Protein nutrition: the basics
The aim of eating dietary protein is to satisfy the body's requirement for amino acids (AA) involved in multiple metabolic pathways. Protein synthesis, however, remains the metabolic pathway that uses most of the dietary AA to renew body proteins. Because proteins are composed of twenty different AA, protein nutrition should cover the requirement for all AA. Indeed, if the requirement for a single AA is not covered, this will have a negative impact on the optimal utilization of all the other AA (limiting AA, mentioned later). Contrary to carbohydrates or lipids, no dedicated AA storage takes place in the body, leading to very limited internal availability outside the food intake period. Protein metabolism must adapt constantly by building and breaking down body proteins without challenging the function of organs or tissues. This is emphasized in human subjects with nine indispensable AA which cannot be synthetized de novo and for which the requirement is 100 % dependent on food intake. Today, the Recommended Daily Allowance (RDA) has been set at 0⋅83 g/kg/d of proteins of ‘good quality’ for healthy young adults(1).
However, there is a growing body of evidence that this RDA is too low for the elderly. Indeed, there seems to be a consensus that the need for protein is increased in the elderly not only to maintain muscle mass(Reference Traylor, Gorissen and Phillips2) but also to maintain muscle functionality(Reference Coelho-Junior, Milano-Teixeira and Rodrigues3). A recent study of the English cohort Newcastle 85+ showed that lower protein intakes (<1 g/kg/d) in people aged over 85 years are associated with higher increases in scores reflecting physical disability and dependence(Reference Mendonca, Granic and Mathers4). In France, the RDA for proteins has recently been increased from 0⋅83 to 1 g/kg/d for adults aged over 65 years(5). Apart from quantity, the quality of protein supply is important. A protein of good quality is a protein whose AA composition covers the requirement of each indispensable AA when ingested at the RDA. In Fig. 1, protein A is therefore of good quality for the healthy adult population when given at the RDA. By contrast, protein B in Fig. 1 does not cover the minimal requirement for lysine, and cannot be considered as a protein of good quality when ingested at the RDA by healthy adults; thus lysine is the limiting AA for optimal body protein synthesis. Protein nutrition follows the barrel/stave theory which has been described extensively in animal nutrition and which is directly applicable to human protein nutrition(Reference Mitchell and Block6). Briefly, the ingested dietary protein is the barrel composed of staves that represent each AA. For a given AA, the height of the stave is the minimum amount at which this AA must be present in order to cover its minimum requirement. The stave with the lowest height corresponds to the limiting AA and influences the maximal capacity of the barrel, i.e. maximal AA retention. With a dietary protein given at the RDA, if the stave of one AA becomes shorter then the volume of the barrel will decrease, i.e. AA retention will decrease. However, in our example (Fig. 1), by doubling the intake of protein B, this dietary protein is then able to fulfil the requirements for lysine. The problem is that to fulfil the minimal requirement of a single AA (lysine), the ingestion of a high amount of protein B will also provide very large amounts of all the other AA. Since these extra AA cannot be stored, they will be wasted and eliminated by oxidation, leading to urea and ammonia production.
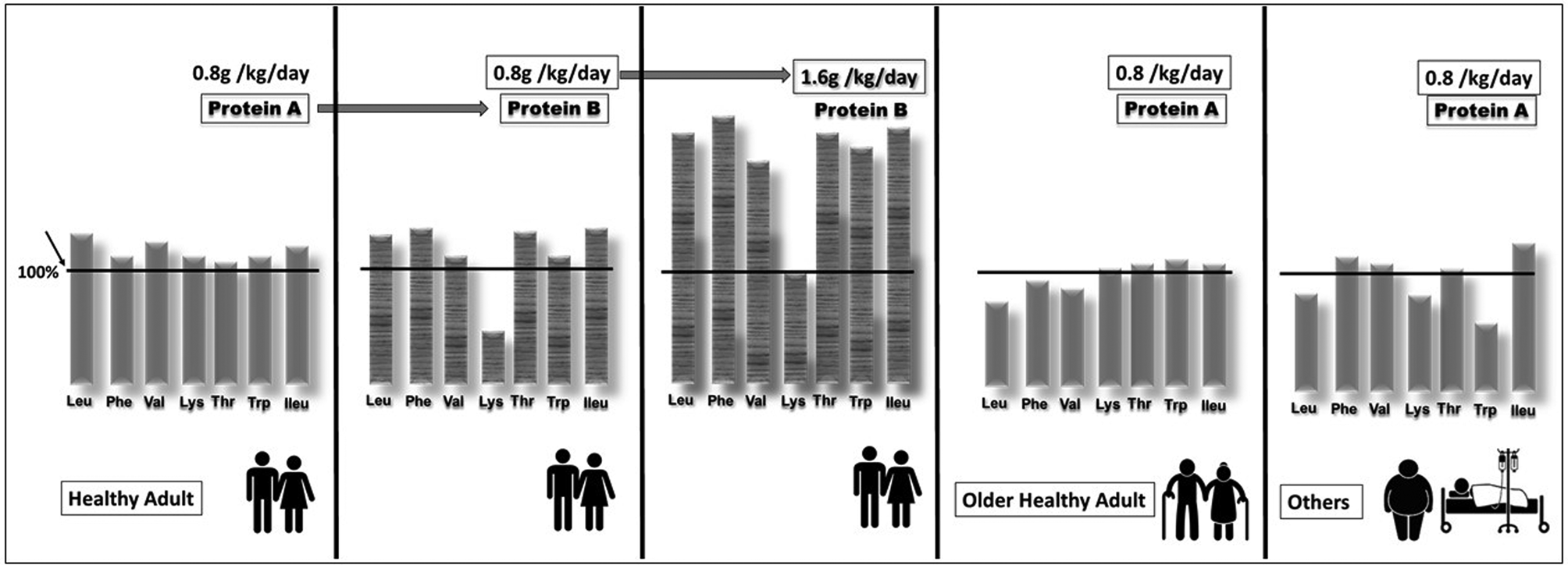
Fig. 1. Schematic representation of the quality of a dietary protein according to its amino acid (AA) composition and to the target population. The vertical bar represents the AA and the height of the bar represents the amount of the amino acid present in the protein ingested as a percentage of the minimum requirement value of the population mentioned and when consumed at the amount specified in the graph (derived from the Food and Agriculture Organization). If the height of the bar is below the minimal requirement (100 %), then the corresponding AA is considered as limiting. Couple by Akshar Pathak from the Noun Project; Couple by Gan Khoon Lay from the Noun Project; Fat man by Gan Khoon Lay from the Noun Project; sick by Gan Khoon Lay from the Noun Project.
Because the requirements of AA may differ according to different populations, i.e. healthy older adults, obese people or hospitalized patients, protein A may not be of good quality when given at the RDA because it cannot, for example, fulfil the leucine, phenylalanine or valine requirements in older adults, or the leucine, lysine or tryptophan requirements in another population (see Fig. 1). The amount of protein A will also have to be increased in such populations even if this dietary protein is considered to be of good quality in healthy adults.
