Introduction
Excavated by the Harappa Archaeological Project (HARP) between 1986 and 2000, the site of Harappa, in modern Pakistan (c. 3700–1900 BC), is a remarkable example of early urbanism (Figures 1 & 2). The populations of urban sites such as Harappa required substantial food supplies. Ethnoarchaeological crop-processing models designed to assess scales of labour needed at harvest time have been developed for a variety of early urban contexts worldwide. Here, we report on the application of these models to the archaeobotanical assemblages from Harappa. Our results suggest that the daily practice of burning dung for fuel, contributed to the crop-processing patterns that characterise some Indus Valley sites, leading to taphonomic equifinality in archaeobotanical assemblages. Recent analysis of macrobotanical remains and phytoliths from small Indus village sites argues that the daily on-site processing of barley and wheat was carried out by individual families rather than under the centralised control of village sites by Indus urban centres (Bates et al. Reference Bates, Singh and Petrie2017). We compare these macrobotanical results to 1144 archaeobotanical samples from the Indus city of Harappa. Although there are striking similarities in patterning between the assemblages, we offer an alternative interpretation.

Figure 1. Map showing the major sites and interaction networks of the Indus Tradition (reproduced with permission from Kenoyer & Meadow Reference Kenoyer, Meadow, Schug and Walimbe2016).

Figure 2. Site plan of the excavations of Harappa (figure by J. Mark Kenoyer).
Crop-processing models
Archaeobotanical crop-processing models have proven invaluable in understanding past agricultural labour practices (Hillman Reference Hillman1973, Reference Hillman, van Zeist and Casparie1984; van der Veen Reference van der Veen, Zeist and Casparie1984; Jones Reference Jones1987; Stevens Reference Stevens2003; Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Bates et al. Reference Bates, Singh and Petrie2017). Although early models distinguished assemblages between ‘consumer’ and ‘producer’ sites (Hillman Reference Hillman, van Zeist and Casparie1984), later iterations focused on the timing of crop storage to distinguish routine and seasonal harvests, and thereby the scales of available labour (Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014).
To understand the point at which charred crop remains entered the archaeological record, these models rely on relative proportions of grain, chaff and weed seeds to infer the scale, organisation and methods used in ancient agricultural systems (Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Stevens Reference Stevens, Marston, Guedes and Warinner2014). Harvesting practices remove weeds, chaff and other plant parts from a crop leaving clean grain and declining numbers of weed seeds as the crop moves through the processing sequence (Reddy Reference Reddy1997; Harvey & Fuller Reference Harvey and Fuller2005; Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Bates et al. Reference Bates, Singh and Petrie2017).
The numbers of glumes to grain decreases as the crop is cleaned through threshing and subsequent winnowing (Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014). Smaller seeds, such as those from weedy taxa, are subsequently removed by fine sieving. Large weed seeds, whose size more closely mimics that of grain, are removed only through a final hand-picking stage. The availability of a large and co-ordinated labour supply for crop processing immediately after harvest should result in low numbers of small weeds and chaff alongside higher proportions of large weed seeds (Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Stevens Reference Stevens, Marston, Guedes and Warinner2014). Alternatively, if crops were processed daily by individual families or small groups, we would expect to see large numbers of small weed seeds, high quantities of chaff and a high proportion of weed seeds to grain.
The specific composition of grains, weed seeds and chaff in an assemblage therefore forms the quantitative basis of these models and their interpretative significance. There are, however, long-running debates about the interpretation of archaeobotanical assemblages, focused on questions of taphonomy and when, exactly, seeds were charred and entered the archaeological record (Miller & Smart Reference Miller and Smart1984; Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014). The taphonomic expectations of crop processing models, combined with potential confounding effects due to the fragility of macrobotanical remains, may lead to site-specific, cultural and taphonomic problems of interpretation.
