This paper contains supplementary material that can be found online at http://journals.cambridge.org
Introduction
Although the Andaman and Nicobar Islands are politically a part of the Indian subcontinent, they have biogeographical affinities with Sumatra and the Lesser Sundas (Rodgers & Panwar, Reference Rodgers and Panwar1988; Das, Reference Das1999). The mammalian fauna of these islands comprises a rich assemblage of rodents and bats (Miller, Reference Miller1902; Hill, Reference Hill1967; Nath & Chatervedi, Reference Nath and Chatervedi1975; Saha, Reference Saha1980; Das, Reference Das1990; Pande et al., Reference Pande, Kothari and Singh1991); 13% of the 5,357 recorded animal species are endemic (Rao, Reference Rao and Saldanha1984). This excludes the marine mammals, none of which are endemic. Endemism is pronounced in 39% of the 270 species and subspecies of birds (Das, Reference Das1971; Abdulali, 1974), 55% of the 58 mammal species, 32% of the 83 reptile species and 20% of the 10 amphibian species (Rao & DevRoy, Reference Rao and Dev Roy1985; Andrews & Sankaran, Reference Andrews and Sankaran2002). Common terrestrial mammals include the Andaman wild pig Sus scrofa andamanensis, crab eating macaque Macaca fascicularis umbrosa, Andaman masked palm civet Paguma larvata tytlerii, Andaman spiny shrew Crocidura andamanensis, Nicobar tree shrew Tupaia nicobarica nicobarica, Andaman horse-shoe bat Rhinolophus cognatus and lesser short-nosed bat Cynopterus brachyotis. Introduced mammals include the spotted deer Axis axis (now occurring throughout South, Middle and North Andaman Islands), goats Capra sp. (on Barren and Narcondum Islands) and elephant Elephas maximus (on Interview Island).
Bats are sensitive to habitat disturbance and landscape changes from anthropogenic activities (Zubaid, Reference Zubaid1993; Struebig et al., Reference Struebig, Kingston, Zubaid, Mohd-Adnan and Rossiter2008) and could be used as indicator species. Previous records (Supplementary Table S1) of bat species in the Andaman and Nicobar Islands are the result of faunal exploration and collection of species (Hill, Reference Hill1967, Reference Hill1971; Abdulali, Reference Abdulali1976a,Reference Abdulalib; Rao et al., Reference Rao, Rao and Devi1994; Das, Reference Das1998, Reference Das1999; Deb, Reference Deb1998). Miller (Reference Miller1902) listed 12 bat species and other small mammals, and provided the first description of the endemic fruit bat Pteropus faunulus, from Car Nicobar Island. Bates & Harrison (Reference Bates and Harrison1997) compiled a list of 23 species from museum specimens and records (e.g. Miller, Reference Miller1902; Hill, Reference Hill1967).
Here we report the first extensive survey of the bats of the Andaman and Nicobar Islands and the threats to these species from hunting and disturbance in their day roosts. We focused on identifying the distribution of species across the islands, validating past records and mapping roosts. We also attempted to initiate community participation in conservation of bats by proposing a voluntary hunting ban and adoption of roosts close to settlements on inhabited islands. This is especially applicable in the Nicobar Islands where the wildlife laws favour the indigenous communities, who have been granted special hunting rights under Section 56 of the Indian Wildlife (Protection) Act, 1972 (Anon, 1994). In a pilot radio-tracking study we successfully tracked 11 P. faunulus, locating a day roost and the foraging range.
