Complex congenital cardiac anomalies may require long-lasting operations and multiple or staged repairs. Some patients may need a long duration of mechanical ventilation and ICU stayReference Harrison, Cox, Davis, Piedmonte, Drummond-Webb and Mee1,Reference LoTempio and Shapiro2 . Immature lungs, congenital anomalies of pulmonary arteries and veins, aortopulmonary collaterals, pulmonary hypertension, genetic anomalies, failure to thrive, immune deficiencies, and repeated pulmonary infections may cause the patients to be more prone to post-operative respiratory complications. In addition to these, some post-operative complications like low cardiac output syndrome, prolonged mechanical circulatory support, pulmonary oedema, neurological disorders, hospital infections, diaphragm, or vocal cord paralysis may increase the risk for a long duration of mechanical ventilatory supportReference Harrison, Cox, Davis, Piedmonte, Drummond-Webb and Mee1-Reference Shi, Zhao and Liu3. Over the last decade, tracheostomy, which facilitates patient care, ambulation, oral feeding, rehabilitation, and weaning from the ventilator has been increasingly performed in children with prolonged mechanical ventilation, whereas there is little consensus about the indications and the right timing of it in childrenReference Shi, Zhao and Liu3,Reference Kanter, Bove, Tobin and Zimmerman4 . Some patients with a tracheostomy may be suitable to be discharged home with the aid of home-type mechanical ventilators. Advantages of HMV include decreased hospital-acquired infections, increased mobility, improved nutrition, and low healthcare costsReference Srinivasan, Doty and White5. However, safety of HMV especially out of the hospital is usually questioned by both doctors and parents. Children with HMV continue to have an increased risk of death after hospital discharge. Some reports suggest that patients with single-ventricle physiology or greater RACHS-1 scores have higher mortality rates or less success in weaning from HMVReference Cotts, Hirsch, Thorne and Gajarski6,Reference Edwards, Kun, Keens, Khemani and Moromisato7 .
The aim of this study is to analyse the outcomes of patients who underwent congenital heart surgery and were followed up with tracheostomy and HMV.
Materials and methods
A total of 1343 congenital heart operations were performed between January, 2014 and June, 2018 in our hospital and 72 (5.3%) patients needed tracheostomy post-operatively. Amongst these patients, 45 were followed up with a home ventilator after tracheostomy and they were reviewed in this retrospective study. Eleven of them had single-ventricle physiology while 34 had biventricular physiology. This study was approved by the local ethics committee.
A standard set of perioperative data were collected retrospectively for all patients. Medical records of demographic features, cardiac diagnosis, congenital heart procedures, and comorbidities were recorded.
The operation for CHD before tracheostomy placement during hospitalisation was used as an index operation. Index operations are shown in Table 1. The patients were divided into two groups according to the procedures performed: palliation or total repair. We inscribed the data for post-operative complications like delayed sternum closure, renal failure, other organ failure, and the need for ECMO support. Bronchoscopy was performed by oto-rhino-laryngologist if there was a suspicion of airway compression, mucus clog, or bleeding.
Table 1. Index operations of the patients
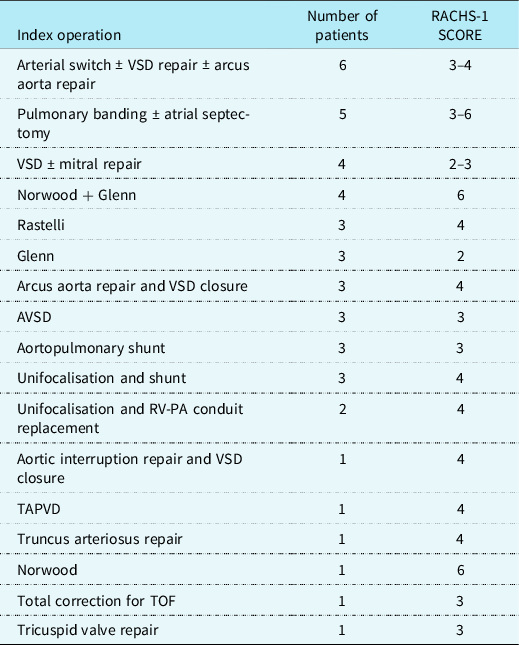
AVSD = Atrioventicular septal defect; PA = Pulmonary artery; RV = Right ventricle; TAPVD = Total pulmonary venous return anomaly; TOF = Tetralogy of Fallot; VSD = Ventricular septal defect.
