan, Foroughinia and Mowla142, Reference Fishel, Watson and Montine143) and oxidative stress(Reference Facchini, Hua and Reaven144).
Influence of whey protein on insulin release
Milk products, in particular the whey fraction, have been found to have an insulinotropic effect both in normal subjects and type 2 diabetics(Reference Nilsson, Stenberg and Frid74, Reference Östman, Liljeberg Elmståhl and Björck145, Reference Frid, Nilsson and Holst146). Whey is a rapidly digested protein, which promotes a higher concentration of amino acids in the plasma following its consumption(Reference Boirie, Dangin and Gachon147). Amino acids are known to stimulate insulin release in the pancreatic β-cell(Reference Floyd, Fajans and Conn148). Nilsson et al. (Reference Nilsson, Holst and Björck149) reported that the branched-chain amino acids leucine, isoleucine and valine, together with lysine and threonine, were the most efficient insulin secretagogues. Table 2 displays the typical amino acids found in whey, together with the quantities (mg/g) from the study by Nilsson et al. (Reference Nilsson, Holst and Björck149).
Table 2 Amino acids in whey*
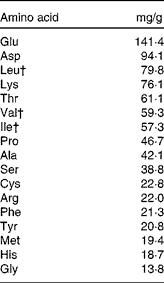
* Adapted from Nilsson et al. (Reference Nilsson, Holst and Björck149).
† Branched-chain amino acids.
The benefit of acute increases in insulin secretion following the consumption of whey protein is that the resultant peak in blood glucose is not as high as it otherwise would be. Frid et al. (Reference Frid, Nilsson and Holst146) reported that the plasma glucose 180 min area under the curve was reduced by 21 %, when whey was included in the lunch meal in comparison with a reference meal containing ham. Reducing the postprandial glucose peak has been suggested as a more effective means of treating diabetes than targeting fasting blood glucose levels(Reference Fonseca150). Epidemiological evidence suggests that, even among overweight individuals, regular dairy product consumption is associated with a significantly lower incidence of the insulin resistance syndrome(Reference Pereira, Jacobs and Van Horn69). In light of the evidence suggesting that consumption of dairy products may have a positive effect on glucose regulation(Reference Ünal, Akalin and Akbulut46), it is feasible that there may be acute, as well as chronic, cognitive benefits associated with regular consumption.
Dairy constituents as direct modulators of cognition
In addition to the cognitive benefits of dairy products associated with improvement to gluco-regulation, there are also a number of components of dairy products that have the potential to influence brain function directly. The composition of milk will be used as an example of the typical components found in dairy products(Reference Goff151): water (85·5–88·7 %), milk fat (2·4–5·5 %) and solids (non-fat, 7·7–10 %). The composition of the milk solids may be summarised as follows: lactose 4·6 %, protein 3·25 % (80 % caseins, 20 % whey proteins, e.g. α-lactalbumin and β-lactoglobulin), minerals 0·65 % (Ca, P, Mg, K, Na, Zn, Cl, Fe, Cu and sulphate), acids 0·18 % (citrate, formate, acetate, lactate and oxalate), enzymes (peroxidase, catalase, phosphatase and lipase) and vitamins (A, B12, C, D, thiamin and riboflavin). A growing body of research has now reported positive effects on brain function and cognitive ability that are associated with a number of the components of dairy products.
Bioactive peptides
Biologically active peptides have been defined as protein fragments that have a positive impact on bodily functions or conditions and may ultimately influence health(Reference Kitts and Weiler152). Milk proteins act as precursors for the formation of bioactive peptides, with the size of the active sequences varying from two to twenty amino acids(Reference Kitts and Weiler152). Casein represents a major proportion (80 %) of the protein content in bovine milk, and contains a large quantity of branched-chain amino acids(Reference Nakamura, Iwamoto and Ogata153). Phosphorylated regions are contained in bovine αs1-casein, αs2-casein and β-casein, regions that may subsequently be released by digestive enzymes(Reference Korhonen and Pihlanto154). The digestive enzymes such as pepsin, trypsin and chymotrypsin have been shown to release a variety of different peptides through hydrolysis of both the casein and whey proteins found in milk. Bioactive peptides are also produced through fermentation of milk with starter cultures, or proteolysis by plant or micro-organism enzymes(Reference Korhonen155). However, yogurt, cheese and probiotic bacteria have been found to produce different bioactive peptides during milk fermentation(Reference Donkor, Henriksson and Vasiljevic156). Additionally, a great variety of bioactive peptides have also been found to be formed during the cheese-ripening process; e.g. Parmigiano-Reggiano, β-casein f(8–16), f(58–87), αs2-casein f(83–88); Gouda, αs1-casein f(1–9), β-casein f(60–68); Festivo, αs1-casein f(1–9), f(1–7), f(1–6); Italian varieties of Mozzarella, Crescenza, Italico and Gorgonzola, β-casein f(58–72)(Reference Korhonen and Pihlanto154).
