Oxidative stress, an unfavourable imbalance between production of reactive oxygen species and antioxidant defence, has been implicated in the aetiology of several chronic diseases including CVD, diabetes and some cancers( Reference Zhang 1 – Reference Barocas, Motley and Cookson 5 ). Although some chronic disease risk factors such as smoking have been consistently associated with higher levels of oxidative stress( Reference Morrow, Frei and Longmire 6 , Reference Aseervatham, Sivasudha and Jeyadevi 7 ), relationships with other lifestyle factors such as diet, alcohol consumption and physical activity have not been well characterised in healthy adults.
Diet may be linked to oxidative stress through the consumption of antioxidants – substances that inhibit the oxidation of body substrates by reactive oxygen species. Nutrients with established antioxidant activity include carotenoids (β-carotene, α-carotene, lycopene, cryptoxanthin, lutein and zeaxanthin), vitamin C (ascorbic acid), vitamin E (α-tocopherol), Se and Zn( Reference Mayne 8 ). While some of these nutrients such as carotenoids act directly by quenching singlet molecular oxygen and free radicals( Reference Di Mascio, Murphy and Sies 9 , Reference Sies, Stahl and Sundquist 10 ), others such as Zn act indirectly as cofactors of antioxidant enzymes( Reference Cruz, de Oliveira and Marreiro Ddo 11 ). Although some studies have observed lower oxidative stress levels with higher dietary intakes of various antioxidant nutrients, such associations have not been demonstrated consistently( Reference Dorjgochoo, Gao and Chow 12 – Reference Thomson, Stendell-Hollis and Rock 17 ). Similarly, consumption of fruits and vegetables, foods rich in antioxidants, has been inversely associated with oxidative stress in some, but not all, observational studies( Reference Dorjgochoo, Gao and Chow 12 , Reference Rossner, Gammon and Terry 18 – Reference Root, McGinn and Nieman 20 ).
Dietary fats may also be related to oxidative stress levels. Though n-3 fatty acids have been associated with lower oxidative stress in some reports( Reference Mas, Woodman and Burke 21 , Reference Kiecolt-Glaser, Epel and Belury 22 ), higher intakes of trans fat and SFA were related to higher oxidative stress in a recent study among midlife women( Reference Tomey, Sowers and Li 13 ). However, human studies investigating the relationships between various dietary fat subgroups and oxidative stress are limited.
Associations between oxidative stress and other behaviours such as physical activity and alcohol consumption are also uncertain. While acute, vigorous exercise appears to increase oxidative stress, chronic, moderate-intensity physical activity may have the opposite effect over the long term( Reference Mastaloudis, Leonard and Traber 23 , Reference Nikolaidis, Kyparos and Vrabas 24 ). Similarly, excess consumption of alcohol is linked to oxidative stress through ethanol metabolism, which involves the production of reactive oxygen species( Reference Das and Vasudevan 25 ). However, the effect of regularly consuming moderate amounts of alcohol remains unclear.
Although numerous biomarkers of oxidative stress exist, F2-isoprostanes (F2-IsoP), generated from free radical-catalysed peroxidation of arachidonic acid, are considered to be among the most accurate( Reference Montuschi, Barnes and Roberts 26 , Reference Milne, Dai and Roberts 27 ). F2-IsoP have been positively associated with chronic disease risk factors such as obesity and smoking( Reference Dorjgochoo, Gao and Chow 28 ). Though prospective studies to date are limited, some evidence has linked elevated F2-IsoP to risk of CVD and certain cancers( Reference Zhang 1 – Reference Dai, Gao and Shu 3 , Reference Barocas, Motley and Cookson 5 ). Urinary F2-IsoP are a particularly stable biomarker of oxidative stress, as they are not subject to autoxidation during sample collection and storage, unlike blood plasma measures( Reference Il’yasova, Scarbrough and Spasojevic 29 ). Furthermore, while local renal production may affect the excretion of unmetabolized F2-IsoP in human urine, the metabolised form of 15-F2t-isoprostane – 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M) – is unaffected by renal production( Reference Morrow, Zackert and Yang 30 ). However, most previous studies on F2-isoprostanes and lifestyle factors have relied exclusively on unmetabolized F2-IsoP. Therefore, the purpose of this cross-sectional study was to evaluate associations of both F2-IsoP and 15-F2t-IsoP-M with physical activity, alcohol consumption, and intakes of dietary fats, fruits and vegetables, and antioxidant nutrients among healthy, premenopausal women.