Intubation time was defined as the time from intubation for cardiac surgery to the time of tracheostomy tube placement. Median duration of pretracheostomy ventilation was 32 days (8–154 days). HMV was planned in these patients because all of them required prolonged respiratory support.
HMV adaptation was started in ICU in patients who were haemodynamically stable without serious infection and with adequate enteral nutrition. Compliant patients to HMV were transferred to the ward with accompanying family members. Continuous electrocardiography and oxygen saturation (SpO2) monitoring were done. Necessary training were given verbally and in written forms to the family members who stayed with the patient during the ward period. The families do hands-on education. They were informed about home ventilators, wearing sterile gloves, suctioning, daily tracheostomy care, skin hygiene, and care, oral care, feeding with nasogastric tube, hand washing, positioning, and changing the tracheostomy tube. If patients were still dependent on tracheostomy, when discharged, the caregivers were given information about tracheal aspiration, bath, and body care, tracheostomy care, observing signs of infection, and protection from it by preventing pressure injuries, preparation of medicines that will be used at home, conditions to consult to a doctor, effective use of medical equipments, home ventilator, pulse oximeter, oxygen concentrator use, fever monitoring, and respiratory physiotherapy. Mean follow-up time was 36.24 ± 11.61 months.
Statistical analyses were performed with the SPSS version 19.0 for Windows (SPSS, Chicago, IL, USA). Data are presented as mean ± SD or median (range) where appropriate and p < 0.05 was taken as significant. Group data were compared by using t-tests analysis of variance, and log-rank tests as appropriate. Pearson χ2 test was used for categorical variables. Kaplan–Meier survival curves were created to compare time-to-event rates.
Results
The median age of the patients was 6.4 months (12 days–6.5 years). Eighteen (40%) of them were female. A total of eight patients (17.7%) had genetic syndromes (Down syndrome, n = 5) and Digeorge syndrome, n = 3) and three patients were born preterm. Delayed sternal closure was used for 10 patients with unstable haemodynamics or post-operative bleeding. Re-exploration for bleeding was performed in two patients. Low cardiac output occurred in eight patients (17.8%) and seven of them needed ECMO support. All of these patients were weaned from ECMO and 6 of them (86%) were able to be discharged from the hospital, the other one died during hospital stay. Renal replacement therapy with hemodiafiltration and/or periton dialysis was done in 15 (33.3%) patients. Chylothorax was developed in six patients with single-ventricle anatomy (13.3%) and thoracic duct ligation was performed in half of them. Permanent pacemaker was implanted in two patients (4.4%) after open-heart surgery. Post-operative diaphragm plication (before tracheostomy) was performed in five patients (11%) due to diaphragm paralysis. Four (80%) of these children were weaned from HMV. All patients in this study needed prolonged mechanical ventilation and/or multiple extubation failures. Amongst these, 15 patients (33.3%) were unable to wean from ventilation due to chronic lung disease. Fourteen of them (31.1%) had pneumonia and six patients (13.3%) had pulmonary hypertension. Severe cerebrovascular event was seen in 2 (4.5%) patients post-operatively.
The median duration of ICU stay after tracheostomy was 27 days (range 2–93 days).
Haemodynamically stable patients who were compatible with home ventilators were taken to the ward. Follow-up time in ward was median 30 days (2–156 days). Fourteen patients (31.1%) were readmitted to ICU and one of them died due to multiorgan failure. A total of 12 patients (26.6%) weaned off of home ventilator and underwent decannulation during the initial hospital stay.
Thirty-two patients (71.1%) were discharged home with home ventilator support (Fig 1). Thirteen of them (40.6%) were transferred out of the city by ambulance. All patients with HMV were called by our nurse routinely after discharge to see if there was any problem. Also in case of hospitalisation need, patients were interned again in our ward or ICU. In 15 patients (46.9%) decannulation was performed in median of 6 weeks (1 week–11 months). Thirteen patients died during follow-up. Total mortality was 31.1% (14 patients). Six patients died after rehospitalisation. Seven patients died at home mostly due to their chronic diseases. The other four patients were still HMV dependent at home at the end of the follow-up. Table 2 categorises the cohort by corrective and palliative operations with respect to survival and weaning status. Mean follow-up for patients with the total repair was 2.77 ± 1.53 years. Mean follow-up for patients with palliation was 2.30 ± 1.6 years. There was no statistically significant difference between these two subgroups about decannulation and mortality (p = 0.39; p= 0.48, respectively). The log-rank test was used to assess the significance, and the survival of the patients with time was lower in those that had palliative surgery, but this did not reach statistical significance (Fig 2).