A range of different functions have been studied in relation to the biologically active peptides. The most intensively studied function has been that of the blood pressure-reducing (hypotensive) peptides, which inhibit angiotensin-converting enzyme I(Reference Murray and FitzGerald157, Reference Clare and Swaisgood158). There is evidence to suggest that the angiotensin-converting enzyme I inhibitory potency of cheese increases during the ripening process(Reference Meisel, Goepfert and Günther159). Casein-derived bioactive peptides with varying angiotensin-converting enzyme I inhibitory potencies have been isolated from a variety of Italian cheeses: Crescenza (37 % inhibition), Mozzarella (59 % inhibition), Gorgonzola (80 % inhibition) and Italico (82 % inhibition)(Reference Smacchi and Gobbetti160). In feeding experiments using spontaneously hypertensive rats, reductions in systolic blood pressure have been found to be significant 6 h following ingestion of the cheese varieties Gouda, Blue, Edam and Harvati(Reference Saito, Nakamura and Kitazawa161).
A number of bioactive peptides have also been found to be opioid receptor ligands with agonistic or antagonistic activities. β-Casomorphins, which are hydrolysed fragments of β-casein, were the first opioid peptides to be discovered. The adult human intestine has not been found to be permeable to casomorphins, as there has been a failure to detect their presence in the blood plasma following ingestion(Reference Teschemacher, Koch and Brantl162). For this reason, the opioid effects of casomorphins are believed to occur only at a peripheral level, causing a reduction in intestinal transit time and modulating the absorption of amino acids and the transport of electrolytes(Reference Daniel, Vohwinkel and Rehner163). However, casomorphins have been detected in the plasma of neonates due to their greater intestinal permeability, and, for this reason, it has been suggested that infant formulas may exert a sedative effect on the newborn child(Reference Sturner and Chang164). Exorphins are another class of milk protein-derived opioids, which include α-lactorphin peptides corresponding to bovine αs1-casein f(90–96) and bovine α-lactalbumin f(50–53); and β-lactorphin peptides corresponding to bovine β-lactoglobulin f(102–105)(Reference Haque, Chand and Kapila165). The exorphins have been shown to exert weak opioid activity in smooth muscles, having a positive effect on the cardiovascular system through vasorelaxation. However, these peptides do not easily cross the blood–brain barrier and have not been found to exert effects in the CNS(Reference Tidona, Criscione and Guastella166).
Peptides with a range of other functions have also been identified, including Ca-binding phosphopeptides as well as antibacterial and immunomodulatory peptides(Reference Korhonen155). With particular relevance to nervous system function and the enhancement of cognitive ability, a number of bioactive peptides have also been found to have antioxidative properties. In a review of antioxidative peptides derived from milk, Pihlanto(Reference Pihlanto167) identified six peptide fragments derived from casein as well as three peptide fragments derived from β-lactoglobulin, which exerted radical-scavenging abilities and inhibited lipid peroxidation: αs1-casein f(144–149), β-casein f(98–105), β-casein f(177–183), β-casein f(169–176), β-casein f(170–176), κ-casein f(96–106), β-lactoglobulin f(19–29), β-lactoglobulin f(145–149), β-lactoglobulin f(42–46). It was noted that antioxidative peptides that had been identified so far all contained one or more residues of histidine, proline, tyrosine and tryptophan. Preclinical and clinical studies with fermented milk products have provided preliminary evidence of antioxidant effects associated with these products. Whey proteins in conjunction with lactic acid bacteria have been shown to have an antiperoxidative action in rats deficient in vitamin E(Reference Zommara, Toubo and Imaizumi168). Similarly, in a human clinical intervention study, 21 d supplementation with 150 g/d fermented goats' milk was found to prolong the resistance of the lipoprotein fraction to oxidation, lower the levels of peroxidised lipoproteins, oxidised LDL, 8-isoprostanes and glutathione redox ratio, and enhance total antioxidative activity(Reference Kullisaar, Songisepp and Mikelsaar169). A recent study by Zemel et al. (Reference Zemel, Sun and Sobhani170) has also revealed a significant reduction in both oxidative and inflammatory stress in overweight and obese participants following a 21 d dairy-rich diet.