When a protein is not of good quality according to its AA composition, this shortcoming can be palliated by increasing the amount ingested until it covers the minimal requirement of the limiting AA. However, the increase in protein intake and more generally the increase of food intake can be difficult to achieve because: (1) the palatability of proteins is generally not good; (2) increased AA requirements are associated with fragility and physio-pathological conditions, the proteins to be supplied could be too high to be compatible with normal intake in targeted populations; (3) these targeted populations are also characterized by poor appetite and undernutrition; (4) as AA in excess of those required cannot be stored, urea production will increase, possibly aggravating weak renal function already present in these populations.
Nutritional strategies targeting protein intake must therefore favour reasonable increases in dietary protein and optimize the composition of what is ingested as much as possible. The present review therefore focuses on the various studies that have formulated a certain number of concepts and also made it possible to identify the various determinants leading to this optimization in the elderly, with, in addition particular attention given to the prevention of sarcopenia.
Pulse protein feeding and leucine, the anabolic amino acid
The main function of skeletal muscle is to provide power and strength for locomotion and posture; what is more this tissue is also the major reservoir of body proteins and AA. Thus, although the loss of muscle proteins has positive effects in the short term by providing AA to other tissues, uncontrolled and sustained muscle wasting impairs movement, leading to difficulties in performing daily activities. It also has detrimental metabolic consequences such as reduced ability to mobilize enough AA in the case of illness and disease. The resulting weakness increases the incidence of falls and the length of recovery, and when advanced muscle wasting correlates to morbidity and increased mortality(Reference Cruz-Jentoft, Bahat and Bauer7). Consequently, one of the challenges we have to face is to supply AA to those tissues with higher requirements in healthy situations, prevent excessive loss of muscle proteins in catabolic states(Reference Obled, Papet and Breuillé8), and ultimately improve muscle recovery.
During the day, protein metabolism is modified by food intake. In adult volunteers, oral feeding is associated with an increase in whole-body protein synthesis and a decrease in proteolysis(Reference Rennie, Edwards and Halliday9–Reference Prod'homme, Rieu and Balage13). Thus, in the case of muscle wasting, muscle protein loss results from an imbalance between protein accretion and breakdown rates that partially stem from a defect in postprandial anabolism. Although each muscle wasting situation is characterized by its specific mechanism(s) and pathways, leading to muscle loss, an increase of catabolic factors such as glucocorticoids, cytokines, oxidative stress, etc., often occurs. It is now well established that these factors have potential deleterious effects on the AA and insulin signalling pathways involved in the stimulation of muscle anabolism after food intake(Reference Balage, Averous and Remond14–Reference Marzani, Balage and Vénien18). These signalling alterations lead to the anabolic resistance of muscle even if the anabolic factor requirements (AA, e.g.) are theoretically covered, i.e. with normal nutrient availability matching the recommended dietary protein allowances in healthy subjects. This anabolic resistance may be partially explained by an increase of the muscle anabolic threshold required to promote maximal anabolism and protein retention(Reference Dardevet, Remond and Peyron19). Since the muscle anabolic threshold is higher, the anabolic stimuli (including aminoacidemia) can no longer reach the anabolic threshold and, by consequence, muscle anabolism is reduced with the usual nutrient intake.
As afore-mentioned, the need to increase protein intake to counteract anabolic resistance is not desirable in the elderly with an ageing renal function. The answer to the question: How can the intake of AA be increased during the meal without increasing the daily protein intake? has been found in pulse protein feeding.
The principle of pulse protein feeding is to make sure that the protein intake is above the anabolic threshold in at least one meal during the day. Since total daily protein intake remains low, it leads to lower protein intakes during the other meals of the day, which is a better solution than to spread protein intake over all meals of the day, since in this case no meal would reach the anabolic threshold. In healthy women, for a total daily protein intake of 1 g/kg/d, it was shown that the pulse protein feeding pattern promoted a better nitrogen balance than the spread feeding pattern in older individuals, whereas there was no difference between the pattern in young individuals(Reference Arnal, Mosoni and Boirie20–Reference Arnal, Mosoni and Boirie23). A difference between the two patterns can be obtained only if the total protein intake is at the RDA. If an even protein intake is used in elderly subjects with at least 30 g protein per meal, good anabolism will ensue, because the anabolic threshold is attained at each meal. However, it could be considered as a high protein intake (90 g/d)(Reference Moore, Churchward-Venne and Witardo24).
Pulse protein feeding is particularly useful for feeding elderly undernourished people. Bouillanne et al.(Reference Bouillanne, Neveux and Nicolis25) showed that after 6 weeks, in malnourished or at-risk patients in an inpatient rehabilitation unit, pulse protein feeding resulted in a better increase in lean body mass than an even protein distribution. In this study, in the even protein distribution group, the amount of protein ingested at each meal varied between 12 and 21 g, which was probably never sufficient to stimulate muscle protein synthesis. On the contrary, in the pulse protein feeding group, one meal daily contained on average 48 g protein, which was above the anabolic threshold, leading to adequate stimulation of muscle protein synthesis. Over the day, it also probably led to a positive influence on muscle protein breakdown, moreover explaining the positive effect on lean body mass.
In the context of global overpopulation with the risk of running out of good quality proteins, pulse protein feeding could also be perceived as a rational and efficient way to meet the protein requirements of healthy elderly subjects.
Despite the recorded effectiveness of pulse protein feeding, lunch remains substantial in quantitative terms to ensure the intake of dietary proteins with real foods and dishes (Fig. 2). It also requires significant meal preparation time, cooking and prior organization to vary menus. In addition, as protein palatability decreases with age, there is a significant risk of the elderly not finishing the dishes and therefore not meeting the AA requirements over the long term.
