Introduction
The genus Echinochloa consists of more than 50 plant species that are widespread in many field crops, such as cotton (Gossypium hirsutum L.), soybean [Glycine max (L.) Merr.], corn (Zea mays L.), sunflower (Helianthus annuus L.), vegetables, and rice (Oryza sativa L.) (Holm et al. Reference Holm, Plucknett, Pancho and Herberger1977; Tabacchi et al. Reference Tabacchi, Mantegazza, Spada and Ferrero2006). The allohexaploid barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] and the allotetraploid late watergrass [Echinochloa phyllopogon (Stapf.) Koso-Pol.; syn.: Echinochloa oryzicola (Vasinger) Vasinger] (Hoste and Verloove Reference Hoste and Verloove2022) are the most widely distributed species in paddy and upland rice fields (Lim et al. Reference Lim, Kim, Noh, Lim, Yook, Kim, Yi and Kim2021). Echinochloa phyllopogon is a polymorphic species that dominates in continuous-cropping rice production systems and exhibits crop mimicry, allelopathy, and considerable abiotic stress resistance (Lim et al. Reference Lim, Kim, Noh, Lim, Yook, Kim, Yi and Kim2021; Panozzo et al. Reference Panozzo, Mascanzoni, Scarabel, Milani, Dalazen, Merotto, Tranel and Sattin2021). In addition, heavy infestations of E. phyllopogon in rice fields are very competitive and result in yield losses ranging from 30% to 50%, and in some cases complete crop loss (Jabran et al. Reference Jabran, Farooq, Hussain, Ehsanullah Khan and Shahid2012; Vasilakoglou et al. Reference Vasilakoglou, Dhima and Gitsopoulos2018).
Herbicides play a vital role in weed control in modern agriculture worldwide, and particularly in rice production systems. Acetolactate synthase (ALS)- and acetyl-CoA carboxylase (ACCase)-inhibiting herbicides are the two most widely used rice herbicides for the control of Echinochloa species. Among the authorized herbicides in rice grown in Greece, the active ingredients with ALS mode of action are azimsulfuron, bispyribac-Na, imazamox (used in tolerant rice cultivars of Clearfield® technology), and penoxsulam, while those with ACCase mode of action are cyhalofop-butyl and profoxydim. In addition, the auxin mimic quinclorac, the photosystem II (PSII) inhibitor propanil, and the elongase inhibitor pretilachlor were exceptionally registered in some recent years, whereas the ACCase inhibitor cycloxydim was also registered recently for control of red rice and Echinochloa species in rice-tolerant cultivars of ProvisiaTM technology. Moreover, the synthetic auxin florpyrauxifen-benzyl was recently registered for effective control of Smallflower umbrella-sedge and broadleaf aquatic weeds and strong suppression of the Echinochloa complex. Finally, the 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor benzobicyclon was exceptionally authorized in Greece in 2021 for preemergence (after seeding) applications in rice to suppress red rice and Echinochloa species and to provide effective sedges control. Therefore, 70% of the authorized rice herbicides in Greece for Echinochloa control belong to the ALS and ACCase groups.