Crop processing at Harappa
Harappa, a large urban settlement in the upper Indus River Valley, has been continuously occupied since at least the fourth millennium BC, and the site is recognised as a critical example of early urbanisation (Kenoyer Reference Kenoyer and Meadow1991, Reference Kenoyer and Long2015; Dales & Kenoyer Reference Dales, Kenoyer and Possehl1993; Figure 2). The notable absence of palaces and temples has led scholars to interrogate the potential lack of economic inequality at the site. Bioarchaeological investigations of human health (Robbins Schug et al. Reference Robbins Schug, Gray, Mushrif-Tripathy and Sankhyan2012; Valentine et al. Reference Valentine, Kamenov, Kenoyer, Shinde, Mushrif-Tripathy, Otarola-Castillo and Krigbaum2015) and research on craft production (Kenoyer Reference Kenoyer, Richards and Van Buren2000) do, however, provide some evidence for social and economic inequality (Kenoyer Reference Kenoyer and Long2015). In this context, crop-processing models provide a valuable means for understanding agricultural organisation. Evidence for the harvesting and processing of crops by large numbers of labourers near cities and villages could support arguments for economic centralisation or the seasonal organisation of Indus communities; evidence for family-scale harvesting, on the other hand, would make the centralisation of agricultural production or large-scale community harvesting unlikely.
Harappa grew from a village into a city of between 60 000 and 80 000 people over the course of roughly 1500 years (Kenoyer Reference Kenoyer1998, Reference Kenoyer, Vol and Witzel2012). In this article, we use the overall chronology for the Indus Tradition established using radiocarbon dates from Harappa and other sites (Meadow & Kenoyer Reference Meadow, Kenoyer, Jarrige and Lefèvre2005; Kenoyer Reference Kenoyer, Nikita and Rehen2024) and following the earlier model proposed by Shaffer (Reference Shaffer and Ehrich1992).The earliest Ravi phase (Period 1, c. 3700–2800 BC) occupation consisted of small huts with reed and clay-plaster walls, as well as some mud-brick structures (Kenoyer Reference Kenoyer1998; Kenoyer & Meadow Reference Kenoyer, Meadow, Taddei and de Marco2000). During this time, it is possible that the inhabitants would have processed crops that were grown in the immediate vicinity of the site. During the incipient urbanisation of the Kot Diji phase (Period 2, c. 2800–2600 BC) and the subsequent urban Harappan phase (Period 3, c. 2600–1900 BC), craft production was concentrated in urban centres and households, and focused on external trade (Kenoyer Reference Kenoyer1998; Wright Reference Wright2010: 189). During this labour transformation, grain may not have been processed in urban areas but rather brought in as clean grain from outlying homesteads where it was threshed and where weeds were removed.
At Harappa, and throughout much of the Indus Civilisation, people depended on two primary seasonal suites of crops. The summer crops (Kharif), grown from July to October, are dependent on rain from summer monsoons. The winter crops (Rabi) rely either on residual rain from the monsoons or on irrigation or floodplain cultivation drawn from the streams and rivers throughout the Indus Valley (Weber Reference Weber, Weber and Belcher2003; Miller Reference Miller, Stanish and Marcus2006; Jones Reference Jones2017; Petrie et al. Reference Petrie2017).
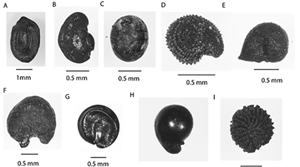
The processing requirements of domesticated crop species vary, requiring the use of different crop-processing models (Reddy Reference Reddy1997). The grain crop species most frequently recovered at Harappa are the Rabi hulled and naked barley (Hordeum sp.) and different wheat varieties (Triticum sp.). Some native millets are present, such as Setaria pumila, Paspalum sp. and Eragrostis sp., though few samples contain significant numbers of these grains (Figure 3, see online supplementary material (OSM) Table S1) (Weber Reference Weber, Weber and Belcher2003). Legumes include lentil (Lens culinaris), pea (Pisum sativum) and small quantities of vetch (Vicia sp.), cowpea (Vigna unguiculata) and chickpea (Cicer sp.) (Figure 3, Table S1). A variety of other oil and fibre (Sesamum sp., Linum sp., Gossypium sp., Brassicaceae) and fruit crops (Phoenix dactylifera, Vitis sp., Ziziphus sp.) have also been found at the site (d'Alpoim Guedes 2024, pers. comm.). Weed taxa include many different types of sedges (Cyperaceae), cf. Medicago/Trifolium and Suaeda sp. (Figure 4, Table S4). Specimens are frequently poorly preserved and unidentifiable to species. In these instances, designations represent the level of taxonomic certainty; for instance, if we were unsure if a partial cotyledon represented pea (Pisum sp.) or lentil (Lens sp.) these are combined into a single Pisum/Lens category. Uncertainty in identification is also indicated using cf. (‘similar to’).

