Introduction and aims
Depressive disorders are one of the leading causes of disability worldwide, with a high impact on individuals and society in terms of medical costs and loss of productivity (Friedrich et al., Reference Friedrich2017). Major depressive disorder (MDD) is one of the most common mental disorders, with an estimated worldwide prevalence rate of 4.7% (Friedrich, Reference Friedrich2017). Notably, approximately 15–30% of MDD patients do not respond to two antidepressant drugs, defining the condition as treatment-resistant depression (TRD), which is associated with more severe cognitive impairment, increased comorbidities, increased risk of suicide and higher medical costs (Du et al., Reference Du, Liu, Du, Chao, Zhang, Wang, Huang, Gao and Tang2017; Garay et al., Reference Garay, Zarate, Charpeaud, Citrome, Correll, Hameg and Llorca2017).
Various treatment strategies have been proposed for TRD, including both pharmacological and non-pharmacological approaches (McIntyre et al., Reference McIntyre, Filteau, Martin, Patry, Carvalho, Cha, Barakat and Miguelez2014). Among the latter, various stimulation techniques have been approved for TRD, such as electroconvulsive therapy, vagus nerve stimulation, deep brain stimulation and repetitive transcranial magnetic stimulation (rTMS) (Akhtar et al., Reference Akhtar, Bukhari, Nazir, Anwar and Shahzad2016). Specifically for rTMS, this technique is progressively gaining ground as a non-invasive, safe and generally well-tolerated treatment option for MDD and its efficacy in patients with TRD has been confirmed in three large, multicentre, randomised controlled trials (RCTs) (O'Reardon et al., Reference O'Reardon, Solvason, Janicak, Sampson, Isenberg, Nahas, McDonald, Avery, Fitzgerald, Loo, Demitrack, George and Sackeim2007; George et al., Reference George, Lisanby, Avery, McDonald, Durkalski, Pavlicova, Anderson, Nahas, Bulow, Zarkowski, Holtzheimer, Schwartz and Sackeim2010; Levkovitz et al., Reference Levkovitz, Isserles, Padberg, Lisanby, Bystritsky, Xia, Tendler, Daskalakis, Winston, Dannon, Hafez, Reti, Morales, Schlaepfer, Hollander, Berman, Husain, Sofer, Stein, Adler, Deutsch, Deutsch, Roth, George and Zangen2015). Briefly, during rTMS sessions, repeated magnetic pulses are delivered through the skull to a specific cortical region; when the magnetic field reaches the neural tissues, a secondary electrical field is generated. The ultimate effect is dependent on stimulation frequency, with high-frequency rTMS associated with increased neuronal excitability and low-frequency stimulations with decreased neuronal excitability (De Risio et al., Reference De Risio, Borgi, Pettorruso, Miuli, Ottomana, Sociali, Martinotti, Nicolò, Macrì, di Giannantonio and Zoratto2020).
Several mechanisms of action have been postulated for rTMS in the treatment of MDD (Chervyakov et al., Reference Chervyakov, Chernyavsky, Sinitsyn and Piradov2015). A prominent hypothesis suggests that rTMS induces neuronal plasticity and a restructuration of neuronal networks (Kozyrev et al., Reference Kozyrev, Staadt, Eysel and Jancke2018). This is relevant since MDD has been associated not only with structural brain alterations, but also with functional connectivity (FC) dysfunctions of major brain networks (Schmaal et al., Reference Schmaal, Pozzi, Ho, van Velzen, Veer, Opel, Van Someren, Han, Aftanas, Aleman, Baune, Berger, Blanken, Capitão, Couvy-Duchesne, Cullen, Dannlowski, Davey, Erwin-Grabner, Evans, Frodl, Fu, Godlewska, Gotlib, Goya-Maldonado, Grabe, Groenewold, Grotegerd, Gruber, Gutman, Hall, Harrison, Hatton, Hermesdorf, Hickie, Hilland, Irungu, Jonassen, Kelly, Kircher, Klimes-Dougan, Krug, Landrø, Lagopoulos, Leerssen, Li, Linden, MacMaster, McIntosh, Mehler, Nenadić, Penninx, Portella, Reneman, Rentería, Sacchet, Sämann, Schrantee, Sim, Soares, Stein, Tozzi, van Der Wee, van Tol, Vermeiren, Vives-Gilabert, Walter, Walter, Whalley, Wittfeld, Whittle, Wright, Yang, Zarate, Thomopoulos, Jahanshad, Thompson and Veltman2020), including the Central Executive Network (CEN), which is involved in cognitive control and emotion regulation, and the Default Mode Network (DMN), which mediates self-referencing and internally oriented processes (Hamilton et al., Reference Hamilton, Farmer, Fogelman and Gotlib2015; Kaiser et al., Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015).