An important issue to consider is the concentrations of bioactive peptides that are required in order to have a clinically significant effect. The natural concentrations of bioactive proteins found in dairy products are quite low(Reference Korhonen and Pihlanto124, Reference Tidona, Criscione and Guastella166). Considering that many of the purported physiological effects of bioactive peptides are currently based on in vitro research(Reference Tidona, Criscione and Guastella166), there is a need to conduct clinical trials in humans in order to determine the dose required for clinical effects. A number of techniques are currently under development in order to isolate and enrich the different proteins found in milk. Microbial fermentation using lactic acid bacteria applied to protein-rich raw material is a technique that has the potential to enable large-scale production of bioactive peptides for human consumption(Reference Korhonen and Pihlanto124).
A number of commercially available bioactive peptides have recently become available(Reference Korhonen and Pihlanto154), which may form the basis for future clinical intervention studies in humans. To date, only a handful of clinical studies, largely in relation to antihypertensive bioactive peptides, have been conducted in humans, using milk protein hydrolysates or fermented milk products(Reference Sekiya, Kobayashi and Kita171–Reference Mizushima, Ohshige and Watanabe175). However, a clinical study by Nakamura et al. (Reference Nakamura, Iwamoto and Ogata153) has been one of the few studies to examine cognitive effects following intake of a milk casein hydrolysate (0·2 g/kg). The authors reported increased oxyhaemoglobin concentration in the prefrontal cortex and improvements to work efficiency following an acute stressor 60 min after ingestion. These findings corroborated the results of a previous study investigating the effects of a soya protein hydrolysate on brain function(Reference Hatakeyama, Yamaguchi and Muramoto176), although the mechanism of action is unclear. Further clinical trials investigating the efficacy of bioactive peptides in enhancing cognitive function, both acutely and chronically, are currently needed.
Colostrinin and proline-rich polypeptides
Proline is an amino acid that occurs widely in the proteins of both prokaryotic and eukaryotic cells, with a high number of proline residues found in milk caseins(Reference Goff151). Proline has an unusual chemical structure, characterised by a side-chain that is cyclised back on to the backbone amide position. For this reason, proline-rich polypeptides create disturbance in protein structure and are highly bioactive molecules(Reference Williamson177). Particularly, high concentrations of proline residues are found in the colostrum, which is the pre-milk fluid produced by the mammary glands of mammals in late pregnancy. The constituents of the colostrum are designed to boost the immunity of the newborn mammal as well as to promote the maturation of the CNS(Reference Boldogh and Kruzel178). A comparison of the major milk proteins found in normal milk and the colostrum is displayed in Table 3. Here, it can be seen that there are higher quantities of α-lactalbumin, β-lactoglobulin and immunoglobulins in the colostrum compared with normal milk. The difference is particularly large for immunoglobulins.
Table 3 Concentration of the major proteins of bovine colostrum and milk*

* Adapted from Korhonen & Pihlanto(Reference Korhonen and Pihlanto124).
Colostrum-derived proline-rich polypeptides are known as Colostrinin™ (CLN), and are obtained from bovine colostrum according to a patented method using alcohol extraction and filtration(Reference Kruzel, Polanowski and Wilusz179). CLN can be taken in the form of a tablet or capsule and has been characterised as a new cytokine that stimulates a general immune response(Reference Janusz, Inglot and Lisowski180). The yield of proline-rich polypeptides is found to be highest within 6 h of delivery(Reference Gladkevich, Bosker and Korf181). CLN consists of approximately 22 % proline, as well as a high proportion of non-polar amino acids, low percentages of glycine, alanine, arginine and histidine and no residues of tryptophan or cysteine(Reference Janusz, Staroscik and Zimecki182).