Figure 1. Results of the patients who were discharged home with HMV. The other 12 patients (26.6%) were weaned off HMV and underwent decannulation during the hospital stay. And one patient died during the hospital stay. EX = Exitus; HMV = Home mechanical ventilation.
Table 2. Children with CHD on home mechanical ventilation by operation status (total repair and palliation operations)
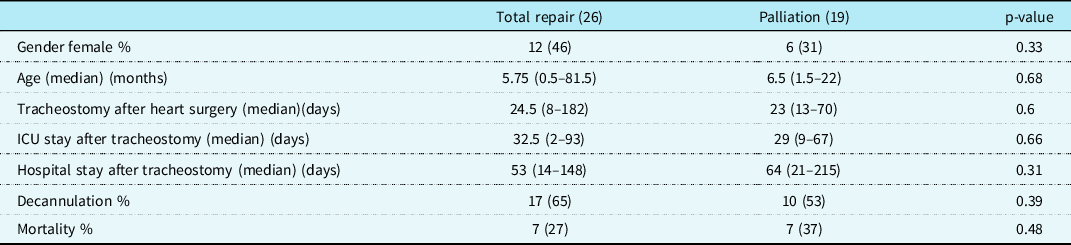
ICU = Intensive care unit.
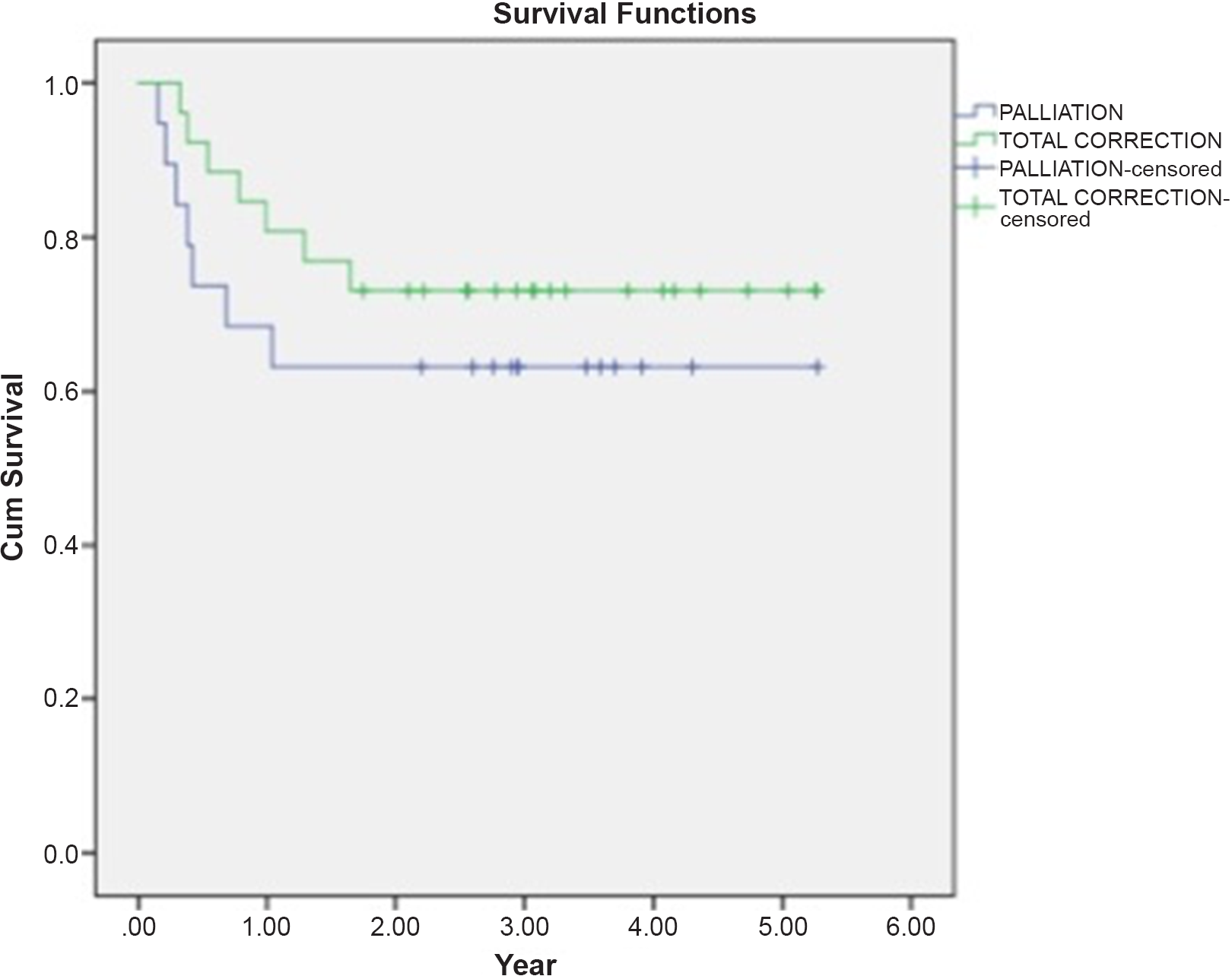
Figure 2. Kaplan–Meier graphic for survival after total correction and palliation surgeries. Estimated survival time for palliation is 3495 ± 0.535 years. Estimated survival time for total repair is 4073 ± 0.386 years. The overall estimated survival time was 3833 ± 0.321 years.