Study area
The Andaman and Nicobar Islands (Figs 1 & 2) lie in the Bay of Bengal between India and Myanmar, parallel to the coast of Myanmar. The Andaman Islands are separated from the Nicobar Islands by the Ten Degree Channel. Both the island groups have a tropical climate throughout the year, with temperatures of 18–34 °C, a mean annual rainfall of 3,000–3,500 mm, and relative humidity of 75–95%. The forest can be broadly classified as tropical evergreen. Grasslands are unique to the Central Nicobar Group (Pande et al., Reference Pande, Kothari and Singh1991). The topography of the islands is hilly and undulating; maximum altitudes are 732 m at Saddle Peak on North Andaman Island and 568 m at Mt Thullier on Great Nicobar Island (Southern Nicobar Group; Oldham, Reference Oldham1885). Among the total of 306 islands in the archipelagos 33 are inhabited and 94 are designated as Sanctuaries, including two islands in the Andaman Islands that are tribal reserves. The entire Nicobar Islands are under the provisions of the Andaman and Nicobar Protection of Aboriginal Tribes Regulation of 1956 and entry to them is restricted to Indian nationals.

Fig. 1 The Andaman Islands, showing the locations of the survey sites (numbered, for names see Supplementary Table S2). The rectangle on the inset indicates the location of the islands in the Indian Ocean.

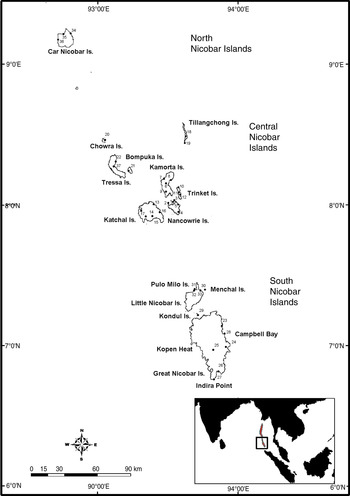
Fig. 2 The Nicobar Islands, showing the locations of the survey sites (numbered, for names see Supplementary Table S2). The rectangle on the inset indicates the location of the islands in the Indian Ocean.
Methods
Surveys of the large islands and representative samples of small islands were carried out on 26 islands (39 sites) in the Andaman Islands and 14 islands (20 sites) in the Nicobar Islands (Figs 1 & 2) during 2003–2006. We made an additional survey after the occurrence of the tsunami that resulted from the Sumatra–Andaman earthquake of 26 December 2004, to record any damage to cave roosts and roosts of Pteropus melanotus, particularly in the mangrove forest belts. Secondary data on hunting, and locations and shift of roosts were regularly obtained from locally trained individuals during 2008–2011.
Standard ground-level mist nets were deployed between 17.00 and 02.00, monitored every 30 minutes, for 121 and 160 nights in the Andaman and Nicobar Islands, respectively. Four nets were deployed at a distance of 500 m from each other at each site, resulting in a total of 1,124 sample nights. Sub-canopy and canopy nets were used for species flying above the lower strata. Nets were deployed in plantations with and without water bodies. The plantations, planted mainly by the horticulture departments of the Andaman and Nicobar Islands, were mostly of banana, mango and guava. These plantations are not for commercial purposes and are mostly to supplement the diet of people in the settlements. Nets were also deployed in primary forests and secondary forest with and without streams. Unique habitats such as freshwater swamps and fringing forest habitats with grasslands, mangroves and littoral forests were also sampled. These unique habitats are restricted in extent. For example, Little Andaman Island is the only island with freshwater swamps, and grasslands are restricted to the Central Nicobar Group. All bats captured were kept in cloth bags and released at point of capture after data collection. Data collected included length of fore arm, sex, weight and pelage colour. In accordance with internationally accepted procedures (Kunz, Reference Kunz2009), nets over streams were set at a height to ensure the last shelf was well above the water, to avoid drowning of captured individuals.
Net nights were calculated by multiplying the number of nets deployed by the number of nights of sampling at each site. Capture success for each species, by island, was calculated as the total number of individuals of a species captured on an island divided by the total number of individuals of all species combined captured on the island, multiplied by 100. Species richness of a site is the total number of bat species recorded.
Day roosts were identified from earlier records (Sankaran, Reference Sankaran1998) through informal discussions with inhabitants, and searches of potential sites. At each roost the species present and the roost type were recorded. Population sizes were estimated by counting the individuals of each species.