Methods
Participants included in this analysis were controls of a case–control study on incident breast cancer nested within the Sister Study – a prospective observational cohort of US women designed to identify risk factors for breast cancer. The objective of the nested case–control study was to evaluate novel biomarkers of premenopausal breast cancer risk. Women aged 35–74 years from the USA and Puerto Rico were recruited for the Sister Study from 2003 to 2009 through a national advertising campaign and a network of breast cancer professionals and recruitment volunteers. All of them had a sister who had been diagnosed with breast cancer, but were themselves free of breast cancer at enrolment. All participants provided their informed written consent. The study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, the National Institutes of Health and the Copernicus Group.
Population for analysis
Women were eligible to be included in the control sample if they were aged 35–54 years, premenopausal, had at least one intact ovary and had a urine sample collected at enrolment. Those who reported one or more menstrual cycles in the previous 12 months were categorised as premenopausal, as were women aged 54 years and younger whose only reason for not experiencing menses was hysterectomy (without bilateral oophorectomy). A total of 912 women, who did not have a breast cancer diagnosis as of 31 December 2012, had urine samples analysed for F2-isoprostanes and were eligible for this analysis.
Measurement of F2-isoprostanes and 2,3-dinor-5,6-dihydro-15-F2t-isoprostane
At enrolment, participants provided samples of first morning urine during a home visit by the study personnel. Reliability studies have demonstrated that a single morning sample adequately reflects daily excretion of F2-IsoP, with concentrations similar to those obtained from a 24-h urine sample( Reference Basu and Helmersson 31 ). Urinary F2-IsoP and 15-F2t-IsoP-M were measured using GC/negative ion chemical ionisation MS at the Eicosanoid Core Laboratory at Vanderbilt University Medical Center. The methods used have been published in detail previously( Reference Milne, Sanchez and Musiek 32 – Reference Morales, Terry and Zackert 34 ). The CV for quality control duplicates were 16·0 % for F2-IsoP and 12·5 % for 15-F2t-IsoP-M. Reported F2-IsoP and 15-F2t-IsoP-M values were adjusted for creatinine concentrations (ng/mg of creatinine) to correct for urine diluteness( Reference Il’yasova, Morrow and Ivanova 35 ).
Questionnaire measures
All dietary and nutrient intakes were ascertained using the Block 98 FFQ( Reference Block, Hartman and Dresser 36 ), completed at enrolment, and refer to average daily intakes in the previous 12 months. Total dietary carotenoids were calculated as the sum of β-carotene, α-carotene, lycopene, lutein+zeaxanthin and cryptoxanthin. Supplement use information was also ascertained from the FFQ and was available for vitamin E, vitamin C, β-carotene, vitamin A, Se and Zn. Scores on the Healthy Eating Index: 1999–2000, a measure of diet quality developed by the US Department of Agriculture( Reference Basiotis, Gerrior and Juan 37 ), were also calculated from the FFQ.