The continuous use of herbicides in rice crops has led globally to the inevitable evolution of Echinochloa populations with cross- or multiple resistance to herbicides (Heap Reference Heap2024). More specifically, Echinochloa resistance has been confirmed for thiobencarb (Dimaano et al. Reference Dimaano, Tominaga and Iwakami2022), propanil (Vasilakoglou et al. Reference Vasilakoglou, Eleftherohorinos and Dhima2000), molinate (Fisher et al. Reference Fischer, Ateh, Bayer and Hill2000a), quinclorac (Gao et al. Reference Gao, Pan, Sun, Zhang, Dong and Li2017), fenoxaprop-P-ethyl (Fisher et al. Reference Fischer, Ateh, Bayer and Hill2000a), cyhalofop (Ruiz-Santaella et al. Reference Ruiz-Santaella, De Prado, Wagner, Fischer and Gerhards2006), clomazone (Norsworthy et al. Reference Norsworthy, Scott, Smith and Wells2007), glyphosate (Vázquez-García et al. Reference Vázquez-García, Rojano-Delgado, Alcántara-de la Cruz, Torra, Dellaferrera, Portugal and De Prado2021), and various ALS inhibitors (Kaloumenos et al. Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013). Regarding E. phyllopogon, molecularly determined multiple target-site resistance to ACCase and ALS inhibitors has only been reported in weed populations grown in rice fields in Spain (Amaro-Blanco et al. Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021), while no molecularly confirmed multiple resistance has also been reported in populations grown in rice fields in California (Fisher et al. Reference Fischer, Ateh, Bayer and Hill2000a) and Korea (Qiang et al. Reference Qiang, Won, Khaitov, Lee and Woong Park2021). In addition to target-site resistance, some cases of field-evolved herbicide resistance in E. phyllopogon were found to be due to enhanced herbicide metabolism via cytochrome P450 monooxygenases (P450s) (Fisher et al. Reference Fisher, Bayer, Carriere, Ateh and Yim2000b) and especially due to the CYP81A subfamily, which is responsible for multiple resistance to herbicides (Dimaano et al. Reference Dimaano, Yamaguchi, Fukunishi, Tominaga and Iwakami2020, Reference Dimaano, Tominaga and Iwakami2022; Iwakami et al. Reference Iwakami, Kamidate, Yamaguchi, Ishizaka, Endo, Suda, Nagai, Sunohara, Toki, Uchino, Tominaga and Matsumoto2019). Similarly, Pan et al. (Reference Pan, Guo, Wang, Shi, Yang, Zhou, Yu and Bai2022) reported that upregulation of a cytochrome P450 gene (CYP81A68) in E. crus-galli endows metabolic multiple resistance to the ALS inhibitor penoxsulam and the ACCase inhibitor cyhalofop-butyl used in intensive rice production systems.
This work began after complaints from many rice growers in northern Greece about poor control of E. phyllopogon populations in rice monoculture fields, with the use mainly of the ALS-inhibiting herbicides bispyribac-Na, imazamox, or penoxsulam, and less frequently with the ACCase-inhibiting herbicide profoxydim and the auxin mimic quinclorac. Thus, a random survey across the main rice production areas located in northern Greece was conducted to (1) confirm the evolution of multiple resistance in E. phyllopogon to ALS- and ACCase-inhibiting herbicides or ALS-inhibiting herbicides and quinclorac, (2) determine the level of the herbicide resistance in E. phyllopogon populations, (3) elucidate the mediated resistance mechanisms to herbicides, and (4) evaluate under field conditions the response of a selected E. phyllopogon population to postemergence-applied rice herbicides.
Materials and Methods
Seed Source of Putative Herbicide-Resistant Echinochloa phyllopogon Populations
The status of herbicide resistance in E. phyllopogon originating from rice production systems of northern Greece was assessed in 17 populations (P1 to P17) using whole-plant pot assays. Echinochloa phyllopogon seeds were sampled just before crop harvest during middle to late September 2021 from rice fields located in the prefectures of Thessaloniki and Serres (northern Greece). In these fields, water-seeded rice grown under flooded conditions as monoculture has been practiced for at least 10 consecutive years. Rice fields in Greece are well leveled by using laser operated equipment during March to May and are flooded to 3- to 7-cm water depth before rice seeding. Then, the water-seeded rice is dispersed into the flooded field with a fertilizer applicator within 2 d after flooding. The water is removed from the flooded fields for the application of the postemergence herbicides (12 to 30 d after seeding), and the treated fields are flooded 2 to 4 d after herbicide application to 7- to 10-cm water depth. Finally, the rice crop remains flooded to 10- to 15-cm water depth during summer, and the water is removed from the flooded fields 2 wk before rice harvest. It was decided to conduct this study because the application of the ALS inhibitors bispyribac-Na (pyrimidynil benzoate), imazamox (imidazolinone), and penoxsulam (triazolopyrimidine), and/or the ACCase inhibitors cyhalofop-butyl (aryloxyphenoxypropionate) and profoxydim (cyclohexanedione), and/or the auxin mimics quinclorac (benzoate) and florpyrauxifen-benzyl (pyridine-carboxylate) failed in recent years to provide satisfactory control of E. phyllopogon infestations. Therefore, seeds of selected putative resistant E. phyllopogon populations were collected from 15 rice fields located in Malgara (40.60007, 22.68000) (populations P1 to P3), Anatoliko (40.65017, 22.69997) (populations P4 to P7), and Halastra (40.61716, 22.71692) (populations P8 to P15) districts from the prefecture of Thessaloniki. Seeds were also collected from two rice fields located in Monoklissia (41.05317, 23.39095) (population P16) and Vamvakia (41.08316, 23.33295) (population P17) from the prefecture of Serres. In addition, seeds were collected from plants growing on the margins of a rice field located in Halastra (40.61716, 22.71692) district that had not received any rice herbicide for the last 5 yr, as this field had been cultivated with cotton/corn according to the farmer. Therefore, this E. phyllopogon population was considered to be susceptible (S). Weed seeds were randomly collected by hand from numerous surviving plants in different patches of each field just before rice harvest. Seeds from each field were pooled together and regarded as a different putative resistant population. The collected seeds were placed in big plastic bags and transported to the laboratory where they were threshed, air-dried, placed in paper bags, and finally stored at 5 to 7 C for use in the experiments.