Caves surveyed were within the forest and at the seashore. Caves within forest were cracks in the rocks, in undulating terrain, below the ground and on inland hills, where they occurred as clefts or cracks under a rocky hill, forming large cave complexes. There are such complexes at Cliff Bay Sanctuary (41 caves) in the North Andaman Group, and Interview Island (34 caves) and Baratang Island (190 inland forest caves, including some small caves) in the Middle Andaman Group. We did not locate any cave complexes in the Nicobar Islands. Where entry by adults to caves was not possible mist nets on entrances were used to ascertain the presence or absence of bats. Upon identification of roosts we attempted to explore all accessible areas, record the species present in each cave and count the approximate numbers in each roost.
Two hundred and seventy interviews were conducted in 150 villages across the islands, during which we collected data on hunting prevalence and intensity and on any preferences for bat meat in the diet. We interviewed both settlers (people not originally from the archipelagos: Tamil, Telugu, Bengali and Karen) and tribal communities (Great Andamanese, Onge and Nicobarese). We did not survey in the tribal areas inhabited by the Jarawa and Sentanalese, in the Andaman Islands, and by the Shompen, on Great Nicobar Island, as entry to these areas is prohibited. In the Nicobar Islands the Nicobarese were interviewed, to gather additional information on any hunting preference for the two sympatric flying fox species, P. melanotus and P. faunulus, and on the presence of P. faunulus in the Central Nicobar Group. We captured these two Pteropus species in plantations close to settlements in the Nicobar Islands but these catches were not included in the mist net captures as they were a deliberate attempt to capture these species and would have biased the reporting of overall percentage capture success.
We conducted pilot radio-tracking during January–March 2006 to locate the day roosts of P. faunulus. Eleven individuals (nine males and two females) were tracked from 18.00 to 03.00, for 45 days, to estimate their foraging range. The roosts of the tagged individuals were identified by triangulation (Kunz, Reference Kunz2009). Type PD-2C collars (Holohil Systems Ltd, Ontario, Canada), weighing 3.25 g and with a battery life of 12 weeks, were used. A TRX 2000S receiver (Wildlife Materials Ltd, Carbondale, USA) with a 3-element Yagi antenna (Wildlife Materials, Carbondale, USA) was used for tracking.
The status of each species recorded was determined using the survey data and their IUCN Red List status (Leary et al., Reference Leary, Seri, Flannery, Wright, Hamilton and Helgen2008; Supplementary Table S1). A total of 52 voucher specimens were retained from the surveys (two females and two males of each species) and were deposited in the Zoological Survey of India, Kolkata.
Results
We identified a total of 25 species of bats, representing 13 genera. In the Nicobar Archipelago we captured bats of eight genera and in the Andaman Archipelago we captured bats of 12 genera (including the eight genera captured in the Nicobar Archipelago; Supplementary Table S1). A list of the species recorded on each island is provided in Supplementary Table S2. Various notes describing the species recorded, including measurements and pelage colour, are provided in Supplementary Material 1.
Mist netting
We captured a total of 612 individuals of 17 species in the Andaman Islands and 772 individuals of 14 species in the Nicobar Islands, in a total of 1,124 net-nights. Insectivorous and frugivorous species comprised 70.8 and 29.2 % of the catches, respectively. Of the various habitats sampled the percentage capture success was highest in gallery forest, followed by secondary forest in which there were freshwater streams.
Species richness was low in habitats such as plantations/monocultures, mangroves and forest fringes in the Andaman Islands and in forest grassland fringes in the Nicobar Islands. Species captured in these habitats were Cynopterus sp., Cynopterus sphinx, Cynopterus brachyotis and Eonycteris spelaea in the Andaman Islands, and C. sphinx and C. brachyotis in the Nicobar Islands. Habitats such as freshwater swamps are restricted to Little Andaman Island. In these habitats the total number of individuals captured in mist nets was low but, compared to other habitats, the number of species was high. In the Andaman Islands species capture success was higher in the North Andaman Group (41.6%, 13 species) and Little Andaman (24.7%, 7 species) followed by the South (18.7%, 8 species) and Middle Andaman Group (14.9%, 9 species). In the Nicobar Islands species capture success was higher in the Central and Northern Nicobar Groups (83.9%, 9 species) than in the Southern Nicobar Group (16.4%, 8 species). However, species richness in the North Nicobar Group was lower, with only five species, than the Central and South Nicobar Group, where we recorded 12 and eight species, respectively. Among the pteropodids the greatest capture success was of Cynopterus spp., in both the Archipelagos. The distribution of Cynopterus sp. and Eonycteris spelaea is restricted to the Andaman Islands. E. spelaea was captured only in plantations, gallery forest and over freshwater streams, whereas Cynopterus sp. was recorded from gallery forests, plantations and over freshwater streams. P. melanotus was identified in day roosts and from specimens hunted in village settlements on both the Andaman and Nicobar Islands. P. faunulus was restricted to the Central Nicobar Group.