CLN has been found to be effective in reducing oxidative stress, with the research by Zablocka et al. (Reference Zabłocka, Janusz and Macała183) providing evidence that CLN regulates cytokine secretion and inhibits the production of the superoxide anion and NO in vivo. There is also evidence to suggest that CLN has a protective effect against mitochondrial dysfunction and Aβ-induced apoptosis of neurons(Reference Boldogh and Kruzel178). Furthermore, CLN has been found to improve both spatial learning and incidental memory in rats(Reference Popik, Bobula and Janusz184). Some preliminary clinical studies have been conducted to investigate the potential of CLN as a treatment of ARCD and AD.
In a double-blind study of CLN in AD, Leszek et al. (Reference Leszek, Inglot and Janusz185) administered CLN, Se or placebo to forty-six AD patients over a 12-month period. A statistically significant improvement in mini-mental state examination score at 12 months was found in those patients receiving CLN who entered the study with mild AD. A trend towards improved outcome was also found for patients receiving CLN who entered the study with moderate or severe AD. CLN was found to be significantly more effective than both placebo and Se in treating AD, with 50 % of the CLN-treated patients showing improvement, while only 5 % of Se-treated patients showing improvement, and none in the placebo group. In a randomised controlled study by Bilikiewicz & Gaus(Reference Bilikiewicz and Gaus186), CLN or placebo was administered to 105 Polish patients with mild-to-moderate AD over a 15-week period. ADAS-cog scores were found to be significantly higher in patients receiving CLN compared with placebo after 15 weeks. The overall benefit analysis revealed that 40 % of patients either stabilised or improved on CLN as opposed to only 21 % on placebo.
α-Lactalbumin
α-Lactalbumin comprises approximately 3·4 % of the total protein content of bovine milk(Reference Swaisgood187) and is the predominant whey protein in human milk, with levels increasing from 21 to 34 % between days 1 and 14 of lactation(Reference Montagne, Cuilliere and Mole188). In addition to the bioactive peptides that result from the partly hydrolysed protein, there are also a number of important amino acids that are released from the fully digested protein. α-Lactalbumin is a particularly good source of the essential amino acids tryprophan and cysteine. Tryptophan is a precursor of serotonin (5-hydroxytryptamine, 5-HT), while cysteine is a precursor of the endogenous antioxidant glutathione(Reference Chatterton, Smithers and Roupas189). α-Lactalbumin protein contains the highest tryptophan content of all food protein sources (6 %)(Reference Heine, Radke and Wutzke190). By increasing the blood plasma ratio of tryptophan:other large amino acids, there is greater transport of tryptophan across the blood–brain barrier, which results in enhanced 5-HT synthesis in the brain(Reference Lehnert and Wurtman191).
Previously, it has been demonstrated that α-lactalbumin containing 1·3 g tryptophan/100 g causes a 48 % increase in plasma tryptophan:large neutral amino acids(Reference Markus, Olivier and Panhuysen192), and more recently, it has been observed that evening consumption of α-lactalbumin with a tryptophan content of 4·8 g/100 g increased plasma tryptophan by 130 %(Reference Markus, Jonkman and Lammers193). Furthermore, in preclinical research with rodents, α-lactalbumin has been found to increase brain 5-HT concentrations(Reference Orosco, Rouch and Beslot194). Raising brain serotonin might have a number of beneficial effects on mood and cognitive function. In terms of stress, raised serotonin might attenuate the effects of reduced neurogenesis that occurs following stress and throughout the ageing process(Reference Jacobs, Van Praag and Gage195). In terms of sleep, serotonin up-regulation may improve sleep deficiencies and abnormalities affecting cognition, which occur in young and elderly individuals due to deficient brain 5-HT activity(Reference Jouvet196).