Rate–Response Pot Assays for Confirmation of Weed Resistance to Herbicides
Echinochloa phyllopogon populations, 17 putative resistant (P1 to P17) and 1 susceptible (S), were studied in rate–response pot assays during the 2022 growing season for possible evolution of cross- or multiple resistance to ALS and ACCase inhibitors and synthetic auxin herbicides registered for chemical control of Echinochloa spp. mainly in rice, but also in corn and broadleaf crops. The experiments were conducted at the farm of the University of Western Macedonia, Florina (40.767°N, 21.4°E). The experiments were established in 10 by 10 by 10 cm plastic pots filled with a mixture of silty clay soil and sand (2:1 v/v). The physicochemical characteristics of the soil used in the pot experiments were clay 48%, silt 33%, sand 19%, organic matter 1.1%, pH (1:1 H2O) 7.4, and CEC 32 meq 100 g−1. Each pot was seeded at a depth of 1 cm with approximately 20 seeds of one E. phyllopogon population. At the 2-leaf stage, weed seedlings were carefully thinned to 6 plants per pot.
The 17 putative resistant populations and the 1 S population were treated with the label rate (1X) or twice (2X) or four times (4X) the label rate of the ALS inhibitors, ACCase inhibitors, 4-HPPD inhibitors and auxin herbicides shown in Table 1. All herbicides were applied with a portable 2.4-m-wide boom field plot propane-pressurized sprayer, carrying six 8002 flat-fan nozzles (TeeJet®, Spraying Systems, Wheaton, IL, USA). The sprayer was calibrated to deliver a water volume of 300 L ha−1 at a pressure of 280 kPa. For herbicide application, weed plants were at GS22-24 (4 to 5 leaves, 1 to 2 tillers) of Zadoks scale (Zadoks et al. Reference Zadoks, Chang and Konzak1974). A nontreated control for the 17 putative resistant populations and the S population was also included. The rate–response pot assay was conducted twice, and the pots within each E. phyllopogon population were rearranged weekly to achieve uniform growth conditions for all plants. The two identical pot experiments were established in a completely randomized design with three replications for each herbicide treatment. Efficacy of all herbicides against E. phyllopogon was assessed by determining the aboveground fresh weight of surviving plants at 4 wk after treatment (WAT). Fresh weight data were expressed as a percent reduction of the nontreated control and subjected to ANOVA. ANOVA was performed across the data obtained from the two whole-plant rate-response experiments, using an 18 (E. phyllopogon populations) by 11 (herbicides) by 3 (rates) split-plot approach, in which the 18 E. phyllopogon populations were considered the main plots and the 11 herbicides by 3 rates were considered the subplots. Differences between means were compared at P < 0.05 using Fisher’s protected LSD test.
Table 1. Source of materials for the products used in the rate–response experiments against the susceptible (S) and the 17 putative resistant Echinochloa phyllopogon populations.