Figure 3. Key Harappa assemblage domesticate taxa: A) Cajanus sp.; B) cf. Avena sp.; C) Lens culinaris; D) Pisum sativum; E) Phoenix dactylifera; F) Setaria pumila; G) Sesamum sp.; H) Linum sp.; I) cf. Macrotyloma sp.; J) cf. Lathyrus sp.; K) Hordeum vulgare (hulled); L) Triticum aestivum; M) Praecitrullus cf. fistulosis; N) Melothria sp. (figure by Jade d'Alpoim Guedes).

Figure 4. Key Harappa assemblage wild taxa: A) Acacia sp.; B) Astragalus sp.; C) Brassica sp.; D & V) Caryophyllaceae; E) cf. Cenchrus sp.; F) Abutilon sp.; G) Chenopodium sp.; H) Suaeda sp.; I) Cleome sp.; J) cf. Bolboschoenus/Schoenoplectus sp.; K) cf. Rumex sp.; L) cf. Carex sp.; M) Cyperaceae type E; N) Cyperaceae type F Bolboschoenus/Schoenoplectus; O) cf. Scirpus sp.; P) cf. Cyperus sp.; Q) Echinochloa sp.; R) Fimbristylis sp.; S) Salsola sp.; T) Trianthema triquetra; U) Trianthema portulacastrum; W) small Fabaceae (cf. Medicago/Meliotus/Trifolium); X) cf. Sophora/Sebania sp. (figure by Jade d'Alpoim Guedes).
As the archaeobotanical assemblage at Harappa is primarily composed of barley and to a lesser extent wheat (Table S2), we employ the models created for these crops. Although the models have been effectively used in a variety of contexts (Song et al. Reference Song, Zhao and Fuller2013; Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Stevens Reference Stevens, Marston, Guedes and Warinner2014), it is important to consider the means by which charred material enters the archaeobotanical record (Miller Reference Miller1984).
Dung burning
Though archaeobotanical assemblages may be created through accidental food spillage and crop waste, or may represent abandoned food stores, the burning of animal dung as fuel is a potential route through which crops may enter the archaeological record. Miller (Reference Miller1984) first noted the use of dung burned as fuel as a possible source of archaeobotanical materials and this recognition has since been integrated into case studies around the world (Miller & Smart Reference Miller and Smart1984; Reddy Reference Reddy1997; Marston & Miller Reference Marston and Miller2014; Spengler Reference Spengler2019). Roughly 15 per cent of barley fed to cattle passes through the digestive tract intact and remains viable for growth (Kaiser Reference Kaiser1999). In modern South Asia, dung remains a significant fuel source and premade dung cakes for burning are available on Amazon.in (Singh Reference Singh2016).
In an ethnographic study of dung burning in Central Anatolia, wild seeds dominated the dung of grazing animals, while animals with a mixed diet of grazing and foddering produced dung with an even ratio of wild seeds to cereal chaff (Anderson & Ertug-Yaras Reference Anderson and Ertug-Yaras1998). Cereal grains often do not survive the burning of a dung cake, though the hard-coated hulled barley proves an exception (Anderson & Ertug-Yaras Reference Anderson and Ertug-Yaras1998). Ethnobotanical analysis found goosefoot (Chenopodium sp.) and other wild species with small seeds (diameter <2mm) are overrepresented in botanical assemblages from Central Asian pastoral dung burning (Spengler Reference Spengler2019). The presence of small weed seeds in assemblages from pastoral communities are often assumed to be the result of grazing (Miller & Smart Reference Miller and Smart1984).