Behavioural findings show that serotonin raised by α-lactalbumin restores sleep in rats that are sleep-deprived through food deprivation(Reference Minet-Ringuet, Le Ruyet and Tome197). In humans, increases in plasma tryptophan availability for uptake into the brain have been shown to enhance sustained alertness early in the morning after an overnight sleep, a finding that has been attributed to improved sleep(Reference Markus, Jonkman and Lammers193). Furthermore, α-lactalbumin has been shown to improve mood and information processing, as well as to attenuate stress-induced cortisol responses in stress-vulnerable subjects (with high neuroticism scores) but not in controls (low neuroticism scores)(Reference Markus, Olivier and Panhuysen198, Reference Markus, Olivier and De Haan199). Research by Schmitt et al. (Reference Schmitt, Jorissen and Dye200) in premenstrual women, who typically display serotonergic hypofunction, revealed that acute administration of α-lactalbumin protein ameliorated memory performance deficits in long-term memory for abstract figures.
Since the serotonin system is important in the regulation of mood as well as cognitive function, the use of α-lactalbumin to relieve depressive symptoms has also previously been investigated. The current treatments for depression largely work by inhibiting the reuptake of serotonin (preventing breakdown and reabsorption in order to increase circulating serotonin). Selective serotonin re-uptake inhibitors have been found to relieve depressive symptoms in both animal and human models(Reference Vaswani, Linda and Ramesh201). Conversely, depletion of tryptophan induces depressive symptoms in depression-vulnerable individuals (for a review, see Booij et al. (Reference Booij, Van der Does and Riedel202)). In preclinical research with rats, α-lactalbumin-enriched diets have been found to enhance serotonin release and induce anxiolytic (antipanic or anti-anxiety agent) and rewarding effects(Reference Orosco, Rouch and Beslot194).
However, in humans, recovered depressive individuals and control subjects who were subjected to a laboratory stressor showed only modest improvements to mood and cortisol response to experimental stress following acute administration of a drink containing 20 g α-lactalbumin(Reference Merens, Booij and Markus203). The authors suggested that acute administration of α-lactalbumin was not sufficient to prevent a stress-induced mood deterioration or cortisol response(Reference Merens, Booij and Markus203) in recovered depressed subjects. In a later study, also examining the effects of α-lactalbumin in recovered depressed patients, again, significant improvement to mood has been observed. However, both recovered depressive subjects and control subjects demonstrated improved cognitive ability following α-lactalbumin administration(Reference Booij, Merens and Markus204). In a recent study by Verschoor et al. (Reference Verschoor, Finlayson and Blundell205), acute administration of a drink containing 20 g α-lactalbumin has not been found to significantly affect the mood or appetite following an acute stressor, although a lower liking for sweet foods has been observed in those with high trait anxiety. Taken together, the data suggest that enhancing 5-HT function through dietary tryptophan may be beneficial for improving sleep, mood and cognitive functioning and may particularly benefit vulnerable individuals coping with high levels of stress. However, it is important to note that the clinical studies reviewed have used drinks with the levels of α-lactalbumin far in excess of that which would be found in normal dairy products. As shown in Table 3, 1·2 g α-lactalbumin per litre is typically found in normal milk, which is a much smaller quantity in comparison with the 20 g typically used in intervention studies(Reference Merens, Booij and Markus203–Reference Verschoor, Finlayson and Blundell205). Thus, in order to see clinically significant effects on cognition or mood associated with α-lactalbumin, a drink with fortified levels would need to be taken.
Vitamin B12
Dairy products are a natural dietary source of vitamin B12, with one cup of yogurt providing about 25 % of the recommended daily intake, and one cup of milk contributing about 10 % of the recommended daily intake of B12 (206). Maintaining adequate dietary levels of B12 is important for healthy brain ageing, with epidemiological research linking vitamin B12 deficiency to a greater risk of developing AD(Reference Haan, Miller and Aiello22, Reference Clarke207, Reference Vogel, Dali-Youcef and Kaltenbach208). A study by Wang et al. (Reference Wang, Wahlin and Basun209) has reported that out of 370 elderly people monitored over a 3-year period, vitamin B12 as well as folate deficiency was associated with double the risk of developing AD. Research by Nilsson et al. (Reference Nilsson, Gustafson and Faldt210) has reported decreased serum vitamin B12 levels in 69 % of demented and non-demented psychogeriatric patients. In another study, this group reported significant improvement to mini-mental state examination scores in a mild-to-moderate dementia group following 2-month treatment with vitamin B12 as well as folate(Reference Nilsson, Gustafson and Hultberg211). Unfortunately, the majority of epidemiological studies do not consider vitamin B12 in isolation from folate, and for this reason, it is difficult to discern the relative contribution of each to the risk of developing dementia. However, current theoretical understanding regarding the relationship between B12 deficiency and accumulation of the amino acid HCy suggests that it plays an important role in the maintenance of healthy brain function(Reference Miller212).