a Nicosulfuron + rimsulfuron (sulfonylureas, acetolactate synthase [ALS] inhibitors) were applied with the surfactant iodecyl alcohol ethoxylate 90% w/v (Trend® 90 SL, Corteva Agriscience, Athens, Greece) at 1 ml L–1; imazamox (imidazolinone, ALS inhibitor) and profoxydim (cyclohexanedione, acetyl-CoA carboxylase [ACCase] inhibitor) were applied with the 37.5% w/w fatty acid esters + 22.5% w/w alkoxylated alcohol-phosphate esters (Dash HC, BASF Hellas) at 4 ml L–1; bispyribac-Na (pyrimidynil benzoate, ALS inhibitor) was applied with the surfactant Biopower® SL (Bayer CropScience, Athens, Greece) at 3.3 ml L–1; penoxsulam (triazolopyrimidine, ALS inhibitor); cycloxydim (cyclohexanedione, ACCase inhibitor); cyhalofop-butyl (aryloxyphenoxy-propionate, ACCase inhibitor); Quizalofop-P-ethyl (aryloxyphenoxy-propionate, ACCase inhibitor); quinclorac (benzoate, auxin mimic); Florpyrauxifen-benzyl (pyridine-carboxylate, auxin mimic); tembotrione (triketone, HPPD inhibitor).
b Abbreviations: EC, emulsifiable concentrate; OD, oil dispersion; SC, suspension concentrate; SL, soluble liquid; WG, water-dispersible granule.
c The rates in bold are the label recommended of the herbicides.
Target-Site (ACCase and ALS Gene) Sequence Analysis
As the rate-response assays indicated that the E. phyllopogon P14 and P15 populations were multiple resistant to ALS and ACCase inhibitors, further experiments were conducted to elucidate their molecular mechanisms of resistance. Echinochloa phyllopogon plants of the P14 and P15 populations, grown in four pots (6 plants per pot) per population, were treated with the labeled maximum field rate of profoxydim at the GS 22-24 of the Zadoks scale (4 to 5 leaves, 1 to 2 tillers); similar treatment with ALS herbicide was not undertaken, because the ALS resistance in E. phyllopogon had been studied previously by Kaloumenos et al. (Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013). In addition, plants of the S population grown in four pots were treated with the same rate of the herbicide, while the plants of four pots remained nontreated. The aim of this treatment was to eliminate possible individual plants susceptible to profoxydim from the two multiple-resistant E. phyllopogon populations and to confirm the susceptibility of the S population. For amplification and sequencing of the ACCase gene fragments, genomic DNA was isolated from four profoxydim-surviving plants (1 new fully expanded leaf per harvested plant from each treated pot) of the P14 and P15 populations, respectively, and from four untreated plants of the S population (1 leaf per harvested plant from each pot), using 20 to 30 mg of young leaf tissue and according to NucleoSpin® Plant II DNA extraction kit protocol (Macherey-Nagel, Duren, Germany). The leaf tissue for DNA extraction was collected at 4 WAT. The quality and quantity of the isolated DNA were checked using a NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
For the molecular resistance study of the ACCase gene, the primer pair CRUSS-F (5′-GATTGGCATAGCCGATGAAG-3′) and CRUSS-R (5′-TGGACAACACCATTGGTAGC-3′) was used to amplify a genomic region of ACCase that contains the codon 1781, producing a fragment of 474 bp (Amaro-Blanco et al., Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021). Cycling conditions consisted of an initial denaturation step of 95 C for 5 min, followed by 35 cycles of 95 C for 30 s, 57 C for 30 s, and 72 C for 1 min, with a final extension at 72 C for 5 min. PCR was performed in 10 μl volumes containing 5 μl (1X) of OneTaq® 2X Master Mix (New England BioLabs, Ipswich, Massachusetts, USA), 3 μl of PCR-grade water (Thermo Fisher Scientific), 0.5 μl of each forward and reverse primer (0.5 μM each) and 1 μl of template DNA (20 ng). Regarding the ALS gene, the primer pair BE1 (5′-GTCTTGGGGCTATGGGATTT-3′) and BE2 (5′-CGACAGAACAAGGGAGAACA-3′) was used for the amplification of the BE region of ALS, giving a fragment size of 594 bp, which ranges from codon 574 to codon 653 (Amaro-Blanco et al. Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021). The PCR reaction carried out was the following: 95 C for 5 min (once), 95 C for 30 s, 61 C for 30 s, and 72 C for 1 min (35 times), and 72 C for 5 min (once). PCR final volume and concentrations/quantities of all reagents used were exactly the same as for the CRUSS-F/CRUSS-R primer pair. PCR products of both ACCase and ALS genes were purified with the microCLEAN DNA cleanup reagent (Gel Company, San Fransisco, USA) according to the manufacturer’s protocol. The purified PCR products were then single-strand sequenced with BigDye Terminator v. 3.1 (Life Technologies, Carlsbad, California, USA) cycle sequencing methodology on an ABI3500 Genetic Analyzer (Applied Biosystems, Waltham, Massachusetts, USA). To detect the presence or absence of point mutations at codon 1781 of the ACCase gene, E. phyllopogon sequences (obtained with the CRUSS-F/CRUSS-R primer pair) were manually checked, aligned, and compared to Alopecurus myosuroides mRNA sequence for acetyl-coenzyme A carboxylase (GenBank accession no. AJ310767.1) using BioEdit v. 7.2.6 software (Hall Reference Hall1999).
In addition, the same plants used for amplification and sequencing of the ACCase gene fragments were sequenced for the possible presence of a coexisting ALS point mutation by comparing the sequences (obtained with BE1/BE2 primer pair) to the E. phyllopogon mRNA sequence for ALS (GenBank accession no. AB636580.1), using the same software.
Herbicide Efficacy against Echinochloa phyllopogon under Field Conditions
Two field experiments were conducted during the growing season of 2023 in Monoklissia district in the prefecture of Serres (41.05°N, 23.4°E), a main rice-growing area of northern Greece, to evaluate the response of the P16 population to a range of postemergence-applied herbicides in the rice crop. According to the farmer, the E. phyllopogon population in this field was not effectively controlled by the tank mixture of imazamox + cyhalofop-butyl or penoxsulam + cyhalofop-butyl during the previous 2 yr. The two experiments, separated by a distance of 100 m, were established in the same rice field where common agronomic practices concerning soil preparation, rice variety, sowing date, and sowing density were implemented. In particular, the imidazolinone-tolerant, Clearfield® variety ‘Luna’ was seeded (the seeds were dispersed by fertilizer applicators into the flooded field) during mid-May at a seeding rate of 200 kg ha−1. The two trials were established in rice fields naturally heavily and uniformly infested by E. phyllopogon. Weed density, assessed before herbicide applications, was found to range from 80 to 120 plants m−2. The herbicide treatments were applied to the rice field where water was removed 3 d before herbicide application and the rice crop was re-submerged 3 d after herbicide applications. The following commercial herbicide formulations were used at the recommended (1X) and two times the recommended (2X) field rate: penoxsulam (ViperTM OD, K&N Efthymiadis SA) applied at 40 and 80 g ai ha−1, imazamox (Pulsar® 4 SL, BASF Hellas) applied at 80 and 160 g ai ha−1, bispyribac-Na (Adora® 400 SC, Bayer CropScience Hellas) applied at 30 and 60 g ai ha−1, quinclorac (Facet® 25 SC, BASF Hellas) applied at 300 and 600 g ai ha−1), cyhalofop-butyl (Clincher NEOTM, Corteva Agriscience Hellas) applied at 300 and 600 g ai ha−1, and profoxydim (Aura® 20 EC, BASF Hellas) applied at 200 and 400 g ai ha−1. Some herbicides evaluated in the rate–response assays were not used in the field study because they are registered for use in corn (e.g., nicosulfuron + rimsulfuron pre mixture, tembotrione) or they are graminicides [e.g., the aryloxyphenoxypropionate (APP) quizalofop-P-ethyl and the cyclohexanedione (CHD) cycloxydim] that can be selectively used against