Analysis of starch grains on cattle teeth at the Farmana site in Haryana (c. 2600–2000 BC), India, suggests that animals could have been foddered with left-over cooked human food, possibly including hulled barley (Kashyap & Weber Reference Kashyap and Weber2010). More widely, livestock may be foddered not only with crops and crop-processing debris, but also with weeds (Reddy Reference Reddy1998; Kashyap & Weber Reference Kashyap and Weber2010; Cappers et al. Reference Cappers, Neef, Bekker, Fantone and Okur2016; Spengler Reference Spengler2019). Research at Indus sites in Gujarat (Chakraborty et al. Reference Chakraborty, Chakraborty, Roux, Miller, Shirvalkar and Rawat2018; Chase et al. Reference Chase, Meiggs and Ajithprasad2020), Rajasthan and Haryana, India (Lightfoot et al. Reference Lightfoot2020), indicates that cattle in these areas were predominately foddered with C4 plants such as millets. At Harappa, the predominant crops of wheat and barley are C3 plants, while weeds are a mix of C3 and C4 taxa (Table S4). Isotope analysis (δ13C) of human tooth enamel from Harappa indicates a heavily C3 diet (Kenoyer et al. Reference Kenoyer, Price and Burton2013), though no corresponding analysis of Harappan cattle diets is currently available to assess whether similar foddering practices were practised.
Dung burning at Harappa
Zooarchaeological cattle remains are ubiquitous at Harappa, as are representations and figurines of cattle and pastoral subsistence systems (Meadow Reference Meadow and Harris1996; Meadow & Patel Reference Meadow, Patel, Weber and Belcher2003). Efforts to identify the presence of dung burning through the analysis of phytoliths and chemical traces indicate that this may be an important taphonomic consideration in the interpretation of the Indus archaeological record (Lancelotti Reference Lancelotti2010; Lancelotti & Madella Reference Lancelotti and Madella2012).
Chaff may be included as ‘temper’ in processed dung cakes (Cappers et al. Reference Cappers, Neef, Bekker, Fantone and Okur2016), as it increases their combustibility, but this lighter material is typically burned up in the process (Shahack-Gross Reference Shahack-Gross2011). Ethnographically, wheat chaff is used more often as a temper than barley chaff, which is relatively brittle and preferred as cattle fodder (Cappers et al. Reference Cappers, Neef, Bekker, Fantone and Okur2016: 745). When not burned directly, incidental charring has the potential to contribute large amounts of chaff to archaeobotanical assemblages (van der Veen Reference van der Veen1992).
These relative proportions of grain to chaff are frequently used to determine the degree to which the crops have been processed (Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014; Stevens Reference Stevens, Marston, Guedes and Warinner2014). In contexts where dung burning could influence the results of crop processing models, the reassessment of the variability of grain to chaff ratios, the ratio of large to small weed seeds and the proportions of weeds to grains, is necessary. If dung burning is represented in the archaeobotanical assemblages at Harappa, we expect to find the following signatures: 1) a high overall proportion of weed seeds, with samples composed predominantly of small-seeded weeds (smaller than 2mm); 2) evidence for dung-producing animals in the zooarchaeological record; 3) the presence of dung or fodder in the samples themselves; 4) the existence of hearths or associated features; and 5) proportions of weed seeds to grain in excess of 50 per cent (Miller & Smart Reference Miller and Smart1984; Reddy Reference Reddy1998; Marston Reference Marston2012; Marston & Miller Reference Marston and Miller2014; Spengler Reference Spengler2019).
Archaeobotanical analysis
The botanical remains in this report were collected during fourteen excavation seasons, from 1986–1990 and 1993–2001. In each excavated 5m unit a stratified random sample was collated from a 1 × 2m area, within which all strata were sampled for sediments and artefacts. The amount of sediment sampled varied depending on the stratigraphy and what was available for sampling. Following sectioning and illustration, all large hearths were sampled stratigraphically in their entirety and selected floor areas in and beyond the proximity of each hearth were sampled to understand the distribution of remains on primary occupation floors. Samples of ash and charcoal were collected from inside and outside of all kilns and firings structures. Sediment samples were collected from post-holes, pits, drains and streets, as well as from different stratigraphic levels within these features to document deposition, and from burial fill and from inside and outside of coffins. Samples were also collected from ancient rodent holes that cut through multiple floors in the Period 1 (Ravi) and early Period 2 (Kot Dijian) levels to both identify and remove potential contaminants from the floor and hearth samples and to document the mixing present in the rodent holes.