Physical activity during the previous 12 months was self-reported via a questionnaire completed at enrolment. Participants were asked to report the number of hours per week they spent engaging in specific activities, and weekly energy expenditures were calculated using the metabolic equivalent (MET) values for each activity as listed in established guidelines( Reference Ainsworth, Haskell and Whitt 38 ). Total physical activity was estimated by summing the MET-h/week of sports or exercise sessions and daily activities. Alcohol consumption was also self-reported on enrolment through questionnaires. Participants reported their average number of drinks per week. This value was multiplied by 14 (the number of grams of alcohol in a standard drink) and used to calculate an average daily intake of alcohol in grams. Current height and weight, used to calculate BMI (kg/m2), were measured during home visits by trained study personnel at enrolment. Information regarding socio-demographic factors and smoking status was collected at enrolment using questionnaires. We excluded women who were missing an FFQ (n 18) or who had implausible values for energy intake (<2092 or >20 920 kJ/d (<500 or >5000 kcal/d; n 6)).
Statistical analyses
Frequencies and percentages were used to describe categorical variables. Medians and quartiles were calculated for continuous variables including dietary intakes, physical activity and alcohol consumption.
Values of F2-IsoP and 15-F2t-IsoP-M were highly skewed, and thus were log-transformed to approximate a normal distribution. Using generalised linear models, geometric means of F2-IsoP and 15-F2t-IsoP-M were calculated by quartiles of all lifestyle variables. Models were adjusted for variables considered a priori as potential confounders. For all exposure variables, geometric means were adjusted for age (continuous), BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, other), physical activity (total MET-h/week, continuous), household income (<$20 000, $20 000–$49 999, $50 000–$99 999, $100 000–$200 000 and >$200 000) and current smoking status (yes/no). Means according to antioxidant nutrients were further adjusted for total energy intake (kJ/d (kcal/d) continuous), and values according to physical activity, alcohol intake, fruit and vegetable consumption, dietary fats intake and Healthy Eating Index scores were further adjusted for use of any multivitamins and/or supplements (yes/no). For physical activity, alcohol intake and dietary fats, geometric means were additionally adjusted for fruit and vegetable servings per day (continuous). Linear regression models, with continuous, log-transformed F2-IsoP or 15-F2t-IsoP-M as the dependent variable, were used to evaluate trends. For antioxidant nutrients, we hypothesised that relationships would be approximately linear within the range of values consumed by women in this population. However, to evaluate potential curvilinear trends, we visually assessed scatterplots of all exposures plotted individually against F2-IsoP and 15-F2t-IsoP-M. In our assessment, no exposure–outcome relationships appeared to be U-shaped. Thus, we proceeded with the evaluation of linear trends using linear regression models. To avoid problems of collinearity in adjusted regression models, related dietary variables were evaluated as covariates individually, rather than in combination.
For analyses of vitamin E, vitamin C, β-carotene, vitamin A, Se and Zn (nutrients for which we had available information on supplement use), we evaluated associations for dietary intakes alone, as well as for combined intakes from both dietary and supplemental sources. In the dietary intake analyses of these nutrients, we performed sensitivity analyses excluding women who reported taking a supplement for that particular nutrient.
In further sensitivity analyses, we excluded women who were current smokers at enrolment. We also performed stratified analyses by BMI (18·5–29·9 v. 30·0+ kg/m2) to investigate potential effect modification. Tests for statistical interaction were conducted by including cross-product interaction terms in regression models.
The number of missing values was <5 % for all variables, and therefore missing values were left as missing in all analyses. Two-sided P values<0·05 were considered to be statistically significant. All statistical analyses were conducted with Sister Study Data Release 4.0 using SAS 9.4 (SAS Institute).
Results
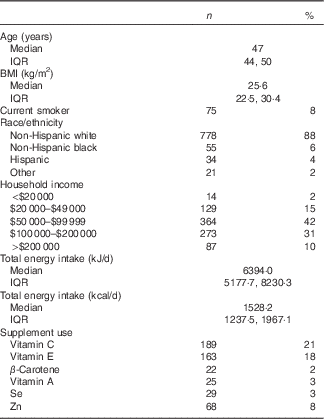
The geometric mean concentrations of F2-IsoP and 15-F2t-IsoP-M were 1·44 (sd 0·75) and 0·71 (sd 0·32) ng/mgCr, respectively. Log-transformed F2-IsoP and 15-F2t-IsoP-M values were highly correlated (r 0·58, P<0·001). Both F2-IsoP and 15-F2t-IsoP-M were positively associated with BMI (F2-IsoP: r 0·25, P<0·001; 15-F2t-IsoP-M: r 0·37, P<0·001). Participants were predominately non-Hispanic white (88 %) with a median age of 47 years and a median BMI of 25·6 kg/m2 (Table 1).