There is strong evidence to suggest that vitamin B12 deficiency brings about cognitive decline due to an excess build-up of the amino acid HCy. Vitamin B12, together with folate, is a cofactor for enzymes that recycle HCy back to methionine, and when they are not present in adequate amounts, the methionine–HCy cycle is disrupted, which has a significant impact on cognitive function(Reference Miller212). HCy, an amino acid produced by the metabolism of methionine, has been found to be a biomarker in its own right for elevated risk of developing AD. HCy is normally metabolised in one of two ways; it is either converted back to methionine by re-methylation or converted to taurine and cysteine through trans-sulfuration. Abnormally high levels of HCy signal a breakdown in these biochemical processes. If not enough HCy is converted back to methionine, this has important implications for brain function(Reference Miller212).
The methionine cycle involves the conversion of methionine to S-adenosylmethionine, which is the most important methyl donor in the human body required for methylation of a host of substances, including DNA and proteins such as myelin. After donating its methyl group, S-adenosylmethionine becomes S-adenosylhomocysteine and then HCy after losing its adenosine. If HCy is not metabolised properly, there will be insufficient S-adenosylmethionine available, and this will result in the inhibition of methylation(Reference Miller212). The gene for the amyloid precursor protein is heavily methylated. Decreased methylation may lead to the promotion of gene mutations involved in the increased expression of amyloid precursor protein and extracellular deposition of the Aβ peptide(Reference West, Lee and Maroun213, Reference Rogaev, Lukiw and Lavrushina214). Furthermore, accumulation of HCy itself, as well as S-adenosylhomocysteine, in the body has been found to cause oxidative stress, excitotoxicity in neurons, as well as DNA strand breakage and mitochondrial membrane damage(Reference Kruman, Culmsee and Chan215). There is also evidence to suggest that excess HCy makes neurons more sensitive to Aβ toxicity(Reference Ho, Collins and Dhitavat216).
Total levels of plasma HCy have been found to increase with age, reaching a plateau at about the age of 60 years(Reference Elias, Sullivan and D'Agostino217). In a study of HCy levels in histologically confirmed AD patients by Clarke et al. (Reference Clarke, Smith and Jobst218), it has been found that people in the top third of HCy levels had a 4·5 times greater risk of AD compared with those in the bottom third. The Framingham Study(Reference Elias, Sullivan and D'Agostino217), which followed up 1092 people for 8 years, has found high HCy levels to be associated with double the risk for AD. A more recent 4·5-year longitudinal study by Haan et al. (Reference Haan, Miller and Aiello22) on 1779 Mexican Americans over the age of 60 years reported 2·39 times the risk of dementia or cognitive impairment associated with high HCy levels at baseline. High levels of HCy are concomitantly observed with low levels of the recycling cofactors vitamin B12 and folate. In a study by Joosten et al. (Reference Joosten, Lesaffre and Riezler219) comparing fifty-two AD patients with forty-nine elderly people living at home and fifty hospitalised non-demented controls, the AD group has been found to have the highest levels of HCy and the lowest levels of vitamin B12. The evidence thus far implicates vitamin B12 as an important vitamin for maintaining proper metabolism of HCy, without which the brain becomes more susceptible to oxidative damage and apoptosis. As an important dietary source of vitamin B12, dairy products are likely to play an important role in ensuring adequate HCy metabolism, particularly during ageing.