The soil samples were between 3 and 20 litres in volume. A total of 1144 samples from the archaeobotanical collection from Harappa are analysed here. Samples were sieved through nested screens into 2mm, 1mm, 0.5mm, 0.25mm and smaller than 0.25mm (pan) fractions. Each fraction except for the pan was sorted and analysed. Wood charcoal from the 2mm fraction was pulled and weighed. Seeds were collected from all analysed fractions, and seed fragments sorted and weighed. Seeds were then identified using reference materials (Fuller Reference Fuller2006; Fuller & Harvey Reference Fuller and Harvey2006; Jacomet Reference Jacomet2006; Cappers et al. Reference Cappers, Neef and Bekker2009; Cappers & Bekker Reference Cappers and Bekker2013). Additional details on sample sorting and calculations are in the OSM.
Results
The proportion of weed seeds to grain in the samples is highly variable. Of the 715 samples with both grain and weed seeds, 382 have proportions of small weed seeds higher than 50 per cent and a noticeable lack of large weed seeds, and 201 have proportions of weed seeds to grain higher than 90 per cent. Crop-processing models suggest that these small weeds should have been removed in the earlier stages of processing, prior to transport to Harappa, through winnowing and sieving (Figures 5 & 6). This pattern is consistent across the majority of the 1144 samples. Of the 224 samples with proportions of grain to weeds higher than 50 per cent, only 41 samples have counts of grain higher than 10. Few of the samples contain chaff (Figures 5 & S2): 63 samples contain at least one rachis fragment and one sample accounts for almost half of the total chaff count (sample 1215, n = 108; see Tables S1 & S2).

Figure 5. Triplot of cumulative proportions of chaff, grain and weeds by time period within each sample. Weeds includes all weed seeds in the assemblage. Most samples indicate high proportions of weeds, no chaff and varying proportions of grain. Arrows indicate increasing proportions of grain, chaff and weeds within each individual sample. Number of samples = 1144 (figure by Nathaniel James).

Figure 6. Triplot of cumulative proportions of chaff, grain and weeds within each sample by context. Weeds includes all weed seeds in assemblage. Arrows indicate increasing proportions of grain, chaff, and weeds within each individual sample. Number of samples = 1144 (figure by Nathaniel James).
The lack of chaff in most samples implies sufficient labour to process the crops off-site shortly after harvest (Figures 5 & 6). The high numbers of small weed seeds appear to contradict this interpretation, however, as these proportions are not typical for assemblages of clean grain and do not fit with the predictions of traditional crop-processing models. The pattern of low chaff, variable grain to weed ratios, the predominance of small weed seeds and corresponding lack of large weed seeds is consistent throughout all time periods (Figure 7, Table S1). The urban Harrapan period 3 (n = 680) comes closest to model expectations about clean heavily processed grain, with a cumulative proportion of 78 per cent grain (whole and fragmented) and 22 per cent weed seeds across all samples; yet, as with other time periods, there is relatively little chaff and the weed assemblage is still dominated by small weeds (Figures 7 & 8).

Figure 7. Biplot of large weed:small weed ratios and weed:grain ratios, expressed as percentages. Most samples contain almost no large weeds, with the overall assemblage dominated by small weeds, but there is large variation in the proportions of weed seeds to grain. Number of samples = 1144 (figure by Nathaniel James).

Figure 8. Proportions of grain, chaff and weed seeds in Harappa assemblages through time, with raw counts displayed. Number of samples = 1144 (figure by Nathaniel James).
Discussion
Three broad patterns emerge from the Harappa archaeobotanical assemblage: 1) variable proportions of weed seeds to grain; 2) a lack of large weed seeds that mimic wheat and barley; and 3) relatively high proportions of small weed seeds. This is contrary to the expectations of macrobotanical crop-processing models, which assume urban ‘consumer’ sites such as Harappa had access to a large pool of labour at harvest.