In this framework, the aim of this review is to summarise the evidence on brain structural and FC changes after excitatory rTMS of the left dorsolateral prefrontal cortex (DLPFC) in MDD, and their potential relation with rTMS therapeutic efficacy.
Methods
We performed a bibliographic search on PubMed, with the following query: ‘(rTMS or TMS or transcranial magnetic stimulation) AND (connectivity or dwi or diffusion) AND (depression)’. No limitation was posed regarding publication date. We included studies using unilateral, excitatory, high-frequency (⩾10 Hz) TMS treatment targeting the left DLPFC. Only studies that investigated brain structural and FC using whole-brain neuroimaging techniques before and after rTMS protocols in depressed patients were included. Studies (a) employing other techniques (e.g. electroencephalogram), (b) based on bilateral or inhibitory rTMS protocols, or (c) targeting areas other than left DLPFC were excluded. The reference list of the selected articles was checked in order to find relevant references not emerged from the main query. Only 13 studies were selected as eligible. Of these, two studies included, in addition to unipolar depressed patients, a small cohort of depressed bipolar disorder type II patients. Finally, nine studies used resting-state functional magnetic resonance imaging (rs-fMRI), three studies employed diffusion tensor imaging (DTI) techniques, and one study employed single-photon emission computed tomography (SPECT).
Results
Connectivity changes induced by rTMS treatment are briefly discussed below, divided according to the investigated connectivity domain. FC results focused on two brain areas, the DLPFC and the subgenual anterior cingulate cortex (sgACC), whose connectivity emerged to be affected by the selected rTMS protocol. Although our focus was on connectivity changes from before to after rTMS, we also listed, when reported, the baseline features predictive of treatment response.
DLPFC functional connectivity changes
In an open-label trial on 58 unipolar and bipolar depressed patients evaluating SPECT FC changes before and after 4 weeks of rTMS, Richieri et al. (Reference Richieri, Verger, Boyer, Boucekine, David, Lançon, Cermolacce and Guedj2018) demonstrated a decrease in FC between the left DLPFC and both the anterior and posterior cingulate cortex and the right medial temporal lobe, key nodes of the DMN. Similarly, in another open-label trial on 17 unipolar and bipolar type II depressed patients and 35 healthy controls (HC) evaluating rs-fMRI connectivity changes before and after 5 weeks of rTMS, Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014) found, at baseline, a decreased FC in patients compared to HC between the left DLPFC and a key region of the DMN, the right parahippocampal gyrus. Moreover, consistently with the results reported by Richieri et al. (Reference Richieri, Verger, Boyer, Boucekine, David, Lançon, Cermolacce and Guedj2018), the authors observed an rTMS-induced decrease in FC between the left DLPFC and many areas of the DMN, such as the ventromedial prefrontal cortex, the posterior cingulate cortex and the right parahippocampal gyrus. Besides the investigation of FC between DLPFC and DMN areas, Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014) also explored FC between DLPFC and regions that are part of the CEN. Specifically, the authors showed, at baseline, a decreased FC in patients compared to HC between the left DLPFC and multiple areas of the CEN, including the premotor cortex, inferior parietal lobule, precuneus, cerebellum and other areas within the lateral prefrontal cortex. However, the FC reductions in these areas did not change after rTMS.