Calcium
It has been estimated that dairy products contribute 70·3 % of Ca in the US diet(220). If dairy products are excluded from the diet, then it is difficult to meet the recommended Ca intake(Reference Gao, Wilde and Lichtenstein221). There is strong evidence from both epidemiological studies and randomised clinical trials to suggest that higher consumption of dairy products may be associated with lower rates of obesity(Reference Astrup, Chaput and Gilbert222). One mechanism by which dairy products may lead to weight reduction is an increase in satiety due to the consumption of dairy proteins(Reference Major, Chaput and Ledoux223). However, perhaps the most significant contribution to weight loss associated with dairy product consumption is due to the impact of Ca on fat excretion. A meta-analysis by Astrup et al. (Reference Astrup, Chaput and Gilbert222) has revealed that increasing dairy Ca consumption by 1200 mg/d resulted in increased faecal fat excretion by 5·2 g/d. Ca forms insoluble fatty acid soaps and other hydrophobic aggregations of bile acids, P and fatty acids in the small intestine, resulting in a greater excretion of fat(Reference Govers, Termont and Van Aken224, Reference Gacs and Barltrop225). Serum cholesterol levels have also been found to be lowered following supplementation with calcium phosphate, as a result of increased bile excretion and regeneration of bile acids from endogenous cholesterol(Reference Ditscheid, Keller and Jahreis226).
In contrast to the positive effects of Ca on obesity and cholesterol, there have been some concerns regarding high intakes of Ca and increased vascular calcification. A cross-sectional study by Payne et al. (Reference Payne, Anderson and Steffens227) has found evidence to suggest that Ca and vitamin D intake was positively correlated with brain lesion volume in a sample of elderly adults. Similar findings were reported by Bolland et al. (Reference Bolland, Barber and Doughty228) in a Ca supplementation study of bone mineral density in elderly women. Those women randomised to receive Ca supplementation were found to be twice as likely of suffering a myocardial infarction in comparison with the placebo group over a 5-year period. A recent supplementation study by Daly et al. (Reference Daly, Ebeling and Khan229) has also reported that abdominal aortic calcification increased in men receiving Ca and vitamin D3 fortified milk in comparison with the control group over a 2-year period. Further research is currently needed in order to determine the dose at which vitamin D and/or Ca increases the risk of vascular calcification.
Ca dysregulation has been proposed as an important factor in brain ageing and neurodegeneration(Reference Thibault, Porter and Chen27, Reference Thibault, Gant and Landfield230) as well as in the metabolic syndrome(Reference Levy, Gavin and Sowers231). Larger Ca2+-dependent afterhyperpolarisation associated with action potentials has been found in cortical and hippocampal neurons of older compared with younger animals(Reference Stutzmann, Smith and Caccamo232–Reference Disterhoft, Thompson and Moyer237). Larger Ca2+ transients during repetitive spike trains(Reference Hemond and Jaffe238, Reference Thibault, Hadley and Landfield239), larger whole-cell Ca2+ currents(Reference Campbell, Hao and Thibault240) and excess Ca2+ influx into neurons via voltage-gated Ca2+ channels have also been found to be associated with ageing, with many of these changes also found to be associated with age-related cognitive deficits(Reference Thibault, Gant and Landfield230, Reference Thibault and Landfield241). Elevated Ca2+ release from ryanodine receptors is a contributing factor to cell death(Reference Thibault, Gant and Landfield230), with ryanodine receptor expression altered in some AD mutations (e.g. presenilin 1)(Reference Stutzmann, Smith and Caccamo232, Reference Smith, Hitt and Green242). However, it is important to note that there is currently no evidence to suggest that dietary intake of Ca is a causative factor in age-related Ca dysregulation.
Probiotics
Probiotics are live microbial food supplements that have a beneficial effect on intestinal microbial balance, with the most frequently used bacteria in commercially available fermented milk and yogurts being the Lactobacillus and Bifidobacterium species(Reference Kopp-Hoolihan243). The research assessing the effects of probiotics on brain function is in its very early stages. However, research suggests that bacteria in the gastrointestinal tract can communicate with the CNS, and may have immune- and non-immune-related effects beyond the gastrointestinal tract(Reference Kopp-Hoolihan243). Studies on clinical populations such as the chronic fatigue syndrome and fybromyalgia, where lower levels of bifidobacterium and higher levels of lactic acid bacteria have been reported, have found evidence to suggest that poorer gut health is correlated with more severe neurological and cognitive deficits such as nervousness, memory loss, forgetfulness and confusion(Reference Butt, Dunstan and McGregor244).