It is important to acknowledge analytical decisions that might impact these results. We note, for example, that we do not include the high numbers of Suaeda sp., in our analysis, whose modern and ancient carbonised seeds look indistinguishable unless broken (Table S4), as these were a mix of modern and ancient seeds. Conversely, our counts of grain include fragmented wheat, barley and Cerealia, significantly inflating our counts of clean grain, particularly in the case of the highly fragmented Cerealia (approximately 17 703 additional estimated grains, Table S1). These heavily charred Cerealia fragments are commonly found together with large numbers of small, charred seeds in household contexts (Table S2). Given the temperatures needed to produce such heavily charred fragments, we expect a proportional impact on small weed seeds. These decisions cumulatively inflated the numbers of grain and weighted the analysis against the existing high numbers of small weeds. Despite this, the presence of so many small weed seeds contradicts the expectations of crop-processing models (Hillman Reference Hillman, van Zeist and Casparie1984; Jones Reference Jones1990; Stevens Reference Stevens2003; Fuller et al. Reference Fuller, Stevens, McClatchie, Madella and Savard2014). Yet, the presence of samples (n = 41) with high numbers of clean grain and an absence of weeds does suggest that highly processed crops were, in some instances, brought into the city (Table S3).
Macrobotanical samples from non-urban Indus village sites also demonstrate an absence of large weeds and chaff alongside large numbers of small weeds (Bates et al. Reference Bates, Singh and Petrie2017). This absence of large weeds is interpreted as the result of handpicking and the discarding of large weeds before waste burning, while the relative absence of chaff is suggested to result from the burning of crop-processing debris. Samples with large numbers of small weeds are therefore the result of burning crop processing waste. Under this interpretation the Indus village assemblages indicate a phase of storage after the winnowing or coarse sieving stage, with the final processing of crops taking place incrementally and the waste subsequently burned. The proposed interpretation is therefore one of ongoing, household-level crop processing, rather than centrally or collectively organised, large-scale processing (Bates et al. Reference Bates, Singh and Petrie2017).
While this interpretation is possible, we argue that, at Harappa, the similar low counts of chaff and resulting ratios of chaff to grain in all time periods imply off-site processing and late-stage storage by a large enough labour force to process the crops immediately after harvest (Figure 5). The presence of high numbers of small weed seeds, which within crop processing model expectations should have been removed, we argue, is the result of dung burning.
The archaeobotanical data from Harappa are consistent with existing expectations from dung burning assemblages, including high weed to grain ratios and high numbers of small weed seeds that cannot otherwise be explained solely by crop processing (Table S5; Miller Reference Miller and Smart1984; Marston Reference Marston2012; Spengler Reference Spengler2019). The use of dung as a critical fuel source at Harappa, for both domestic and industrial purposes (e.g. in pottery or faience kilns) could explain the patterning (Miller & Smart Reference Miller and Smart1984; Kenoyer Reference Kenoyer, Parpola and Koskikallio1994; Reddy Reference Reddy1998; Meadow & Patel Reference Meadow, Patel, Weber and Belcher2003). Simultaneously, the presence of some samples with very few small weed seeds and high numbers of grains (Table S3) means that a single explanation for the Harappa assemblage is unlikely. Rather, a combination of all three activities—dung burning, crop processing and accidental food waste—was at work, in concert with a variety of depositional and taphonomic processes.
Many of the weed taxa in the Harappa assemblage are weeds of cultivation such as Chenopodium, Suaeda sp. and Trianthema sp., as well as various sedges (Cyperaceae) and grasses (Poaceae) that may have been consumed by ruminant livestock (Hillman Reference Hillman, Harris and Thomas1991; Marwat et al. Reference Marwat, Usman, Khan, Khan, Khan, Khan and ur Rehman2013; Spengler Reference Spengler2019) (Figure 4, Tables S1 & S3). Sedges, a major class of weeds in the Harappan assemblage, may have been consumed by cattle grazing along the riverbanks and marshes around the site (Simpson Reference Simpson2010: 200), or as fodder (Shaheen et al. Reference Shaheen, Qureshi, Qaseem and Bruschi2020). Other weed seeds, including low-growth habit legumes such as vetches (Vicia sp.) and medick/alfalfa (cf. Medicago/Meliotus/Trifolium sp.), could also derive from fodder (Miller Reference Miller, Stanish and Marcus2006; Cappers et al. Reference Cappers, Neef, Bekker, Fantone and Okur2016).