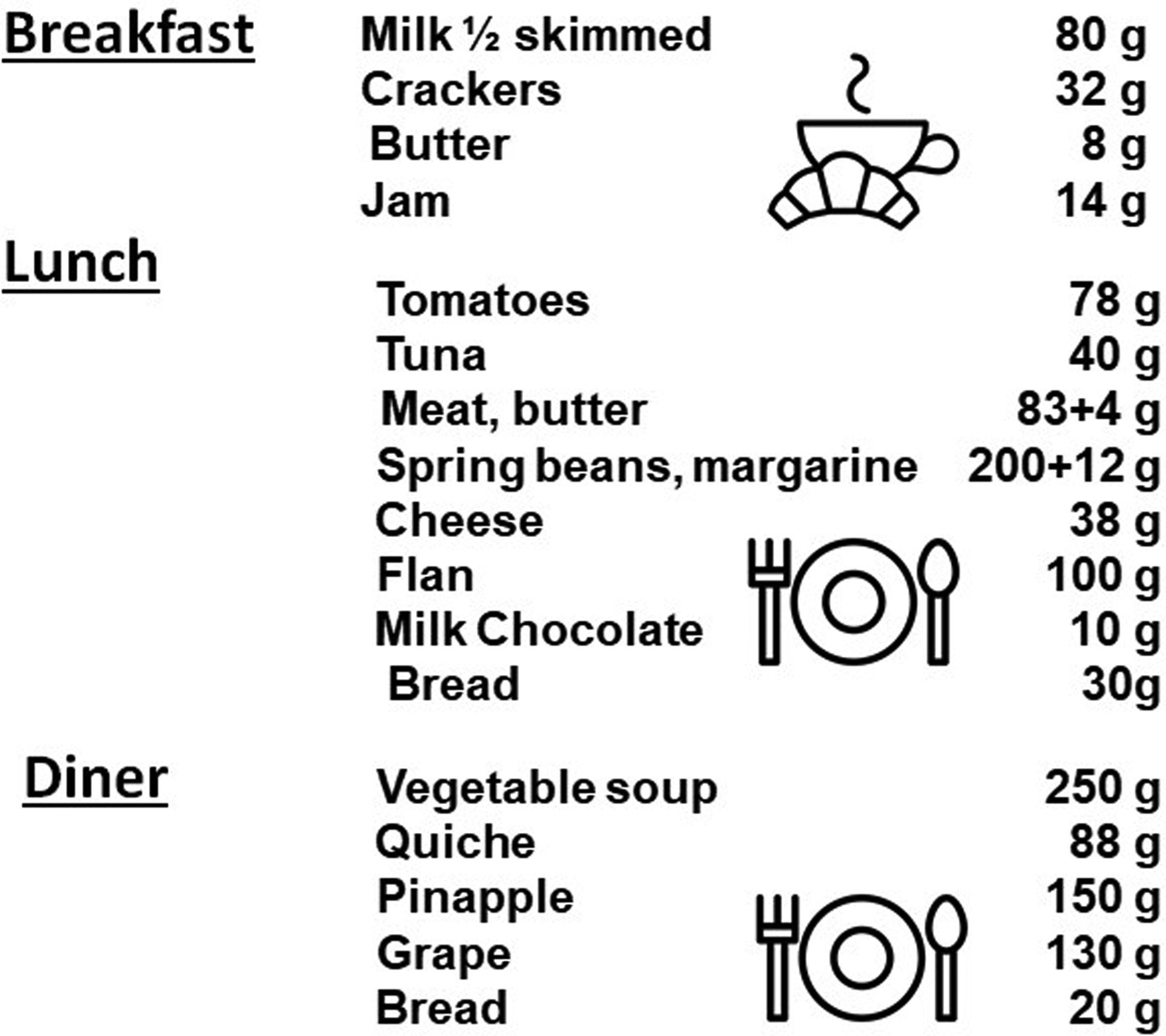
Fig. 2. Example of meals given for breakfast, lunch and dinner with a ‘protein pulse feeding’ protocol in the study of Arnal et al.(Reference Arnal, Mosoni and Boirie23) (personal communication). Breakfast by Agung Cahyo; Breakfast by rahmat from the Noun Project.
During ageing, the loss of muscle sensitivity to the anabolic effect of dietary AA has been linked to an essential AA: leucine(Reference Dardevet, Sornet and Balage26–Reference Guillet, Zangarelli and Mishellany29). Indeed, leucine is a signal AA which is not only a substrate for protein synthesis, it also stimulates an intracellular muscle signalling pathway involved in the initiation of protein synthesis: the mTOR signalling pathway. This suggests that increasing leucine availability may represent a nutritional strategy to overcome the increase in the anabolic threshold observed during ageing. Studies in both elderly human subjects and rodents subjected to free leucine supplementation have shown that such supplementations significantly improve muscle protein balance after food intake by increasing muscle protein synthesis and decreasing muscle proteolysis in the postprandial state(Reference Balage and Dardevet30–Reference Dreyer, Drummond and Pennings32). However, the few chronic studies conducted with such free leucine supplementations did not succeed in promoting the prevention of sarcopenia(Reference Verhoeven, Vanschoonbeek and Verdijk33,Reference Zeanandin, Balage and Schneider34) . Choosing free leucine as a supplement over a normal protein diet triggers de-synchronization between the leucine signal and the increase of all AA(Reference Dardevet, Remond and Peyron19). Indeed, free leucine is absorbed immediately whereas the other AA are released later after gastric emptying and proteolytic digestion in the gut. This non-synchronization between the stimulation of muscle leucine-associated protein metabolism pathways and the delayed availability of AA as substrates may be the reason why protein anabolism is stimulated for only a very short time during the postprandial period and thus could not result in significant muscle protein accretion. Studies on synchronized leucine signals and AA availability have been performed using leucine-rich proteins that are rapidly digested (whey proteins)(Reference Dangin, Boirie and Guillet35). With such proteins, leucine availability is increased simultaneously with that of the other AA to reach the increased muscle anabolic threshold. However, as observed for free leucine supplementation, when such dietary proteins were given, muscle anabolism was improved considerably(Reference Pennings, Boirie and Senden36). However, in the long term in elderly rodents, muscle mass remained unchanged(Reference Rieu, Balage and Sornet37). Positive results could only be recorded if whey proteins were given in the form of a chronic high protein diet (twice the RDA)(Reference Mosoni, Gatineau and Gatellier38). However, Magne et al.(Reference Magne, Auzeloux and Migne39,Reference Magne, Savary-Auzeloux and Rémond40) showed that in elderly rodents recovering from acute muscle atrophy, leucine-rich proteins were nevertheless efficient in improving the recovery of muscle mass whereas free leucine supplementation remained ineffective.
During ageing, protein requirement is increased, partly explained by anabolic resistance to AA and in particular to leucine. Dietary protein intake must be increased but vigilance is required regarding the potential deleterious effects of high protein diets in this population and the difficulty of supplying more AA. The pulse feeding diet has shown positive results but its implementation remains complex and may require oral nutritional supplements that are not widely accepted by this population(Reference Hubbard, Elia and Holdoway41). Free leucine supplements do not seem to show a positive effect due to the desynchronization of the arrival of AA in the peripheral blood. Leucine-rich and quickly digested proteins such as whey proteins enhance the anabolic effect of food intake but remain non-optimal in the elderly for the preservation of muscle mass if given at the RDA.
Energy substrates for enhancing the anabolic potential of dietary proteins in the elderly
Back in the 1980s, Reeds et al.(Reference Reeds, Fuller, Nicholson, Garrow and Halliday42) and Waterlow and Millward(Reference Waterlow, Millward, Wieser and Gnaiger43) calculated the daily energy cost of protein turnover as approximately 18 kJ (4⋅3 kcal)/kg body weight, or about 20 % of the BMR, showing that protein metabolism is an energy-requiring process. The first conclusion is therefore that in order for the anabolic effects of dietary proteins to have an optimal effect, they must be combined with energetic substrates, even more so in the elderly for whom this effect is precisely non-optimal. In this review, we discuss the effect of the nature and the timing of this energy supply on the mechanisms regulating muscle mass during ageing. However, we also point out the potential sarcopenic effect of the ingestion of simple sugars.