A possible explanation for the link between gut health and cognition is the effect of pro-inflammatory cytokines in the CNS. It has been suggested that the effect of probiotics on systemic inflammatory cytokines and oxidative stress may ultimately lead to an increase in brain-derived neurotrophic factor(Reference Logan and Katzman245). A preclinical study by Desbonnet et al. (Reference Desbonnet, Garrett and Clarke246) has revealed that 14 d of treatment with the probiotic Bifidobacterium infantis resulted in a significant attenuation of pro-inflammatory cytokines, together with a significant increase in the serotonin precursor tryptophan. These findings are indicative of an antidepressant effect, and have led to the recent suggestion that probiotics may be used as an adjunct treatment for major depressive disorder(Reference Logan and Katzman245).
There have been few studies to directly assess the effects of probiotics on cognition. A study by Benton et al. (Reference Benton, Williams and Brown247) has been one of the few chronic intervention studies to directly investigate the effects of probiotics on cognition. However, the effects on cognition were not in the direction that might have been expected. At day 20 of the intervention, individuals in the probiotic group were found to perform significantly worse on a test of semantic memory in comparison with placebo. However, considering the scarcity of other studies to investigate the cognitive effects associated with probiotics, further research is required to corroborate these findings.
Summary
The use of dairy products in the prevention or amelioration of normal ARCD and dementia is of growing interest. A number of components present in dairy products may have a substantial impact on the physiological factors associated with ageing and dementia. As with other dietary interventions which influence cognitive function, the impact of dairy products on neurocognition is modulated by individual differences. Some of these may be specific to dairy, such as lactose intolerance(Reference Lomer, Parkes and Sanderson248), although the area is rather under-researched. Dairy consumption, in particular low-fat dairy, has been found to be associated with a lowered incidence of the metabolic syndrome, with positive effects on cognition through improved glucose regulation and weight management associated with whey protein and Ca. In order to reduce the health risks associated with saturated fats, it is recommended that low-fat dairy be consumed as part of the regular diet in preference to high-fat dairy. A number of bioactive peptides originating from dairy products have been found to have a beneficial effect on cardiovascular function, as well as on antioxidant and anti-inflammatory properties. However, the natural concentrations of these peptides are relatively low, and manufacturing techniques are currently needed in order to further isolate and enrich these beneficial peptides. CLN is a form of dairy product that has been found to be effective in reducing oxidative stress and inflammation, and has shown potential in the treatment of patients with mild AD. α-Lactalbumin from whey protein has been found to be beneficial in increasing the levels of serotonin, with preliminary data suggesting potentially beneficial effects on sleep, mood and cognition, particularly in individuals vulnerable to stress. However, currently, the quantity of α-lactalbumin found in normal milk is inadequate to achieve clinically significant effects, and for this reason, the quantity must be fortified. Vitamin B12 has been found to be an important dietary constituent that is required for effective HCy metabolism, with dairy products being a major source of vitamin B12 in the diet. Dairy products are also a major source of dietary Ca, with Ca consumption associated with lower rates of obesity and lowered serum cholesterol levels. However, there has been some concern that increased Ca intake may be linked to an increase in vascular calcification. Probiotics have also been found to have a positive effect on neurocognitive health by attenuating pro-inflammatory cytokine activity and increasing levels of tryptophan and brain-derived neurotrophic factor. In conclusion, current evidence suggests that the regular consumption of low-fat dairy products as part of a balanced diet may have a number of positive effects on neurocognitive health in ageing.
Acknowledgements
The present study was supported by Dairy Innovation Australia Limited. There are no conflicts of interest concerning any authors involved with the preparation of the manuscript. D. A. C. conducted the literature review as well as preparation of the final manuscript. L. O. was also involved in conducting the literature review. A. P. contributed information to the manuscript regarding the cognitive effects of ageing. A. B. S. was involved in editing the final manuscript. C. S. was involved in editing the final manuscript.