Moreover, in an open-label trial on 27 unipolar depressed patients and 27 HC, Zheng et al. (Reference Zheng, Yu, Du, Liu, Zhang, Xu, Xiang and Du2020) analysed rs-fMRI connectivity changes before and after 2 weeks of rTMS through functional connectivity density, defined as the FC between a voxel and the rest of voxels across the whole brain. Consistently with the results found by Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014), the authors observed, at baseline, a decreased FC in patients compared to HC within the CEN. However, this decreased FC improved after rTMS, contrasting with the null effect of rTMS reported by Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014). Additionally, in an RCT on 21 unipolar depressed patients evaluating rs-fMRI connectivity changes after 2 weeks of real v. sham rTMS, Kang et al. (Reference Kang, Lee, Jhung, Kim, An, Yoon, Kim, Namkoong and Lee2016) demonstrated a decreased FC in active compared to sham group between both targeted (left) DLPFC and contralateral DLPFC and between the left DLPFC and left caudate. Consistently with these results, in an RCT on 33 unipolar depressed patients evaluating rs-fMRI connectivity changes before and after 4 weeks of rTMS, Eshel et al. (Reference Eshel, Keller, Wu, Jiang, Mills-Finnerty, Huemer, Wright, Fonzo, Ichikawa, Carreon, Wong, Yee, Shpigel, Guo, McTeague, Maron-Katz and Etkin2020) observed a decreased FC in active compared to sham group between both targeted (left) DLPFC and controlateral DLPFC and between the left DLPFC and bilateral amygdala. Moreover, the authors demonstrated an increased targeted (left) DLPFC global FC in active compared to sham group. All these post-rTMS changes brought patients closer to the FC values demonstrated in the HC group. Interestingly, the authors also investigated the modulating role of the left DLPFC stimulation on contralateral DLPFC and bilateral amygdala through the analysis of the fMRI blood oxygen level-dependent signal after the left DLPFC single-pulse TMS. The authors found that, in HC, DLPFC stimulation deactivated bilateral amygdala, causing no change in contralateral DLPFC, whereas in patients it failed to deactivate bilateral amygdala and aberrantly activated contralateral DLPFC. Finally, in an RCT on 27 unipolar depressed patients, Iwabuchi et al. (Reference Iwabuchi, Auer, Lankappa and Palaniyappan2019) found no difference, after TMS, in FC in the circuit between right amygdala and DLPFC.
sgACC functional connectivity changes
In the already cited study carried out by Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014), the authors also explored the sgACC connectivity. Specifically, the authors demonstrated, at baseline, an increase in FC between sgACC and multiple DMN areas, including the ventromedial prefrontal cortex, pregenual anterior cingulate cortex (pgACC) and precuneus, in patients compared to HC, which reverted after rTMS. A similar reversal of the FC alterations between sgACC and pgACC was demonstrated by Baeken et al. (Reference Baeken, Marinazzo, Wu, Van Schuerbeek, De Mey, Marchetti, Vanderhasselt, Remue, Luypaert and De Raedt2014), in a study on 20 unipolar depressed patients evaluating rs-fMRI connectivity changes induced by rTMS. Also, Taylor et al. (Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018), in an RCT on 32 unipolar depressed patients exploring rs-fMRI connectivity changes before and after 4 weeks of real v. sham rTMS, investigated FC between sgACC and both DMN and CEN. Specifically for the DMN, they demonstrated, in responders (both to real and sham stimulation), a decrease in FC between sgACC and DMN, consistently with the result reported by Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014). Interestingly, no specific effect of rTMS (real v. sham) on the Montgomery-Åsberg Depression Rating Scale (MADRS) and FC was demonstrated, suggesting that reduction in FC between sgACC and DMN may parallel the reduction in depressive symptoms, with no specific effect of active v. sham rTMS. Moreover, with regards to the CEN, the authors found a decrease in FC between sgACC and CEN. This last result is in contrast with the one reported by Liston et al. (Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014), who found no difference in FC connectivity between sgACC and CEN before and after rTMS.
Finally, Ge et al. (Reference Ge, Downar, Blumberger, Daskalakis and Vila-Rodriguez2020), in an open-label study on 50 unipolar depressed patients exploring rs-fMRI connectivity changes before and after 4–6 weeks of rTMS, demonstrated, at follow-up, a decrease in FC between sgACC and DLPFC, fusiform gyrus and middle occipital cortex, both in responders and in non-responders.