Could sugars be sarcopenic?
The first answer to this question that comes to mind is ‘of course not’. In the context of a normal and balanced diet, the nature of the energy consumed has only marginal effects on the regulation of muscle protein metabolism. In the short term, for one meal, co-ingestion of sugar with proteins should lead to higher insulin secretion. This anabolic hormone increases muscle anabolism essentially through reduced muscle proteolysis. However, in the long term, and considering that more and more people do not eat a normal and balanced diet, the answer to this question is different.
Indeed, since the 1950s, added sugar consumption (i.e. sugars that are not naturally present in our diet as, for example, in fruits but that are added artificially) has been steadily increasing, leading to high-fructose intake, either in the form of sucrose (sucrose is half glucose, half fructose) or directly as pure fructose, in particular since the introduction of high-fructose maize syrups(44,Reference Bray, Nielsen and Popkin45) . This consumption is chronic, daily, and continues for months and years. Such a diet (high chronic intake of added sugar) is thought to trigger many metabolic disorders. High fructose intake induces an increase in liver lipid content, leading to insulin resistance and then to dyslipidaemia, hypertension, oxidative stress, inflammation and non-alcoholic fatty liver disease(Reference Tappy and Lê46). As already explained, inflammation, oxidative stress and insulin resistance, which are also hallmarks of ageing, are probably responsible for the increase in the muscle anabolic threshold observed during ageing. High chronic intake of added sugar could therefore accelerate the rate of ageing, and indeed be sarcopenic(Reference Gatineau, Polakof and Dardevet47).
Indeed, it was shown that after 5 months of a high sucrose diet in old rats, sucrose fed rats lost significantly more lean body mass than starch fed rats (−8⋅1 v. −5⋅4 %, respectively) and final muscle mass was 11 % higher in starch than in sucrose fed rats(Reference Gatineau, Savary-Auzeloux and Migne48). Accordingly, meal-induced stimulation of muscle protein synthesis was significantly lower in sucrose (+7⋅3 %) than in starch fed rats (+22 %). This result was in line with an insulin sensitivity index divided by 2(Reference Gatineau, Savary-Auzeloux and Migne48).
More generally, liver damage, as induced by fructose, was shown to impact muscle, not only through insulin resistance, inflammation and oxidative stress, but also through endoplasmic reticulum stress, a decrease in the peripheral availability of anabolic factors such as hormones and AA, and through the production of catabolic effectors such as various hepatokines, methylglyoxal and uric acid(Reference De Bandt, Jegatheesan and Tennoune-El-Hafaia49). In agreement with these results, a prospective study showed that the consumption of added sugars in the diet of older people was associated with frailty(Reference Laclaustra, Rodriguez-Artalejo and Guallar-Castillon50). Thus, given all these elements, it seems likely that sugars, or more precisely high chronic intake of added sugar, are sarcopenic(Reference Gatineau, Polakof and Dardevet47).
De-synchronized energy boluses during the postprandial period
As developed earlier (see earlier), when given in the long-term, whey proteins alone do not appear to be an optimal nutritional strategy to prevent or slow down muscle wasting during ageing or catabolic states(Reference Revel, Jarzaguet and Peyron51–Reference Nicastro, Artioli and dos Santos Costa54). This could be explained by the nature and intensity of the catabolic state and by the fact that the digestion of whey proteins may be too rapid during a catabolic situation to sustain the anabolic postprandial AA requirement necessary to elicit an optimal anabolic response(Reference Lacroix, Bos and Léonil55). Indeed, the stimulation of muscle protein synthesis has a defined and limited duration during the postprandial period(Reference Atherton, Etheridge and Watt56,Reference Bohé, Low and Wolfe57) . Atherton et al. showed that whey ingestion is only able to stimulate muscle protein synthesis for 2 h and has been named the muscle full effect(Reference Atherton, Etheridge and Watt56). The leucine content of a complete meal drives peak activation but not the duration of skeletal muscle protein synthesis(Reference Norton, Layman and Bunpo58). Interestingly, it has been shown recently that the duration of the postprandial stimulation of muscle protein synthesis in healthy conditions can nevertheless be prolonged by maintaining the cellular energy status with the supplementary ingestion of a desynchronized carbohydrate load at approximately 150 min after food intake(Reference Wilson, Layman and Moulton59). Such a prolongation could be a complementary strategy to improve the whey protein supplement effect during ageing, or in other catabolic situations in which these proteins are still not optimal. This hypothesis has been tested in preclinical models of ageing (rodents) and catabolic states (mini pigs) in which rapid sugars such as glucose and sucrose were given to sustain energy supply to skeletal muscle 90–120 min after the beginning of food intake. Unfortunately, this desynchronized energetic bolus during the postprandial period has shown no beneficial effect on the intensity or the duration of anabolism initiated by whey proteins in either model(Reference Mosoni, Jarzaguet and David60).
Energy boluses during the post-absorptive state to attenuate muscle loss during the night
The overnight sleep period represents the longest period during the day with no AA intake and as a consequence, results in both reduced rates of muscle protein synthesis and increased rates of muscle protein breakdown(Reference Meek, Persson and Ford61). It can be hypothesized that the additional muscle protein gain initiated by the ingestion of whey protein intake at lunch may then be lost at night, explaining the disappointing results of whey protein supplementation to reduce and slow down sarcopenia in the long term. Thus, attenuating the overnight losses of lean tissue mass (including muscle) would potentially be beneficial in helping to maintain lean tissue/muscle mass in the elderly by sparing the anabolic effect of whey protein ingested at lunch.
Several strategies have been explored to take advantage of this nutritional window, including pre-sleep protein ingestion, combined or not with exercise. The impact of such interventions on muscle, lean mass and protein synthesis is variable, although some evidence suggests a beneficial effect, as has been reviewed elsewhere(Reference Snijders, Trommelen and Kouw62,Reference Karagounis, Beaumont and Donato-Capel63) . While these strategies aim at improving overnight protein synthesis (normally low(Reference Beelen, Tieland and Gijsen64)) another possibility would consist in tackling the protein breakdown that takes place concomitantly during the food deprivation period. Indeed, early studies on food-deprived animals have shown that fructose feeding induced a reduction in hepatic AA catabolism(Reference Pedersen, Alnor and Vilstrup65), resulting, as with other carbohydrates(Reference Takada and Saitoh66,Reference Levine and Saltzman67) in a protein sparing effect. This interaction between carbohydrates and AA metabolism in the post-absorptive state has been known since the first studies of Cuthbertson and Munro(Reference Cuthbertson and Munro68) and Gamble(Reference Gamble69) on human subjects and on both young and older adults(Reference Gelfand and Sherwin70,Reference Fukagawa, Veirs and Langeloh71) in particular regarding physiological conditions, such as in catabolic states in which energy intake is limited(Reference Wilmore72,Reference Mikura, Yamaoka and Doi73) . In addition, carbohydrates have also been shown to exert a better nitrogen-sparing effect than fat, particularly under circumstances of limited energy supply(Reference Boirie, Walrand, Beaufrere and Cynober74).