We also analysed the outcomes of the patients according to RACHS-1 scores. Twenty-five patients had greater RACHS-1 scores (>3). There was no statistically significant difference for decannulation between patients with RACHS-1 scores > 3 and patients with RACHS-1 scores ≤3 (p= 0.54). For children with single-ventricle physiology (n=11, 24.4%), mortality incidence was 18.2% and seven of them were decannulated. Three single-ventricle patients in our cohort have completed three-stage palliation of the Fontan procedure after weaned from HMV. Mortality in the biventricular group was 32.4%.
Discussion
Although survival rates of CHD improved a lot in time, some children with complex cardiac anomalies may still need multiple operations or staged procedures. Infants with CHD are likely to have chronic lung disease, so they may require prolonged respiratory support after heart surgery. Prolonged intubation compromises patient comfort, feeding, and mobilityReference Hoskote, Cohen, Goldman and Shekerdemian8. Preoperative diagnosis and the complexity of the operation, underlying pulmonary disease, residual cardiac lesions, tracheobronchomalacia resulting from chronic airway compression from cardiac structures, diaphragm, or vocal cord paresis/paralysis, lung disease due to preoperative or post-operative courses are shown to be risk factors for tracheostomy need after cardiac surgeryReference Kanter, Bove, Tobin and Zimmerman4,Reference Hoskote, Cohen, Goldman and Shekerdemian8,Reference Brown, Ridout, Goldman, Hoskote and Penny9 . Also, younger age, prematurity, prolonged cardiopulmonary bypass time during cardiac reoperations, and early sepsis are found to be other risk factorsReference Kanter, Bove, Tobin and Zimmerman4,Reference Hoskote, Cohen, Goldman and Shekerdemian8,Reference Brown, Ridout, Goldman, Hoskote and Penny9 . Berry and colleaguesReference Berry, Graham and Roberson10 demonstrated an increased risk of in-hospital mortality following tracheostomy in children with CHD. Rosner et al.Reference Rosner and Mastropietro11 mentioned that prior cardiac surgery is independently associated with decreased survival after tracheostomy. Hoskote et alReference Hoskote, Cohen, Goldman and Shekerdemian8 reported an overall survival rate of 60.5% for patients with tracheostomy after cardiac surgery. Hospital survival is worse in infants with complex lesions and single ventricleReference Cotts, Hirsch, Thorne and Gajarski6,Reference Pizarro, Murdison, Derby and Radtke12 . Our cohort of 45 children with CHD on HMV is a heterogeneous group with various cardiac lesions. Edwards et al informed that HMV provides only a limited long time survival in children in higher RACHS categories or with single-ventricle anatomyReference Edwards, Kun, Keens, Khemani and Moromisato7. Our cohort of 45 children with CHD on HMV is a heterogeneous group with various cardiac lesions. In our study 11 (24.4%) patients had single-ventricle pathology and 7 of them were decannulated. Mortality of these patients was 18.2%. Mortality in the biventricular pathology group was 32.4%. We assume that this result is due to the high number of complex cardiac diseases and underlying pulmonary disease in the biventricular group. Twenty-six patients underwent total repair for their CHD while 19 of them underwent palliative operations. There was no statistically significant difference between these two subgroups about decannulation and mortality although the survival with time was lower in those that had palliation. Also, in this study, 20 patients had RACHS-1 scores ≤3 and 25 patients had RACHS-1 scores > 3. There wasn’t any significant difference for decannulation between these subgroups (p = 0.54).