Structural connectivity changes in white matter tracts
Three studies examined the effect of rTMS on fractional anisotropy (FA), a marker of white matter microstructure, measured through DTI (Kozel et al., Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al., Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012; Tateishi et al., Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019). All these studies included unipolar depressed patients. While Tateishi et al. (Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019) did not use a control group, the other two studies employed a sham stimulation group (Kozel et al., Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al., Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012) and a group of HC (Peng et al., Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012). Specifically, Peng et al. (Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012) observed that unipolar depressed patients showed, at baseline, decreased FA in the left middle frontal gyrus compared to HC. After real rTMS, the authors also found increased FA in the left middle frontal gyrus whereas Tateishi et al. (Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019) found higher FA values in the right superior frontal gyrus. Interestingly, Tateishi et al. (Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019) observed increased FA only in rTMS non-responders. In contrast, Peng et al. (Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012) found higher FA changes to be correlated with more pronounced improvement in depressive symptomatology.
Finally, in the sample studied by Kozel et al. (Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011), no significant difference was found in prefrontal FA values between active and sham rTMS treatment. Indeed, the authors found an FA increase in the left prefrontal white matter only at a trend-level significance.
Baseline features associated with treatment response
Stronger baseline FC between sgACC and multiple areas of the DMN and the CEN was found to be positively associated with rTMS response (Liston et al., Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014). In contrast, weaker baseline FC between the left DLPFC and cingulate cortex, medial frontal cortex and bilateral medial temporal limbic areas (Richieri et al., Reference Richieri, Verger, Boyer, Boucekine, David, Lançon, Cermolacce and Guedj2018) as well as between the bilateral DLPFC and left caudate was found to be positively associated with rTMS response (Kang et al., Reference Kang, Lee, Jhung, Kim, An, Yoon, Kim, Namkoong and Lee2016). Another area whose baseline connectivity was reported to be associated with rTMS response was the right anterior insula (rAI). Specifically, stronger baseline FC between rAI and both DLPFC (Iwabuchi et al., Reference Iwabuchi, Auer, Lankappa and Palaniyappan2019) and posterior cingulate cortex (Taylor et al., Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018) was found to be positively associated with rTMS response.
Discussion
In this review, we summarised the results of studies investigating structural and FC changes after excitatory rTMS on the left DLPFC. Interestingly, the results showed that FC changes in key areas involved in the emotion regulation (i.e. DLPFC and sgACC) and major resting-state networks (DMN, CEN, Salience Network (SN)) were found to be associated with rTMS treatment, possibly mediating rTMS therapeutic efficacy.
In particular, in the reviewed studies, a decrease in FC between DLPFC (Liston et al., Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014; Richieri et al., Reference Richieri, Verger, Boyer, Boucekine, David, Lançon, Cermolacce and Guedj2018) or sgACC (Baeken et al., Reference Baeken, Marinazzo, Wu, Van Schuerbeek, De Mey, Marchetti, Vanderhasselt, Remue, Luypaert and De Raedt2014; Liston et al., Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014; Taylor et al., Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018) and DMN areas consistently emerged. Also, stronger baseline rAI connectivity was consistently found to be positively associated with rTMS response, both with DLPFC (Iwabuchi et al., Reference Iwabuchi, Auer, Lankappa and Palaniyappan2019) and with the posterior cingulate cortex (Taylor et al., Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018).