Associated with the consumption of soft drinks and pastries rich in high-fructose maize syrup, rates of obesity and other metabolic dysregulations are increasing and have been suggested by some authors to be linked to fructose intake(Reference Lustig75). However, during the night with only limited to no energy supply, it is clear that fructose is converted mostly into glucose and glycogen, and no deleterious effects on insulin sensitivity or lipid homeostasis have been observed so far. In this context, the specific positive effects of fructose could be expected to occur through its interaction with AA metabolism. We observed(Reference Dardevet, Mosoni and David76) that, although both fructose and glucose spared body weight and lean mass, fructose feeding was more efficient than glucose in sparing liver, skeletal muscle and intestinal masses. Fructose also significantly spared fat mass, which may be of specific interest in muscle wasting conditions: (1) because conserved fat mass reduces the risk of morbidity and mortality in hospitalized patients, particularly in elderly people(Reference Bouillanne, Dupont-Belmont and Hay77,Reference Hickson78) ; and (2) because during protein-energy restriction larger fat stores can delay AA utilization. Overall, this study reported an inter-organ metabolic strategy resulting in reduced hepatic AA utilization for neoglucogenesis able to preserve the mass of major tissues, and more generally the lean mass of the body (Fig. 3). We also showed(Reference Mikura, Yamaoka and Doi73), indirectly, that some tissues such as skeletal muscle remained metabolically stable and benefited from deep modifications in other organs, such as the liver, to improve their carbohydrate storage and maintain their masses. The fact that fructose feeding preserved both lean and fat mass without inducing hyperlactatemia and while maintaining low uric acid and TAG suggests that this nutritional intervention, in addition to protein supplements given at lunch, may be of potential use in the elderly in the form of a pre-sleep long-lasting carbohydrate release bolus.
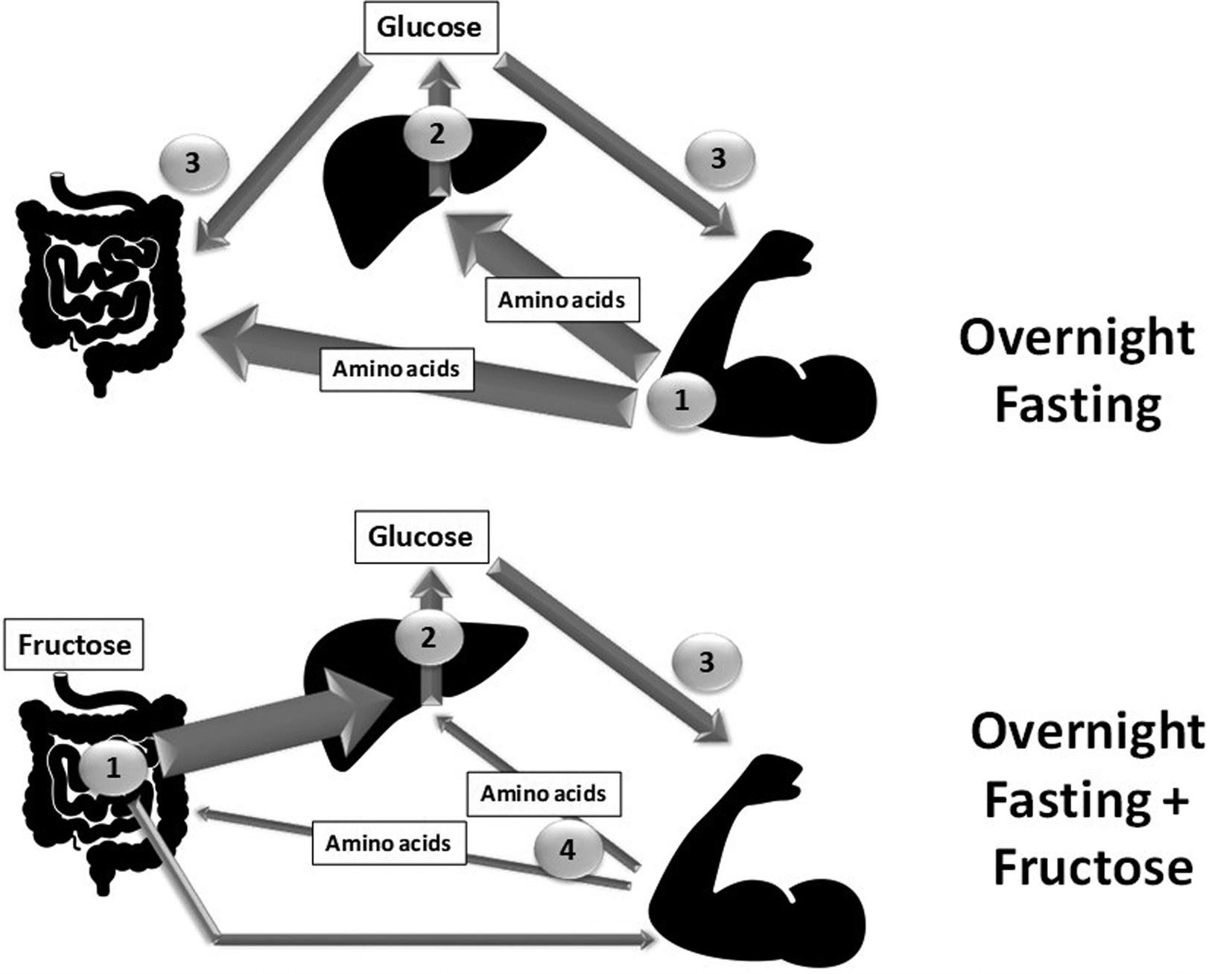
Fig. 3. Schematic representation of the metabolic pathways involved between the different organs in a night-fasting situation with or without a fructose bolus given apart from dinner and just before bedtime. The thickness of the arrow represents either the amount of the nutrient involved or the intensity of the corresponding metabolic pathway. The number represents the order in which the nutrient flows are involved. Gut, liver by Philip Hogeboom; Muscle by Adrien Coquet from the Noun Project.
Simple sugar can be sarcopenic if ingested in excessive amounts on a daily basis.
Targeted energetic boluses given 90 min after food intake may not be efficient to sustain postprandial muscle protein synthesis initiated by whey proteins.
Energetic boluses given apart from dinner (≥2 h), during the night with no other nutrient intake, could limit post-absorptive muscle breakdown and thus preserve the anabolic effect of whey protein supplementation. Fructose could be favoured in this specific strategy as it is more efficiently transformed into glucose/glycogen and is able to spare more fat mass than glucose.