Table 1 Participant characteristics(Numbers and percentages; medians and interquartile ranges (IQR); n 888)
After multivariable adjustment, total MET-h/week of physical activity was inversely associated with F2-IsoP (P trend=0·003) (Table 2). A weaker, non-significant trend was observed for 15-F2t-IsoP-M. Although alcohol intake appeared to be inversely associated with concentrations of F2-IsoP, this association was not statistically significant. Healthy Eating Index scores were not significantly associated with either F2-IsoP or 15-F2t-IsoP-M in adjusted models. No significant relationships were observed between F2-IsoP or 15-F2t-IsoP-M and total fat, total dietary n-6 fatty acids, total dietary n-3 fatty acids, SFA, MUFA, PUFA or total dietary short-chain n-3 fatty acids. Intake of total dietary long-chain n-3 fatty acids was inversely associated with 15-F2t-IsoP-M (P trend=0·03), but was marginally associated with F2-IsoP (P trend=0·06). Higher intake of trans fat was associated with higher F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend=0·002).
Table 2 Quartiles of physical activity, alcohol and dietary fat subgroupsFootnote * (Geometric means and 95 % confidence intervals of F2-isoprostanes (F2-IsoP) and 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M); n 888)

MET, metabolic equivalent.
* Values of F2-IsoP and 15-F2t-IsoP-M are expressed in ng/mg creatinine.
† Adjusted for age, BMI, race/ethnicity, physical activity, household income, current smoking status, fruit and vegetable servings per day, and any multivitamin and/or supplement use.
‡ Adjusted for age, BMI, race/ethnicity, physical activity, household income, current smoking status, and any multivitamin and/or supplement use.
Although fruit consumption was inversely associated with F2-IsoP only (P trend=0·04), vegetable consumption was inversely associated with both F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001) (Table 3). Inverse associations were observed between vitamin E and both F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001). Higher vitamin C intake was associated with lower F2-IsoP (P trend=0·01), with a similar, although non-significant, association with 15-F2t-IsoP-M (P trend=0·1). β-Carotene was inversely associated with F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001). Similar associations were observed for vitamin A with both F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001). Se intake was inversely associated with F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001). Higher Zn intake was associated with lower F2-IsoP (P trend=0·01) and marginally associated with lower 15-F2t-IsoP-M (P trend=0·07). Strong inverse associations were found between lutein+zeaxanthin and both F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001), although lycopene was not associated with either F2-IsoP or 15-F2t-IsoP-M. α-Carotene was inversely associated with F2-IsoP (P trend=0·03) but not significantly associated with 15-F2t-IsoP-M (P trend=0·5). Cryptoxanthin was not associated with F2-IsoP or 15-F2t-IsoP-M. Total carotenoid intake was strongly associated with both F2-IsoP (P trend<0·001) and 15-F2t-IsoP-M (P trend<0·001). For dietary vitamin E, vitamin C, β-carotene, vitamin A, Se and Zn, patterns remained similar when supplement users for these nutrients were excluded (data not shown).
Table 3 Quartiles of fruit and vegetable servings and intake of dietary antioxidant nutrientsFootnote * (Geometric means and 95 % confidence intervals of F2-isoprostanes (F2-IsoP) and 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M); n 888)

RAE, retinol activity equivalents.
* Values of F2-IsoP and 15-F2t-IsoP-M are expressed in ng/mg creatinine.