annual and perennial grass weeds only in broadleaf field crops. A portable field plot AZO sprayer with a 2.4-m-wide boom was used for herbicide applications. The sprayer carried six 8002 flat-fan nozzles and was calibrated to deliver 300 L ha−1 of water at a pressure of 280 kPa. Herbicide applications were performed when E. phyllopogon plants were at the GS22-24 (4 to 5 leaves, 1 to 2 tillers) of the Zadoks scale and rice plants were at the 3- to 4-leaf stage. Herbicide treatments were performed in plots arranged in a randomized complete block design. There were four replicates for each treatment, with a plot size of 6 by 3 m. All blocks were separated by a 2-m buffer zone. A nontreated control (four replicate plots) was also included. Smallflower umbrella-sedge (Cyperus difformis L.) and other broadleaf weeds were effectively controlled with the application of a tank mix of propanil (STAM FLO 480 SC, BASF Hellas) + MCPA (LiMPCA® Forte SL, K&N Efthymiadis SA) at their maximum recommended field rates (100 + 100 g ha−1). Visual crop phytotoxicity (rice injury) assessments were made at 15, 30, and 45 d after treatment (DAT) by comparison with plants grown in nontreated plots (controls) to record any phytotoxicity of the herbicides on rice plants during the growing season, while herbicide efficacy was evaluated at the same periods, using a scale from 0 to 100, where 0 indicates no weed plant injury (as compared with the nontreated plants) and 100 indicates no plant survival. Only herbicide efficacy at 30 DAT is presented as no significant changes were observed thereafter.
An ANOVA was performed across the data obtained from the two field experiments, using a six by two factorial approach (six herbicides by two application rates). Differences of treatment means were compared at P < 0.05 using Fisher’s protected LSD test. As the homogeneity of variances of the two field trials, checked using Bartlett’s test (Snedecor and Cochran Reference Snedecor and Cochran1989), indicated no significant departure from normality, the data were analyzed over the two field experiments. Normal distribution of the data was tested analytically with the Shapiro-Wilk test.
Results and Discussion
Rate–Response Pot Assays for Confirmation of Weed Resistance to Herbicides
The ALS inhibitor imazamox application at 1X, 2X, and 4X field rates reduced the fresh weight of 10, 9, and 10 E. phyllopogon populations by 0% to 14%, 0.5% to 27%, and 18% to 54%, respectively (Figure 1). Similarly, the respective rates of penoxsulam reduced the fresh weight of 12, 9, and 11 weed populations by 0% to 15%, 11% to 42%, and 21% to 58%, whereas bispyribac-Na applied at the recommended field rate (1X) reduced the fresh weight of 12 out of 17 populations by 0% to 30%, while the respective reduction due to 2X and 4X rates was 0% to 45% and 19% to 63%. Also, the 1X rate of nicosulfuron + rimsulfuron (corn herbicides) reduced the fresh weight of 8 populations by 0% to 11%, whereas their application at 4X rate caused 0% to 67% reduction of fresh weight in 10 E. phyllopogon populations. It is worth mentioning that the 1X rate of penoxsulam reduced the fresh weight of the P4 population by 32%, whereas the respective reduction due to the 1X rate of imazamox, bispyribac-Na, and nicosulfuron + rimsulfuron was 81%, 72%, and 89%. However, the 1X, 2X, and 4X rates of the abovementioned dissimilar ALS inhibitors reduced the fresh weight of the P3, P8, P9, and P12 E. phyllopogon populations by 92% to 100%, 100%, 81% to 100%, and 100%, respectively.

Figure 1. Fresh weight reductions (% of untreated control) of 18 Echinochloa phyllopogon populations due to the application of the recommended 1X, 2X, and 4X rates of the acetolactate synthase (ALS) inhibitors bispyribac-Na, imazamox, penoxsulam, and nicosulfuron + rimsulfuron. LSD0.05 = 3 for the comparison of population by herbicide by rate means (six replicates per treatment).