Oral health and its impact on amino acid availability
The digestive process starts in the mouth and food bolus characteristics (that will be further degraded in the rest of the gut) are highly dependent on masticatory function(Reference Peyron, Woda and Bourdiol79,Reference Rémond, Shahar and Gille80) . The purpose of mastication is to prepare the food bolus via the mechanical disruption of the morsel of food into small particles and moisturizing by saliva foods that the can swallowed. Some chemical digestive processes also start in the mouth due to the activation of α-amylases and lipases. During normal ageing, several determinants of oral function can be progressively impaired such as decreased tongue thickness, the cross-sectional area of the masseter, mixing ability and tongue movement(Reference Lamster, Asadourian and Del Carmen81). However, masticatory function can be maintained through minor adaptations such as an increase in the number of masticatory cycles to offset reduced masticatory forces, and generate a safe, swallowable bolus(Reference Yoshida and Kazuhiro82). On the contrary, when the dental state is impaired (decreased number of teeth, decreased occlusal force) and when the quantity and quality of the saliva are lower, masticatory function can be impacted negatively. This leads to the insufficient breakdown of the bolus and a higher proportion of large particles(Reference Peyron, Santé-Lhoutellier and François83). This masticatory function remains impaired in edentulous individuals even when wearing a denture(Reference Mishellany-Dutour, Renaud and Peyron84).
This alteration of oral health and masticatory function is particularly present in sarcopenic, pre-frail and frail populations. Indeed, several studies (observational and longitudinal) have demonstrated that a frail group of elderly adults present relatively fewer (functional) teeth, lower occlusal force/masseter thickness and overall impaired masticatory function along with a lower muscle mass index and nutritional state compared to a robust elderly group(Reference Watanabe, Hirano and Arai85,Reference Horibe, Ueda and Watanabe86) . Consistent with the earlier discussion, alterations in dental status, chewing and swallowing are considered to be among the determinants of malnutrition in elderly adults(Reference O'Keeffe, Kelly and O'Herlihy87). This raises the question whether impaired masticatory function is a cause or a consequence (or both) of the overall muscle mass loss and nutritional state observed in frailty. It has been repeatedly demonstrated that tooth loss or edentulism is associated with diet selection (fewer vegetables and fruits, leading to a lower intake of certain vitamins and dietary fibres) and overall decreased energy and protein intake(Reference Marcenes, Steele and Sheiham88–Reference Sheiham, Steele and Marcenes90). This diet selection is partially driven by the incapacity of edentulous elderly people to generate a swallowable food bolus (afore-mentioned)(Reference Mioche, Bourdiol and Peyron91). This alone, via a lower energy and protein intake, could participate in worsening sarcopenia and frailty directly through a decrease in dietary protein supply. However, food choice is not the only parameter that can reduce AA availability in elderly people. Indeed, since edentulous and denture wearing elderly people chew less, a reduction in the availability of nutrients (including AA) can occur due to insufficient mechanical disruption of proteins in the mouth. To test this hypothesis, we carried out a clinical study on elderly volunteers in which AA bioavailability from beef meat was assessed in fully dented individuals compared to edentulous ones wearing complete dentures(Reference Rémond, Machebeuf and Yven92). We showed that denture wearers presented delayed absorption of proteins associated with an overall lower AA occurrence in the blood during the post-meal period. It was also associated with a lower anabolism at the whole-body level (Fig. 4)(Reference Rémond, Machebeuf and Yven92). This means that, in a situation of similar dietary protein intake, reduced AA bioavailability due to impaired mastication functionality resulted in non-optimal protein nutrition to sustain the AA requirement.
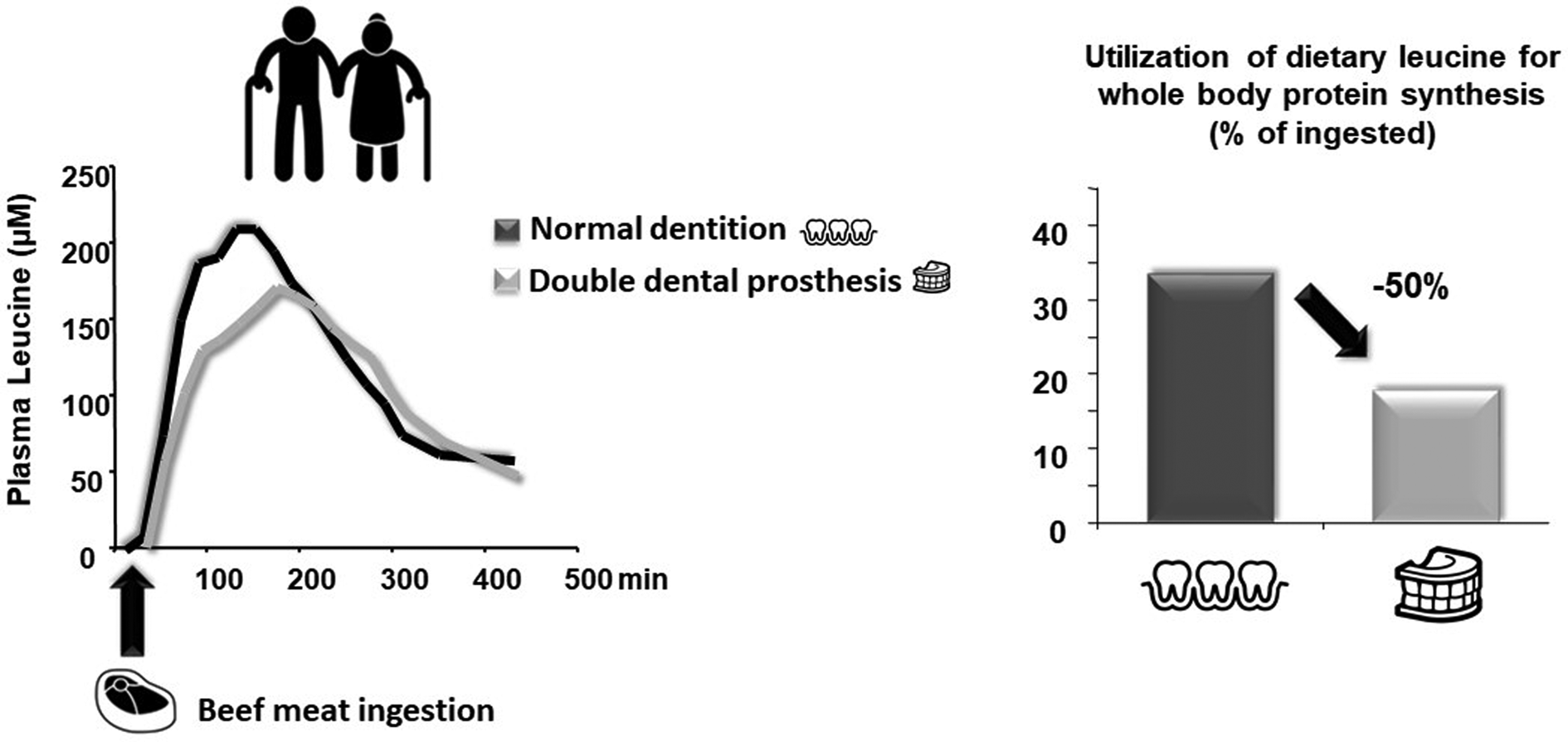
Fig. 4. Effect of masticatory deficiency on plasma amino acid availability and whole-body anabolic effect (from Rémond et al.(Reference Rémond, Machebeuf and Yven92)). Denture by Jems Mayor; Teeth by Adrien Coquet; Steak by Sandra from the Noun Project.