† Adjusted for age, BMI, race/ethnicity, physical activity, total energy intake, household income and current smoking status; fruit and vegetable servings additionally adjusted for any multivitamin/supplement use.
Associations of F2-IsoP and 15-F2t-IsoP-M with combined dietary and supplemental intakes of vitamin E, vitamin C, β-carotene, vitamin A, Se and Zn were generally similar to those observed for dietary intakes of these nutrients alone (online Supplementary Table S1). However, associations with combined dietary and supplemental sources appeared to be somewhat weaker for vitamin E and stronger for vitamin C and Zn, compared with associations with dietary intakes alone.
All trends remained similar when analyses were restricted to non-smokers (data not shown). In stratified analyses, patterns were largely similar between those with a BMI of 18·5–29·9 kg/m2 and those with a BMI of 30·0 kg/m2 or greater. Although inverse associations between F2-IsoP (P trend=0·002) and 15-F2t-IsoP-M (P trend=0·03) and physical activity were only apparent among women with a BMI of 18·5–29·9 kg/m2, the interaction test was not statistically significant (F2-IsoP: P interaction=0·9; 15-F2t-IsoP-M: P interaction=0·4; online Supplementary Table S2). Likewise, Zn intake was inversely associated with F2-IsoP (P trend=0·008) and 15-F2t-IsoP-M (P trend=0·03) only among women with a BMI of 18·5–29·9 kg/m2, although the interactions were not significant (F2-IsoP: P interaction=0·3; 15-F2t-IsoP-M: P interaction=0·4). Fruit intake was inversely associated with F2IsoP only among women with a BMI of 18·5–29·9 kg/m2 (P trend=0·009). However, the interaction test did not indicate a significant difference according to BMI (P interaction=0·4; online Supplementary Table S3).
Discussion
In this study, we found that oxidative stress, as measured by F2-isoprostane and its metabolite, was associated with a number of dietary and lifestyle factors. Lower oxidative stress was observed with greater intake of fruits and vegetables, antioxidant nutrients and long-chain n-3 fatty acids, whereas higher oxidative stress was found among women with a greater intake of trans fats. In addition, our findings suggest a possible inverse relationship between total physical activity and oxidative stress. Associations were similar for non-obese and obese women and remained largely unchanged when current smokers were excluded.
Individual nutrients most strongly associated with both F2-IsoP and 15-F2t-IsoP-M in the present study included vitamin E and the carotenoids β-carotene and lutein+zeaxanthin – findings consistent with the antioxidant properties of these compounds. Vitamin E, or α-tocopherol, is a lipid-soluble, chain-breaking antioxidant, whereas carotenoids are lipid-soluble compounds that scavenge singlet oxygen( Reference Helmersson, Arnlov and Larsson 16 ). Other studies of F2-IsoP have observed similar strong associations with these antioxidant nutrients. A recent cross-sectional study among healthy, middle-aged men found that β-carotene and lutein+zeaxanthin were the carotenoids with the strongest inverse associations with urinary F2-IsoP( Reference Cocate, Natali and Alfenas 14 ). The results from the Study of Women’s Health Across the Nation (SWAN) showed dietary intakes of vitamin E, lutein and β-carotene, as well as vitamin A and vitamin C, to be negatively correlated with F2-IsoP( Reference Tomey, Sowers and Li 13 ). The relative strengths of associations with the antioxidant nutrients we evaluated may be partly explained by their efficiency in reacting with various free radicals and pro-oxidants and their ability to interact with other antioxidants( Reference Sies, Stahl and Sundquist 10 , Reference Young and Lowe 39 ). Tocopherols (vitamin E), for example, are the most abundant and efficient scavengers of peroxyl radicals in biological membranes, and their antioxidant activity is supported by their interaction with vitamin C( Reference Sies, Stahl and Sundquist 10 ).