Species captured occasionally in nets were Tylonycteris pachypus at Webi (Middle Andaman Island), Hesperotenus tickelii (Webi and Interview Island; Middle Andaman Group), Hipposideros pomona in Menchal Island (South Nicobar Group) and H. tickelii in the Andaman Islands. These species were captured in gallery forest in nets set over freshwater streams. Murina cyclotis was captured on four occasions over freshwater streams and in gallery forests in Interview Island and at Chippo Village in the North Andaman Group, and in the Nicobar Islands it was captured in similar habitats on 11 of the 14 islands surveyed. Details of the capture success of each species in the various habitats are given in Supplementary Table S3.
Roosts
The survey of roosts included caves, bridges, crevices, road culverts, abandoned buildings, foliage and tree hollows (Supplementary Table S4). We surveyed a total of 116 and 54 caves in the Andaman and Nicobar Islands, respectively (Supplementary Table S5), including caves newly discovered by us (two on Point Island in the North Andaman Group, four on Little Andaman Island and six on Tressa Island in the Central Nicobar Group; Aul, Reference Aul and Vijayakumar2003, Reference Aul and Marimuthu2006, Reference Aul2007). We found only one species in 87 caves, 2–3 species in 28 caves and in 55 caves we found no bats. In the Andaman Islands bats that occupied caves were C. brachyotis, E. spelaea, Taphozous melanopogon, Megaderma spasma, Rhinolophus yunanensis, R. cognatus, Rhinolophus famulus. Hipposideros fulvus, Hipposideros larvatus, Myotis horsfieldii dryas and Pipistrellus javanicus. In the Nicobar Group the species were T. melanopogon, Hipposideros nicobarulae, H. pomona, Hipposideros diadema nicobarensis, Miniopterus pusillus, Pipistrellus javanicus and Pipistrellus coromandra camortae. We sighted an albino H. diadema nicobarensis in the cave on Katchal Island in February 2005 (Aul & Marimuthu, Reference Aul and Marimuthu2006). Pit vipers Trimeresures spp. and a monitor lizard Varanus salvator were recorded feeding on swiftlets and bats in one cave on Cliff Bay and three on Interview Island (North Andaman Group) and two seashore caves on Little Nicobar Island (South Nicobar Group) and two on Katchal Island (Central Nicobar Group; Aul & Vijayakumar, Reference Aul and Vijayakumar2003; Aul, Reference Aul2007).
Pteropus spp. and Cynopterus spp. were found roosting in trees. P. melanotus, in colonies of 200 to > 1,500 individuals were found on Interview, North Reef, Point, Paget and the Landfall Islands of the North Andaman Group, Havelock and Outram Islands in the South Andaman Group and in Dugong creek on Little Andaman Island. These roosts were located in mangrove and inland forest canopy trees. Pteropus hypomelanus was recorded only from Barren Island, in the Middle Andaman Group, roosting in trees < 4 m in height on slopes. In the Nicobar Islands we recorded two colonies of P. melanotus, one with 50 to < 600 individuals on Tillangchong Island and one with 50 to < 200 individuals in a mangrove forest on Great Nicobar Island.