The last question that remains is the following: can the altered AA availability observed in elderly people presenting altered masticatory functions be reversed? Few studies have tackled this issue but they tend to show that this area of research remains to be investigated in the field. One potential lever to optimize AA availability is the use of foods textured to fit elderly oral capabilities to overcome impaired masticatory function. Another path is to assess the impact of the food matrix itself or in association with masticatory impairments(Reference Aguilera and Park93) (see earlier). This was tested by Pennings et al.(Reference Pennings, Groen and van Dijk94) on elderly men wearing dentures offered minced or non-minced meat in a cross-over experimental design. In the denture-wearing volunteers presenting impaired masticatory function(Reference Mishellany-Dutour, Renaud and Peyron84), beef proteins were more rapidly digested and AA availability improved after eating minced meat. Pre-processing of foods targeted at elderly edentulous/denture-wearing populations is therefore an area of research that requires further investigation. Another way to improve both protein intake and digestibility is to enhance masticatory function via training. This has been investigated by Kikutani et al.(Reference Kikutani, Enomoto and Tamura95) who showed increased body weight and plasma albumin in frail elderly Japanese volunteers after both oral functional training and protein/energy supplementation compared to supplementation alone. Again, such beneficial effects of oral training interventions require further investigation, with a detailed assessment of the nutritional state of elderly volunteers.
Oral health is certainly an important concern during ageing that can directly affect food choice linked to texture, mastication, food bolus formation and swallowing. Ageing itself has a slight impact and some adjustment of mastication can compensate for a decrease in masticatory force or tongue motility, for example.
The challenge of maintaining correct food intake and optimal absorption in the elderly presenting severe oral deficiencies is to develop/design food textures that match both their oral sensory–motor capabilities and their nutritional needs.
The food matrix as a strong determinant of amino acid availability and dietary protein anabolism potential in the elderly
For a protein source to be of good nutritional value and content quality, the indispensable amino acids (IAA) contained in it must be well balanced with respect to body requirements. They must also be bioavailable, meaning that the proteins have to be highly digestible to ensure the optimal release of AA in the bloodstream. The digestibility of food proteins depends on the intrinsic structure of the proteins and on their physical and chemical environment, the so-called matrix effect.
The effect of protein structure on digestibility is illustrated by the very low intestinal digestibility of zein (maize protein), a very compact protein of low solubility, when compared to whey proteins (30 v. 90 %)(Reference Calvez, Benoit and Fleury96). Despite its leucine content, i.e. 15 %(Reference Matthews, Kunkel and Acton97) which is interesting for the elderly (see earlier), its usefulness as a protein source for older adults is nevertheless lessened by its low digestibility. Indeed, it would be necessary to double its intake if used as a single source of dietary proteins. In addition, the molecules associated with dietary proteins within the matrix or the meal can also limit the efficiency of protein digestion. This is particularly the case for compounds that block the proteolytic activity of digestive enzymes (trypsin inhibitors, polyphenols, etc.). Cooking generally leads to the inactivation of trypsin inhibitors. It improved the intestinal digestibility of a soya protein concentrate that was considerably improved by autoclave treatment(Reference Caine, Sauer and Verstegen98). It has also been shown that the fermentation of seeds before cooking significantly reduces the activity of these enzymes. Regarding polyphenols, we observed that the digestion of meat proteins was not impacted by the presence of polyphenol-rich fruit and vegetables in the meal, whereas the intake of the same polyphenols in equivalent quantities, in the form of extracts, significantly decreased the apparent digestibility of proteins(Reference Dufour, Loonis and Delosiere99). It is known that, by complexing with dietary proteins or digestion enzymes, polyphenols can reduce the efficiency of digestion. However, it is interesting to note that the effect of these bioactive plants can be very different depending on whether they are in free form, as an extract (in food supplements, or as a food preparation additive) or in their original matrix. This could partly explain the lack of effect of supplementation with polyphenols in free form, as natural antioxidants, on muscle mass preservation during ageing(Reference Mosoni, Gatineau and Gatellier38,Reference Mosoni, Balage and Vazeille100) . This is because the beneficial antioxidant effect is counter-balanced by a decrease in protein digestion and in AA bioavailability.
In addition to digestibility as such, digestion speed, which is the incremental increase of AA over time, can be very different between dietary proteins and lead to very different hyper-aminoacidemia levels when they are ingested at the same amount (Fig. 5). In older adults, it has been shown that the dietary proteins with fast digestion speeds and which generate the highest amino acidemia were much more efficient in initiating muscle anabolism (see earlier). A case study was performed to compare two milk proteins with similar digestibility, i.e. casein and whey proteins(Reference Dangin, Guillet and Garcia-Rodenas101). Whey proteins are rapidly digested whereas caseins, which coagulate in the stomach, are the best example of slowly digested animal proteins. In older adults, whey proteins are much more efficient than casein in promoting muscle anabolism when ingested at 30 g/meal. Ranking proteins according to their speed of digestion remains a challenge. Indeed, beyond the specific properties of each protein, the technological processes used to prepare foods, such as cooking, can alter digestion kinetics. With meat matrices, we have shown that the digestion speed of proteins increases by heating up to a temperature of 70–75°C, above which it declines(Reference Bax, Buffière and Hafnaoui102). Heat treatment leads to the denaturation of the protein, which results in the externalization of the hydrophobic AA that become accessible to digestive proteases. At temperatures above 70°C, aggregation and spherical congestion are initiated by oxidation reactions, hydrophobic interactions or covalent binding, which reduce accessibility to proteases and thus decrease the speed of meat protein digestion(Reference Bax, Buffière and Hafnaoui102,Reference Bax, Aubry and Ferreira103) . Although cooking temperature has little effect on the total bioavailability of AA in meat (digestibility), which in all cases remained very high(Reference Bax, Buffière and Hafnaoui102,Reference Oberli, Marsset-Baglieri and Airinei104) , we observed that by increasing the digestion speed, it can have a very significant positive effect on the bioavailability and assimilation of meat AA in the elderly(Reference Buffière, Gaudichon and Hafnaoui105).

Fig. 5. Schematic representation of the plasma kinetic of amino acid appearance, digestion speed and maximal plasma amino acid levels following the same amount of slow and fast digested dietary proteins with identical digestibility.
The second example is milk processing. We indeed observed that gelling milk (with rennet or gluconodeltalactone) delays the duodenal occurrence of proteins and therefore decreases the digestion speed of dietary protein, leading to a modification of the absorption of dietary AA(Reference Barbé, Ménard and Le Gouar106,Reference Barbé, Ménard and Gouar107) . Several hypotheses could explain this result: very long gastric retention (associated with considerable digestive secretions), and increased resistance to digestive proteolysis. It is also interesting to note that between milk and yoghurt, ileal digestibility is not modified(Reference Gaudichon, Roos and Mahé108). As with heat treatments of meat, gelation affects protein digestion speed but not the quantity digested.