Our findings regarding antioxidant nutrients likely explain, in large part, the inverse associations observed for fruits and vegetables, foods rich in carotenoids and other antioxidants. Although adjustment for multivitamin/supplement use, physical activity, BMI and other confounding factors attenuated associations with fruit intake in our sample, this was not the case for vegetable intake. A stronger trend for vegetables than for fruits in relation to urinary F2-IsoP has been observed previously( Reference Tomey, Sowers and Li 13 ), and may be explained by the specific types of fruits and vegetables commonly consumed among women of this age group, or by the greater range of vegetable servings consumed in this population (0–15), relative to fruit servings (0–5).
We also found that dietary Zn intake was inversely associated with F2-IsoP, with a similar but non-significant association with 15-F2t-IsoP-M. A similar association with F2-IsoP was found among participants in SWAN( Reference Tomey, Sowers and Li 13 ). The antioxidant activity of Zn, a ubiquitous trace element in the body, is proposed to occur through several different mechanisms, one of which involves its role as a cofactor for superoxide dismutase, an important component of antioxidant defence( Reference Prasad 40 ). In our sample, associations between F2-IsoP and 15-F2t-IsoP-M and Zn appeared somewhat stronger for combined intake of Zn from both dietary and supplemental sources, compared with associations with dietary Zn intake alone. Yet, some trials have suggested that Zn supplementation has little effect on markers of lipid peroxidation, such as F2-IsoP( Reference Andriollo-Sanchez, Hininger-Favier and Meunier 41 , Reference Seet, Lee and Lim 42 ). Human studies remain scarce, particularly in healthy populations, and further investigation is needed to understand the role of Zn in oxidative stress reduction.
In our sample, a higher intake of dietary long-chain n-3 fatty acids, which has been associated with a lower risk of cardiovascular events( Reference Delgado-Lista, Perez-Martinez and Lopez-Miranda 43 ), was significantly predictive of lower 15-F2t-IsoP-M and marginally predictive of lower F2-IsoP. Supplementation with EPA and DHA, two long-chain n-3 fatty acids found in fatty fish, has led to decreases in F2-IsoP in some trials( Reference Mas, Woodman and Burke 21 , Reference Mori, Woodman and Burke 44 – Reference Skarke, Alamuddin and Lawson 46 ) and has had no effect in others( Reference Kirkhus, Lamglait and Eilertsen 47 , Reference Wu, Lu and Wang 48 ), contrary to previous concerns that higher overall intake of unsaturated fatty acids would increase lipid peroxidation( Reference Mas, Woodman and Burke 21 ). Mas et al.( Reference Mas, Woodman and Burke 21 ) suggest that the reduction in F2-IsoP is related to the anti-inflammatory effects of n-3 fatty acids. Arachidonic acid, from which F2-isoprostanes are derived, is a primary component of inflammatory cell membranes, but may be partially replaced in membranes by EPA, thereby leading to decreased production of arachidonic acid-derived products.
We observed strong positive associations between trans fat intake and both F2-IsoP and 15-F2t-IsoP-M concentrations. Trans fats are PUFA, which occur naturally in ruminant fats, but are also formed during the hydrogenation of vegetable oils in industrial processes( Reference Kuhnt, Wagner and Kraft 49 ). Some trials have found higher urinary F2-IsoP among participants given trans-fatty acid supplementation( Reference Kuhnt, Wagner and Kraft 49 , Reference Smit, Katan and Wanders 50 ), and it has been suggested that an increase in lipid peroxidation may partially account for the relationship between trans fat and CHD risk( Reference Smit, Katan and Wanders 50 ). Among women enrolled in the SWAN study, Tomey et al.( Reference Tomey, Sowers and Li 13 ) reported an increase in urinary F2-IsoP with higher trans fat intake. However, while their results also suggested positive associations with total fat, SFA, linoleic acid (a PUFA) and oleic acid (a MUFA), we observed no consistent relationships between urinary F2-IsoP and the majority of dietary fat subgroups that we evaluated. Although human investigations remain limited, some evidence from intervention studies has also suggested that F2-IsoP excretion may not be strongly affected by the overall fat content of the diet( Reference Marina, von Frankenberg and Suvag 51 – Reference Egert, Kratz and Kannenberg 54 ). Future studies are needed to evaluate associations between specific dietary fat subgroups and oxidative stress as assessed by F2-IsoP.