P. faunulus is endemic to the Central Nicobar Group and was rediscovered, after 100 years, during the present survey (Aul, Reference Aul2006). It was not recorded from its type locality (Car Nicobar Island; Miller, Reference Miller1902) and is probably locally extinct from this island. Nine males and two females of P. faunulus were tracked to locate their day roosts on Kamorta Island. Roost sites were distinct from foraging areas, with the males appearing to roost closer to foraging areas (2.11–12.35 km) than females (7.49–10.82 km). One radio-collared individual was tracked to a hunter's home, from whom the tag was recovered.
C. brachyotis was found in groups of < 5 individuals, occupying the fronds of Cocos nucifera. We observed single individuals of C. brachyotis in trees close to the entrance of the caves on Interview Island in the North Andaman Group. Megaderma spasma and R. cognatus used tree hollows as roosts on the Andaman Islands. Such roosts were not abundant on the Nicobar Islands and were recorded only on Tillangchong and Tressa Islands in the Central Nicobar Group, and were utilized by P. coromandra and H. fulvus (Supplementary Table S4).
Threats
In 270 interviews, discussions and observations we noted that hunting for sport was the prime threat to flying foxes, followed by habitat clearance for settlements. It was apparent that there is no dependence on bat meat as a protein supplement. Hunting is restricted to foraging bats and to roosts close to human settlements (of settlers in the Andaman Islands and of the Nicobarese in the Nicobar Islands). The size of colonies roosting away from settlements (e.g. Middle and Little Andaman Islands and Central Nicobar Group) was usually larger (100 to > 2,000 individuals) than that of colonies roosting closer to settlements (< 200 individuals). At Dugong creek the Onge people live in the vicinity of one of the largest P. melanotus roosts but no hunting of bats was recorded at this site.
Our further observations confirmed the prevalence of hunting in villages where settlers reside in the Andaman Islands. Some communities, particularly of the Karen from the North Andaman Group, travel as far as Barren Island to hunt bats and feral goats. The Nicobarese, excluding the Shompen tribes, mostly hunt flying foxes whilst the bats are foraging (18.00–20.00) during the flowering of Ceiba sp. during January–March. Although air guns are commonly used, catapults are occasionally used in the Nicobar Islands.
Secondary sources confirmed the decline of populations of Pteropus spp.. It appears there is no preference for hunting particular Pteropus species. There is a prevalent belief in the medicinal properties of bat meat but no confirmation of its healing properties was validated by people who had consumed bat meat. Reports in 2010 indicated that hunting during the flowering period of Ceiba sp. had been voluntarily banned in the settlements on Kamorta and Trinket Islands, where hunting was formerly common.
Indirect threats are primarily to the cave dwelling species, resulting from disturbance during collection of nests of the edible nest swiftlet Collicalia fuciphaga, mainly in the Andaman Islands. Baratang Island is an important site as it has nine species of bats and one of the largest cave complexes (with 190 caves) in the Andaman Islands. Excessive cave tourism on Baratang Island (Middle Andaman Group), particularly during October–March, caused bats to abandon their roosts. We cannot ascertain the time of abandonment but during the previous survey, in 2003, these caves contained bats; in 2006 they were vacant. Bright lighting and the influx of tourists might have driven the bats away from some of these caves. Disturbance in caves is minimal in the Nicobar Islands and is restricted to poachers from Myanmar and Thailand during May–June.
Impact of the 2004 tsunami
The 2004 Indian Ocean tsunami partially affected the mangroves of the Andaman Islands and completely destroyed the mangroves of the Nicobar Islands. In the Andaman Islands no shifting of roosts of P. melanotus were recorded whereas in the Nicobar Islands the colonies of P. melanotus in Great Nicobar and Tillangchong Islands moved to inland forest areas. During a resurvey in 2006 in Tillangchong Island we confirmed that recovery of Nypa fruticans had facilitated the return of the colonies of P. melanotus.
According to Manchi & Sankaran (Reference Manchi and Sankaran2009) caves were minimally affected and a limited number of larger caves had observable damage at Challis Ek (North Andaman Group). During our resurvey in 2007 we found that the roosts in these caves had not been abandoned. However, coastal caves in the Nicobar Islands were affected, with bats vacating caves whose entrances were partially or completely submerged.