Decreased FC between sgACC and DMN areas
The results on sgACC showed that after rTMS, the increased activity of sgACC (Mayberg et al., Reference Mayberg, Brannan, Tekell, Silva, Mahurin, McGinnis and Jerabek2000, Reference Mayberg, Lozano, Voon, McNeely, Seminowicz, Hamani, Schwalb and Kennedy2005) and the increased connectivity between sgACC and multiple areas, in particular within the DMN (Greicius et al., Reference Greicius, Flores, Menon, Glover, Solvason, Kenna, Reiss and Schatzberg2007), found to be associated with depressive symptomatology, seemed to normalise. Notably, this evidence is in line with previous findings showing a similar decrease in hyperactivation and hyperconnectivity of sgACC after very different therapeutic options, such as deep brain stimulation (Mayberg et al., Reference Mayberg, Lozano, Voon, McNeely, Seminowicz, Hamani, Schwalb and Kennedy2005), electroconvulsive therapy (Argyelan et al., Reference Argyelan, Lencz, Kaliora, Sarpal, Weissman, Kingsley, Malhotra and Petrides2016), vagus nerve stimulation (Nahas et al., Reference Nahas, Teneback, Chae, Mu, Molnar, Kozel, Walker, Anderson, Koola, Kose, Lomarev, Bohning and George2007), rTMS targeting the bilateral excitatory dorsomedial prefrontal cortex (Salomons et al., Reference Salomons, Dunlop, Kennedy, Flint, Geraci, Giacobbe and Downar2014), inhibitory right DLPFC rTMS (Kito et al., Reference Kito, Fujita and Koga2008), antidepressant drugs (Mayberg et al., Reference Mayberg, Brannan, Tekell, Silva, Mahurin, McGinnis and Jerabek2000; Drevets et al., Reference Drevets, Bogers and Raichle2002) and the administration of placebo pills, which can result in a clinical response very similar to the one of antidepressant therapies (Mayberg et al., Reference Mayberg, Silva, Brannan, Tekell, Mahurin, McGinnis and Jerabek2002). Interestingly, the latter therapeutic option could have occurred in the study performed by Taylor et al. (Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018) since the decreased FC between sgACC and the DMN and its association with the amelioration of depressive symptomatology reported by this study in the sham group probably suggests a placebo effect of the sham rTMS.
Decreased FC between DLPFC and DMN areas
The DMN is a network of brain areas active when attention is not focused on the outside world, but is engaged in self-referential processing (Gusnard et al., Reference Gusnard, Akbudak, Shulman and Raichle2001; Raichle et al., Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001). This network, which comprises the medial prefrontal, medial posterior parietal cortex and posterior cingulate cortex, has been repeatedly associated with rumination (Zhou et al., Reference Zhou, Chen, Shen, Li, Chen, Zhu, Castellanos and Yan2020) and (meta)cognitive style in depression (Gusnard et al., Reference Gusnard, Akbudak, Shulman and Raichle2001; Raichle et al., Reference Raichle, MacLeod, Snyder, Powers, Gusnard and Shulman2001). Indeed, hyperconnectivity within the DMN has been consistently reported by FC studies in MDD patients (for a review, see Kaiser et al., Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015). Moreover, when attention is focused on the outside world, the FC between DLPFC and DMN decreases (Piccoli et al., Reference Piccoli, Valente, Linden, Re, Esposito, Sack and Salle2015; Denkova et al., Reference Denkova, Nomi, Uddin and Jha2019; Bauer et al., Reference Bauer, Rozenkrantz, Caballero, Nieto-Castanon, Scherer, West, Mrazek, Phillips, Gabrieli and Whitfield-Gabrieli2020) and, therefore, the decrease in FC between DLPFC and DMN areas observed in the reviewed studies after rTMS treatment in depressed patients could have facilitated the attention towards the outside world, thus avoiding rumination and improving depressive symptomatology.
Baseline connectivity
Concerning baseline features associated with treatment response, an association between stronger baseline rAI connectivity and rTMS response consistently emerged from the reviewed studies.
Anterior insula (AI) is part of the SN, a key circuit directing attention and cognitive control (Menon, Reference Menon2015), and comprising not only the AI but also the dorsal anterior cingulate cortex, amygdala, ventral striatum and substantia nigra/ventral tegmental area. Specifically, AI, and especially the rAI, is crucial to detect and select salient stimuli as well as to interact with other neurocognitive systems, including the DMN and the CEN, by activating or deactivating them according to circumstances (Menon, Reference Menon2015). Notably, this structure has been often found impaired in depressive disorders (Grimm et al., Reference Grimm, Boesiger, Beck, Schuepbach, Bermpohl, Walter, Ernst, Hell, Boeker and Northoff2009; Sheline et al., Reference Sheline, Barch, Price, Rundle, Vaishnavi, Snyder, Mintun, Wang, Coalson and Raichle2009). Therefore, the stronger baseline FC between rAI and selective areas within the CEN (i.e. DLPFC) and within the DMN (i.e. posterior cingulate cortex), which was consistently found to be positively associated with rTMS response (Taylor et al., Reference Taylor, Ho, Abagis, Angstadt, Maixner, Welsh and Hernandez-Garcia2018; Iwabuchi et al., Reference Iwabuchi, Auer, Lankappa and Palaniyappan2019), could point towards the hypothesis that rTMS treatment improves the communication between the rAl and these neurocognitive systems, which in turn may have positive effects on depressive symptomatology.