The decision of tracheostomy is a multidisciplinary approach involving cardiovascular surgeon, cardiac ICU, and oto-rhino-laryngologist. In patients who are not able to wean from ventilation and who fail extubation recurrently, reasons like phrenic nerve injury, residual cardiac lesions, and tracheobronchomalacia should be excluded before deciding tracheostomy. In our institute, bronchoscopy was performed by ORL if there was a suspicion of airway compression or mucus clog, thorax CT was done to patients in case of a serious infection or a suspicion of compression to the ventilatory tract. Flouroscopy was performed for phrenic nerve injury, and we performed post-operative diaphragm plication in five patients before tracheostomy. Eighty per cent of these patients (four patients) had decannulation. LoTempio et alReference LoTempio and Shapiro13 mentioned that they performed plication up to 40% of their CHD patients. All of the infants and children in our cohort underwent open surgical tracheostomy. Concerning the risk of respiratory tract infection, accidental extubation, vocal cord dysfunction, subglottic stenosis during orotracheal intubation, tracheostomy is a good and reasonable alternative. Easy inhalation, safe airway control, easy separation from mechanical ventilation, rapid weaning from sedation, and patient mobilisation are amongst the benefits of tracheostomyReference Mohr, Rutherford, Cairns and Boysen14. However, there are some disadvantages like cannula obstruction, accidental decannulation, acute haemorrhage, local infection, and pneumothoraxReference Carr, Poje, Kingston, Kielma and Heard15,Reference Goldenberg, Golz, Netzer and Joachims16 . We haven’t seen any complications like mediastinitis, serious local infection at the site of the tracheostomy in our patients. We must balance between the risks and benefits of tracheostomy and decide the optimal timing.
Transfer from the ICU to the paediatric cardiovascular service occurs when patients are haemodynamically stable on consistent ventilator support with a home ventilator. When the patient is medically stable with a home ventilator, he is discharged homeReference Noyes17. Optimising quality of life, rehabilitation, and growth and development are the main goals of home mechanical ventilation in childrenReference Amin and Fitton18. There is an overwhelming stress in the air for the caregivers during discharge time as they take charge. Srinivasan et alReference Srinivasan, Doty and White5 demonstrated that in 150 patients requiring HMV, ventilator failure occurred relatively infrequently, and there were no adverse outcomes as a result of equipment failure at home in their study. They speculate that equipment failure is not a frequent or serious problem for ventilator-assisted patients treated at home. Also, this study showed improper equipment care, damage, or tampering by caregivers being responsible for 13% of reported ventilator failures, and 30% of failures being due to functional equipment being used incorrectly by caregivers. This emphasises the importance of patient teaching prior to initial discharge from the hospital to home. In our institute, the caregivers were given all kind of information about home ventilators, and we believe that these information and hands-on training are very important for upcoming conditions that can happen at home. Sufficient parental skills and a good domestic environment are required for a safe HMV period. In our study, seven patients had respiratory arrest at home and died. Edwards et alReference Edwards, Q’toole and Wallis19 found out that the most common obstacles to discharge a child home on tracheostomy-dependent ventilation were funding for staffing and equipment, and local organisational delays, which both occurred in 41% of their cases. In 33% of their cases, the families’ housing was unsuitable or needed major alterations. In our report, 32 patients (71.1%) were discharged home with home ventilator support. Thirteen of them (40.6%) were transferred out of the city by ambulance. In our study, total mortality is 31.1%. Properly selected patients can be safely ventilated at home with HMV.
Our study has some notable limitations. Principally, it is a retrospective study of one single centre in a group of patients with heterogeneous cardiac lesions and co-morbidities. This study was not designed to identify predictors of patients requiring tracheostomy after congenital heart surgery, but it can suggest that underlying chronic lung disease, residual heart defects, and hospital infections may be the main risk factors for tracheostomy. Multicentre analyses may help to adjust co-morbidities like preoperative respiratory insufficiency, diaphragmatic paralysis, serious neurological problems, and associated chromosomal abnormalities.
Conclusion
HMV via tracheostomy can be used in the treatment of chronic respiratory failure after congenital heart surgery. By reducing the hospitalisation period, care can be maintained at home and this can be beneficial to the majority of patients both physically and mentally although there are potential risks.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and has been approved by the institutional committee.