Discussion
Species inventories are important for identification of priority areas for conservation but the efficacy of such inventories is dependent on the quality of inventory data available (Struebig et al., Reference Struebig, Christy, Pio and Meijaard2010). Although bats comprise 20% of all mammalian species the understanding of factors influencing their community structure in fragmented habitats is limited (Racey & Entwistle, Reference Racey, Entwistle, Kunz and Fenton2003; Frick et al., Reference Frick, Hayes and Heady2008). Lack of adequate information about bat diversity and distribution hamper effective conservation (Costa et al., Reference Costa, Leite, Mendes and Ditchfield2005; Palmeirim et al., Reference Palmeirim, Champion, Naikatini, Niukula, Tuiwawa and Fisher2007 ). Earlier reports on the bat fauna of the Andaman and Nicobar Islands (Miller, Reference Miller1902; Bates & Harrison, Reference Bates and Harrison1997) included Taphozous saccolaimus and Scotophilus kuhlii (Hill, Reference Hill1967) but these two species were not found during our survey. Das (Reference Das1998) reported the presence of Hipposideros cineraceus on Mt Harriet but visual identification of the image in the article suggests it is M. cyclotis. As we did not record them, T. saccolaimus, S. kuhlii and H. cineraceus are excluded in our list of species for the islands.
We identified a total of 25 bat species, including two new records and two new subspecies (Hipposideros ater nicobarulae has renamed H. nicobarulae; Bounsavane et al., 2011; Murina cyclotis has been reassessed as a new sub species in the Nicobar Islands; Soisook et al., 2013). Mist netting in a range of habitats provided preliminary information on bat assemblages across the islands and their various habitats. Information on Pteropus spp. in the archipelagos is sporadic, with no mention of roosts or diet, and thus their role as pollinators can only be presumed from studies on other islands, where Pteropus spp. are important pollinators and seed dispersers. Pteropus vampyrus has been recorded as a seasonal migrant to the Nicobar Islands (Rao et al., Reference Rao, Rao and Devi1994) but was not recorded during our survey and was not included in our final list of species for the islands. The rediscovery of P. faunulus and its apparent extinction from its type locality emphasizes the importance of having baseline information that includes habitat requirements, diet and threats. Such information will be critical for developing management and developmental plans for the islands. P. hypomenalus has been reported from Narcondum and Barren Islands and although we recorded it from Barren Island it seems morphometrically similar to P. melanotus.
Biodiversity loss on islands is a result of deforestation and anthropogenic activities (Fritts & Roda, Reference Fritts and Roda1998; Cincotta et al., Reference Cincotta, Winsnewski and Engleman2000; Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000) that simultaneously modify the composition or structure of the landscape and vegetation (Coreau & Martin, Reference Coreau and Martin2007). Threats to the bat fauna of the Andaman and Nicobar Islands are mainly from hunting and habitat modification (Deb, Reference Deb1998), the latter principally from the loss of potential roost sites. Wild animals, particularly pteropodids, are part of the diet of local human communities (García & Goodman, Reference García and Goodman2003; Golden, Reference Golden2009) and are threatened throughout their geographical range (Cheke & Dhal, Reference Cheke and Dhal1981; Wiles, Reference Wiles1987; Rainey, Reference Rainey1990; Craig et al., Reference Craig, Trail and Morrell1994; Wiles et al., Reference Wiles, Engbring and Otobed1997; Brooke & Tschapka, Reference Brooke and Tschapka2001; Mohd-Azlan et al., Reference Mohd-Azlan, Zubaid and Kunz2001; Mickleburgh et al., Reference Mickleburgh, Waylen and Racey2009). Although hunting of insectivorous bats has been observed in Madagascar (Goodman, Reference Goodman2006; Cardiff et al., Reference Cardiff, Ratrimomanarivo, Rembert and Goodman2009) there are no such reports from the Andaman and Nicobar Islands. In the Andaman and Nicobar Islands insectivorous bats are being disturbed by collection of nests of the edible nest swiftlet, and by cave tourism on Baratang Island. Hunting is prevalent on the islands despite inclusion of some of the hunted species in the protected list of the Indian Wildlife (Protection) Act, 1972, and the Amended Acts, 2002. In addition to the hunting of bats we witnessed the hunting of a crocodile Crocodylus sp., the green turtle Chelonia mydas and pigeons Ducula spp. in the Central and Southern Nicobar Groups.