With adjustments for confounders such as age, BMI and daily intake of fruits and vegetables, total physical activity was inversely associated with F2-IsoP, with a similar but non-significant trend for 15-F2t-IsoP-M. To avoid problems of collinearity, we chose not to control for multiple related dietary factors in the same model. Thus, adjustment only for fruits and vegetables may be insufficient to account for confounding by diet, given strong correlations between most dietary intakes and total physical activity in our sample. Although our findings are consistent with several aerobic exercise trials among women( Reference Devries, Hamadeh and Glover 55 – Reference Campbell, Gross and Potter 58 ), our interest was in the combination of activity from both exercise and daily activities, and thus the results may not be directly comparable. Habitual physical activity is thought to potentially decrease oxidative stress through adaptive processes, in which levels of antioxidant enzymes and water- and lipid-soluble antioxidants may increase( Reference Aldred 59 ). However, observational studies of habitual physical activity and F2-isoprostanes among premenopausal women have been conflicting( Reference Rudra, Wactawski-Wende and Hovey 60 , Reference Sowers, McConnell and Jannausch 61 ), and further investigation is warranted.
The evaluation of 15-F2t-IsoP-M, a biomarker used in a few previous studies, is a unique strength of this study. In addition, owing to extensive baseline data collection in the Sister Study, we were able to control for the major factors known to affect oxidative stress. However, there are some limitations including the reliance on self-reported measures of diet, alcohol consumption and physical activity. Measurement error is inherent to the FFQ, and under- or over-reporting of physical activity and alcohol consumption may be a concern. Although more objective measures may be preferable, they would likely be infeasible in a sample as large as ours. Furthermore, the CV for the assays of F2-IsoP and 15-F2t-IsoP-M were somewhat high, suggesting caution in the interpretation of our results. Participants in this study were largely homogeneous with respect to demographic characteristics, limiting our ability to generalise to males or non-white populations. Given the large number of associations that we evaluated, there is also a risk of false-positive results. However, all tests were based on a priori hypotheses. Finally, we were unable to address differences in F2-IsoP concentrations by intensity of physical activity or type of alcohol consumption. The effects of long-term, moderate-intensity physical activity on oxidative stress may differ from those of acute, vigorous activity( Reference Mastaloudis, Leonard and Traber 23 , Reference Nikolaidis, Kyparos and Vrabas 24 ), whereas the effects of red wine may differ from those of other alcoholic beverages due to its antioxidant content( Reference Das, Sato and Ray 62 , Reference Covas, Gambert and Fito 63 ). Evaluation of such associations could further our understanding of the influence of lifestyle factors on oxidative stress.
In summary, the results of this study suggest that physical activity and specific dietary factors, such as antioxidant nutrients and long-chain n-3 fatty acids, may be inversely associated with oxidative stress among premenopausal women. Our findings also suggest that higher intake of trans fats may be associated with higher levels of oxidative stress. Future studies are warranted to evaluate additional biomarkers of oxidative stress in more diverse populations.
Acknowledgements
The authors appreciate the helpful comments of Dr Kelly Ferguson and Dr Yong-Moon Park.
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005), the Avon Foundation (02-2012-085) and by the National Center for Advancing Translational Sciences (KL2-TR001109).
C. A., H. B. N., D. P. S. designed the study; D. P. S., G. L. M. conducted the study; C. A., H. B. N. analysed the data; all the authors contributed to writing of the paper and had primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516003226