The characteristics of the food matrix significantly influence the digestion speed of dietary proteins and the bioavailability of AA, and modulate protein assimilation efficiency in the elderly.
Technological processing, by acting on the food matrix, helps in optimizing digestive parameters according to the specific characteristics of the elderly.
Thus, for the elderly, in whom protein requirements tend to increase while their spontaneous intake decreases, optimized food matrices could decrease the risk of inadequate intakes of IAA, and improve assimilation efficiency.
Could the optimization of protein nutrition include plant proteins in the elderly?
Due to the negative environmental impact of intensive animal production, and because of the benefits to health of consuming fruit, vegetables and grains, dietary guidelines in several countries tend to advise increasing plant protein intake with a corresponding increase of the plant:animal protein ratio in human nutrition(109,Reference Boland, Rae and Vereijken110) . In addition, consumers appear to agree with this transition to more plant proteins due to ethical considerations with regard to animal welfare and because of nutritional messages that accuse red meats as being harmful to health if consumed in large amounts(Reference De Gavelle, Davidenko and Fouillet111,Reference Mariotti and Gardner112) .
The search for alternative protein sources and transitioning towards more sustainable, plant-based nutrition has received much attention in the past decade(113). Indeed, although new protein sources such as algae, micro-algae and insects are currently attracting growing interest, the most relevant option in the, urgent, short term is to take better advantage of the huge amount of plant proteins already available. However, besides certain technical and organoleptic issues, one of the main obstacles to using plant proteins in human nutrition is generally the low nutritive value classically attributed to them, because of their unbalanced composition in IAA, and because they are generally less digestible than animal proteins(114). Solutions to optimize IAA supply with plant foods include combining plant foods with complementary AA compositions, amino-acid complementation, consuming higher amounts of plant-based products on a more frequent basis and enhancing the nutritional quality of crops through genetic engineering(Reference Sands, Morris and Dratz115).
During ageing, the impact of lower quality plant proteins was exemplified in older healthy volunteers by Gorissen et al.(Reference Gorissen, Horstman and Franssen116). When given at 35 g/meal, casein is unable to stimulate muscle protein synthesis, illustrating anabolic resistance to normal protein intake during ageing. Whey proteins, speedily digested and rich in leucine, were able to stimulate anabolism when given at the same amount whereas, like casein, wheat protein remained inefficient. The absence of efficiency is explained by the lower content of lysine in cereal protein sources (about 50 %), so this AA was limiting (see earlier). Although when wheat proteins were almost doubled (60 g), an anabolic effect was again recorded in skeletal muscle. This study clearly showed that if a plant protein with an unbalanced AA composition is consumed, the quantities that must be ingested to stimulate protein anabolism would be of a magnitude impossible to maintain in the elderly in the long term. It is noteworthy that this is also true in younger individuals but it is aggravated during ageing due to anabolic resistance. It is known that plant proteins have to be mixed, for instance cereals with leguminous seeds, in order to re-equilibrate the IAA composition when ingested. This strategy, if followed correctly, has been shown to be safe enough to cover the requirement of the essential AA and is therefore optimal for anabolism in the healthy young adult population(Reference Mariotti and Gardner112). However, is this strategy sufficiently safe to be applied directly to the elderly for whom an increased need for AA has been demonstrated, even in individuals considered to be in good health? This was tested in a pre-clinical study by Berrazaga et al.(Reference Berrazaga, Salles and Laleg117). They showed that if wheat proteins were mixed with either faba bean, lentil or pea proteins, the resulting diet remained inefficient in stimulating muscle protein synthesis in old rats by contrast to whey proteins consumed at the same amount. This clearly shows that a significant increase in protein intake must be considered for the elderly when consuming a diet whose protein sources are solely of plant origin, and even if they are AA-balanced for younger adult individuals. This finding was supported by the unpublished results of Jarzaguet et al., who tested a plant protein, i.e. soya protein which, despite being well balanced in essential AA, remained ineffective in stimulating anabolism in old rats if given at a level that was efficient in younger adults(Reference Jarzaguet, Polakof and David118). This difference in anabolic effect can be explained by the lower content of IAA, especially leucine, in plant proteins v. animal proteins(Reference van Vliet, Burd and van Loon119).
This implies that protein intake with only plant proteins must be higher than with animal proteins, which would be undoubtedly tricky to achieve in an elderly population. However, to significantly ‘green’ dietary proteins intended for the elderly, a portion of high-quality animal protein should remain in the diet in order to keep the total protein intake at values compatible with the food intake of these individuals. Recently, it has been shown that a blend of 70/30 % of balanced AA plant proteins/whey proteins can be as efficient as whey alone at stimulating muscle anabolism in old rats if the total protein intake per meal is increased by only 25 %(Reference Jarzaguet, Polakof and David118).
In conclusion, although nearly all epidemiological studies converge regarding the protective effect of meat (animal) consumption on sarcopenia(Reference Nikita, Alexandrov and Cécile120), the health benefits of plant proteins have yet to be investigated extensively(Reference Lonnie, Hooker and Brunstrom121) and more studies are needed to evaluate the effectiveness of plant proteins in the prevention of muscle mass and strength loss.
Plant proteins are generally of lower nutritional quality than animal proteins, but this does not hinder ‘greening’ the dietary proteins of the elderly if simple rules are followed: (1) Vegetal protein sources should be mixed to avoid unbalanced protein AA consumption; (2) A portion of high-quality animal proteins in the diet should be maintained; (3) The daily intake of total proteins should be increased. Above 50 % of vegetable proteins in the diet, the risk of inadequate protein intake is first linked to the quantity of proteins consumed, then above 70 % the nature of the proteins consumed becomes significant, and the percentage of legumes, nuts and seeds consumed must be increased to prevent lysine deficiency in particular(Reference de Gavelle, Huneau and Bianchi122).
More studies are also needed to evaluate the effectiveness of plant proteins in the prevention of muscle mass and strength loss.
Conclusions
Ageing is associated with multiple deficiencies, which challenge the efficiency of protein nutrition even when the RDA for dietary proteins is respected. These alterations which are both metabolic (anabolic resistance) and functional (oral health) will have an impact that will be more or less important depending on the age but also the physiopathological state of the person. In this review, we have highlighted several potential determinants that may be taken into account or on which, attention should be paid, depending on the clinical and health examination of the person (see graphical abstract). As a result, the strategy put in place to optimize the efficiency of the protein supply must be multi-modal because a single lever cannot be sufficient but also because some of them cannot be implemented depending on the state of senior's health. The equation is indeed complex to solve, but taking into account the individual and personalized limitations of each person still allows the implementation of the solutions that we have mentioned in this review.
Financial Support
None.
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.