Section 56 of the Indian Wildlife (Protection) Act, 1972 provides special hunting rights for the indigenous communities of the Nicobar Islands. Such rights need to be reassessed, however, and hunting for subsistence or protein supplement should be clearly defined following an identification of the needs of the local communities. Pteropus species are the largest native mammals on the archipelagos and could be critical in the dispersal and pollination of some key plant species. Protection of their foraging sites in and around settlements is therefore required.
Fruit bat roosts are larger when they are further from human settlements (Krueger & O'Daniel, Reference Krueger and O'Daniel1999). We observed > 2,000 Pteropus in roosts situated away from settlements but < 500 in roosts closer to settlements. Our observation of the destruction of major Pteropus roosts in mangroves by the 2004 tsunami indicates that natural calamities can also have serious effects on bat populations.
Establishment of protected areas may be particularly effective for the conservation of species with wide geographical distributions on larger land masses (Rodrigues et al., Reference Rodrigues, Resit, Andelman, Bakarr, Boitani and Brooks2004) but scattered protected areas, even in smaller regions, may not include all the species that require conservation. In the Nicobar Archipelago only four islands (Battimalv, Tillangchong, Megapode and Great Nicobar) are protected. Little Nicobar and Bompuka, which have many endemic plant and animal species, are not included in the protected area network (Abdulali, Reference Abdulali1976a,Reference Abdulalib; Sankaran, Reference Sankaran1997, Reference Sankaran2001). Any protected area in the Nicobar Group would need to be established with the support of the tribal communities, to ensure effectiveness. It would also be of value to establish a flagship bat species (e.g. P. faunulus) that could reinforce an inherent cultural association or belief and illustrate the economic and ecological values of conservation (Mickleburgh et al., Reference Mickleburgh, Hutson and Racey1992; Bowen-Jones & Entwistle, Reference Bowen-Jones and Entwistle2002).
We recommend the establishment of a conservation programme involving local stakeholders, and identification of a flagship species (Aul, Reference Aul2006). The need to conserve endemic species such as P. faunulus arises from the fact that an extremely narrow geographical range increases the likelihood of extinction (Brooks et al., Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands and Konstant2002). Our research on the endemic bats of the Andaman and Nicobar Islands has resulted in the formation of a team of trained local people and a voluntary ban on hunting of bats near settlements on Kamorta Island. Roosts closer to settlements are being monitored and protected from land clearance, and information is regularly collected by the local team on any change in bat populations across the islands.
Acknowledgements
We thank the Conservation Leadership Programme, Lubee Bat Conservancy, Rufford Foundation and the Andaman and Nicobar Environmental Team for project logistics and support to BA, the Andaman and Nicobar Administration, Chief Wildlife Warden (Andaman and Nicobar) for providing the necessary permit, and Professor Paul Racey and Dr John Raisweiler IV for their guidance and advice. Additional support was provided by the UGC (SAP)-CAS Programme.
Biographical sketches
Bandana Aul is researching the ecology of endemic species on the Andaman and Nicobar Islands and is currently working with The Bombay Natural History Society, Mumbai. Paul Bates has spent the last 30 years researching the bats and small mammals of South and South-east Asia, and has helped develop a network of South-east Asian taxonomists working on mammals, birds and amphibians. David Harrison has been involved in research projects in South and South-east Asia. Currently, he is researching the fossil mammal fauna of Tertiary and Quaternary Britain. G. Marimuthu specializes in the biology and behaviour of bats. He is founder Chairman of the Chiroptera Conservation Information Network of South Asia and member of the Scientific Advisory Board of Bat Conservation International.