Structural connectivity changes
The three DTI studies here reviewed (Kozel et al., Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al., Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012; Tateishi et al., Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019) reported increased FA, which suggests an improvement of white matter tracts integrity, in regions within the prefrontal lobe after rTMS treatment. These findings suggest the presence of a normalizing effect of rTMS treatment on prefrontal tracts, which have been often found to be characterised by reduced FA in MDD (Korgaonkar et al., Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams2011; Chen et al., Reference Chen, Hu, Li, Huang, Lui, Kuang, Ai, Bi, Gu and Gong2016), similarly to what has been found for antidepressant treatments (Zeng et al., Reference Zeng, Liu, Liu, Shen, Li and Hu2012; Gryglewski et al., Reference Gryglewski, Seiger, Baldinger-Melich, Unterholzner, Spurny, Vanicek, Hahn, Kasper, Frey and Lanzenberger2020). Therefore, these studies support the hypothesis of a relationship between white matter abnormalities and depressive symptomatology (Walther et al., Reference Walther, Hügli, Höfle, Federspiel, Horn, Bracht, Wiest, Strik and Müller2012; Coloigner et al., Reference Coloigner, Batail, Commowick, Corouge, Robert, Barillot and Drapier2019; Heij et al., Reference Heij, Penninx, van Velzen, van Tol, van der Wee, Veltman and Aghajani2019), although a clear relationship between white matter deficits and MDD is currently lacking, mainly due to the heterogeneities observed between the studies (Coloigner et al., Reference Coloigner, Batail, Commowick, Corouge, Robert, Barillot and Drapier2019). This is true also for the results reported by the three reviewed studies, since increased prefrontal FA was observed both in the left (Kozel et al., Reference Kozel, Johnson, Nahas, Nakonezny, Morgan, Anderson, Kose, Li, Lim, Trivedi and George2011; Peng et al., Reference Peng, Zheng, Li, Liu, Zhang, Shan, Zhang, Yin, Liu, Li, Zhou, Li, Yang and Zhang2012) and in the right (Tateishi et al., Reference Tateishi, Nishihara, Kawaguchi, Matsushima, Murakawa, Haraguchi, Kunitake, Maekawa, Kato, Asami, Mizoguchi and Monji2019) sides. Therefore, these contrasting results warrant the need for future studies to better clarify the relationship between rTMS treatment and structural connectivity changes in MDD.
Limitations and conclusions
The reviewed studies suffer from some limitations. First, the sample size was often modest and some studies (Liston et al., Reference Liston, Chen, Zebley, Drysdale, Gordon, Leuchter, Voss, Casey, Etkin and Dubin2014; Richieri et al., Reference Richieri, Verger, Boyer, Boucekine, David, Lançon, Cermolacce and Guedj2018) also included a mixed sample of unipolar and bipolar depressed patients, possibly decreasing the statistical power of the statistical analyses. Second, the majority of patients were concomitantly treated with medications, so the observed connectivity changes could be linked to concomitant psychotropic drugs, or placebo effects in open-label studies. Third, the stimulation parameters of rTMS were heterogeneous in terms of TMS frequency, number of sessions, timing and concomitant treatments, possibly influencing the connectivity changes observed.
In conclusion, the abovementioned results support the hypothesis that rTMS induces neuronal plasticity and reorganisation of key networks in the pathogenesis of unipolar depression. However, whether these changes underlie the antidepressant effect of rTMS is not defined yet. Further studies including larger and more homogeneous samples are needed to better clarify the effect of rTMS on brain connectivity and the relationship with its therapeutic effect in unipolar depression.
Data
All data described in this review have been included in Table 1.