Dung burning does not appear to have been necessary due to a lack of wood, as analysis of wood charcoal at Harappa indicates the extensive use of both locally available scrub and riverine woods, as well as the importation of mountain pine (Lancelotti Reference Lancelotti2010). Dung burning was, as it is today, a cultural practice that allowed for a slow steady heat for cooking and made use of a readily available fuel. Small pieces of dung identified during excavation and in palaeoethnobotanical samples further support the practice of dung burning at Harappa (Table S5). Multi-proxy approaches that incorporate phytoliths and faecal spherulites have also demonstrated both the presence of dung (Lancelotti & Madella Reference Lancelotti and Madella2012) as well as chaff (Bates et al. Reference Bates, Singh and Petrie2017) at Indus sites that might not have been preserved in the macrobotanical record, which means that a microbotanical analysis at Harappa is critical to confirming and expanding on our understanding of the patterns we report here.
We argue that the daily burning of dung was a sufficiently pervasive practice at Harappa to impact on the ability of traditional crop-processing models to effectively assess labour organisation or the timing of crop storage. This does not argue against the potential applicability of these models but underscores the need to account for taphonomic processes that potentially shape the formation of archaeobotanical assemblages. Our analysis indicates that crops likely arrived at Harappa in a highly processed state, with subsequent dung burning introducing large numbers of small weed seeds to depositional contexts across the site.
Conclusion
Two alternative interpretative models have been advanced to explain the patterns found in Indus archaeobotanical assemblages, each with its own significant implications for understanding the interactions between rural and urban food supplies. Bates and colleagues (2017) have recently argued that the predominance of small weed seeds at Indus villages indicates crop processing at a household level at regular intervals. In contrast, Weber (Reference Weber, Weber and Belcher2003) asserts that crops were likely processed away from Indus cities like Harappa by a workforce large enough to process and store the clean crop before transportation to the city (Stevens Reference Stevens, Marston, Guedes and Warinner2014). Here, we have analysed 1144 archaeobotanical samples from Harappa; the relative lack of chaff across the assemblage supports the latter interpretation. Moreover, we argue that the Harappan archaeobotanical assemblage was heavily impacted by dung burning, reflected in the high numbers of small weed seeds. Similarities in the compositions of macrobotanical assemblages from Harappa and from non-urban contemporaneous Indus sites (Bates et al. Reference Bates, Singh and Petrie2017) are intriguing as they imply that cultural practices may have been the same at sites that were very different in scale and organisation. The crop processing patterns identified here also exhibit strong continuity over time (Figure 9). Despite the various political and economic transformations of urban Indus settlements (Possehl Reference Possehl1997; Kenoyer Reference Kenoyer, Jarrige and Lefèvre2005), subsistence systems and agricultural practices seem to have continued much as they were at the height of Harappan urbanisation (Weber et al. Reference Weber, Barela and Lehman2010).

Figure 9. Relative proportions of large (>2.00mm) and small (<2.00mm) weed seeds through time. Number of samples = 1144 (figure by Nathaniel James).
Fuller and colleagues’ (2014) extension of earlier crop-processing models argues that the sum of macrobotanical remains from a site reflects the dominant daily activities performed there. They emphasise that crops processed at a site by a large workforce before storage would potentially be charred and preserved, while crops processed after storage on a small scale would likely represent daily household food preparation. Into both of these possibilities we would add the need for consideration of the contribution of dung burning, a key practice at many sites in South Asia and around the world. Archaeological assemblages and models therefore need to be parsed for the contribution of each of these different processes.
Through the findings presented here, the archaeobotanical assemblage from Harappa helps sharpen understanding of the social organisation of this site and the wider Indus Civilisation. Ongoing work at sites historically framed as ‘peripheral’ to core urban centres is crucial for informing questions of social organisation throughout the Indus region (Petrie et al. Reference Petrie2017; Nayak et al. 2021). Our results contribute to a growing awareness of commonalities of practice at sites of dramatically different social and economic scales, and to wider understandings of food supply logistics in early cities and relationships between urban and non-urban populations, both in the Indus Valley and throughout the world.
Acknowledgements
This article is dedicated to the memory of Steven Alec Weber (1954–2020) who led the Harappan Archaeobotanical Project. His influence guided the early stages of this manuscript, and he was invariably generous and kind to everyone he worked with. We also dedicate this article to the late Dr George F. Dales (1927–1992).
Funding statement
This research received no specific grant from any funding agency or from commercial and not-for-profit sectors.
Online supplementary materials (OSM)
To view supplementary material for this article, please visit https://doi.org/10.15184/aqy.2024.196 and select the supplementary materials tab.