Table 1. Connectivity changes in major depressive disorder after rTMS: a review of functional and structural connectivity data
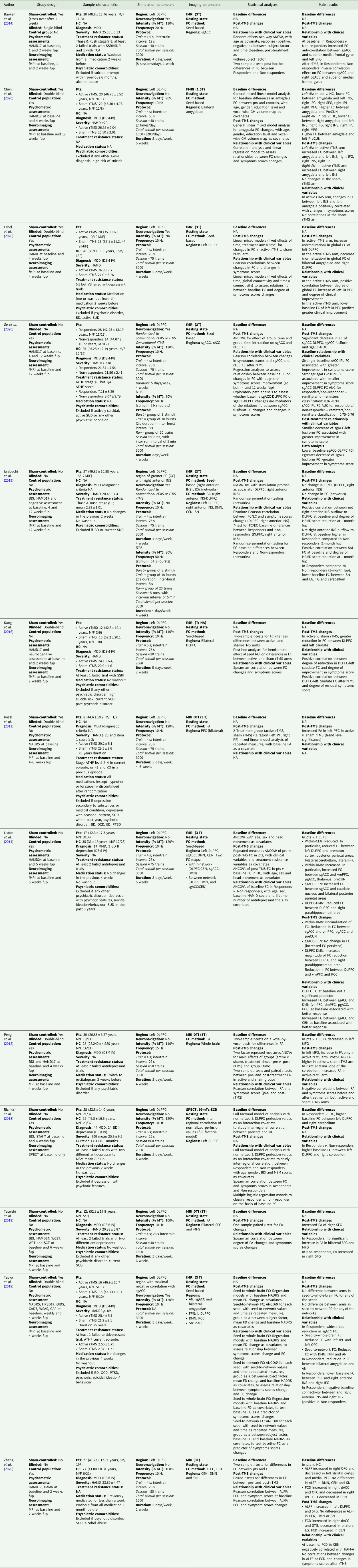
99mTc-ECD, 99mTc-ethyl cysteinate dimer; AI, anterior insula; ALFF, amplitude of low frequency fluctuation; AN, Affective Network; ATHF, antidepressant treatment history form; BD, bipolar disorder; BDI, Beck Depression Inventory; CEN, Central Executive Network; DLPFC, dorsolateral prefrontal cortex; DMN, Default Mode Network; DSM, Diagnostic and Statistical Manual of Mental Disorders; DTI, diffusion tensor imaging; EC, effective connectivity; ED, eating disorder; FC, functional connectivity; FCD, functional connection density; FD, framewise displacement; fMRI, functional magnetic resonance imaging; fup, follow-up; GAD7, General Anxiety Disorder, 7 items version; GAF, Global Assessment of Functioning; GC, Granger causality; GM, grey matter; HAMA, Hamilton Anxiety Rating Scale; HAMD17/24, Hamilton Depression Rating Scale (17/24 items version); HC, healthy controls; ICA, independent component analysis; IFG, inferior frontal gyrus; INS, insula; IPL, inferior parietal lobule; iTBS, intermittent theta burst stimulation; LG, lingual gyrus; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; MFG, medial frontal gyrus; MSM, Maudsley Staging Method; MT, motor threshold; OCD, obsessive compulsive disorder; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; preCUN, precuneus; Pts, patients; PTSD, post-traumatic stress disorder; QIDS, Quick Inventory of Depressive Symptomatology; rACC, rostral anterior cingulate cortex; rTMS, repetitive transcranial magnetic stimulation; SCT, Stroop Color Test; SFG, superior frontal gyrus; sgACC, subgenual anterior cingulate cortex; SN, Salience Network; SPECT, single-photon emission computed tomography; SSRI/SNRI, selective serotonin/norepinephrine reuptake inhibitors; STAI-Y, State-Trait Anxiety Inventory, Y version; STG, superior temporal gyrus; SUD, substance use disorder; TCA, tricyclic antidepressants; vmPFC, ventromedial prefrontal cortex; FA, fractional anisotropy; WCST, Wisconsin Cart Sorting Test; WFT, Word Fluency Test; WSAS, Work and Social Adjustment Scale.
Financial support
This study was supported by a grant from the Italian Ministry of Health (GR-2018-12367789 to EM).
Conflict of interest
None.