The ACCase inhibitor cyhalofop-butyl applied at 1X rate reduced the fresh weight of 12 and 4 E. phyllopogon populations by 0% to 39% and 46% to 70%, respectively, whereas the 2X rate reduced the fresh weight of 11 and 3 populations by 68% to 86% and 95% to 98% (Figure 2). The 4X rate of this herbicide reduced the fresh weight of 15 populations by 90% to 100%, whereas its 1X, 2X, and 4X rates resulted in 0%, 0%, and 1%, fresh weight reduction of both P14 and P15 E. phyllopogon populations, respectively. Quizalofop-P-butyl applied at 1X rate reduced the fresh weight of 13 and 2 populations by 90% to 100% and 85% to 86%, respectively, whereas its application at 2X and 4X rates provided excellent control (100%) of 15 populations. However, the three rates of this herbicide resulted in 0% to 11% and 5% to 10% fresh weight reduction of the P14 and P15 populations, respectively. The ACCase inhibitors profoxydim and cycloxydim applied at all rates reduced the fresh weight of 15 populations by 100%, whereas the 1X, 2X, and 4X rates of profoxydim resulted in 0%, 3%, and 24% fresh weight reduction of the P14 population, and 3%, 30%, and 44% reduction of the P15 population. Similarly, the three rates of cycloxydim reduced the fresh weight of the P14 population by 12%, 24%, and 50% and of the P15 population by 21%, 31%, and 46%, respectively.

Figure 2. Fresh weight reductions (% of untreated control) of 18 Echinochloa phyllopogon populations due to the application of the recommended 1X, 2X, and 4X rates of the acetyl-CoA carboxylase (ACCase) inhibitors cyhalofop-butyl, quizalofop-P-ethyl, profoxydim, and cycloxydim. LSD0.05 = 3 for the comparison of population by herbicide by rate means (six replicates per treatment).
These results support the evidence of evolved cross-resistance to the ALS inhibitors bispyribac-Na, imazamox, penoxsulam, and nicosulfuron + rimsulfuron in 13 E. phyllopogon populations, whereas the remaining P3, P8, P9, and P12 populations were found to be susceptible to these herbicides. However, the P14 and P15 populations, along with their evolved cross-resistance to ALS inhibitors, were also cross-resistant to the ACCase inhibitors cycloxydim, cyhalofop-butyl, profoxydim, and quizalofop-P-ethyl. This is the first report of E. phyllopogon populations with multiple resistance to five ALS and four ACCase inhibitors being field selected in rice production systems of Greece.
The 1X, 2X, and 4X rates of the auxin mimic quinclorac reduced the fresh weight of the P1, P4, P7, and P10 populations by 0% to 29%, 10% to 36%, and 18% to 39%, whereas the same rates reduced the fresh weight of 12, 13, and 13 populations by 70% to 100%, 79% to 100%, and 100%, respectively (Figure 3). The reduced control of these four E. phyllopogon populations with quinclorac could be attributed to either reduced initial sensitivity or evolved multiple resistance in the P1, P7, and P10 populations to both ALS inhibitors and auxin-type quinclorac (Figures 1 and 3). Extremely high multiple-resistance levels to quinclorac and propanil (PSII inhibitor) in a junglerice [Echinochloa colona (L.) Link] population from Arkansas have been reported by Rouse et al. (Reference Rouse, Roma-Burgos and Barbosa Martins2019), but the resistance of the weed plants to quinclorac was due to their ability to detoxify this herbicide utilizing the UDP-glycosyltransferase enzymes (Rangani et al. Reference Rangani, Rouse, Saski, Noorai, Shankar, Lawton-Rauh, Werle and Roma-Burgos2022).

Figure 3. Fresh weight reductions (% of untreated control) of 18 Echinochloa phyllopogon populations due to the application of the recommended 1X, 2X, and 4X rates of the auxin mimic quinclorac, auxin mimic florpyrauxifen-benzyl, and the 4-Hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor tembotrione. LSD0.05 = 3 for the comparison of population by herbicide by rate means (six replicates per treatment).
The 1X rate of the auxin mimic florpyrauxifen-benzyl reduced the fresh weight of 9 and 8 populations by 74% to 79% and 80% to 90%, respectively, while the respective fresh weight reductions of all populations by the 2X and 4X rates were 90% to 98% and 100% (Figure 3). The reduced efficacy of the recommended rate of this herbicide against some populations could be attributed to either initial tolerance due to the involvement of reduced herbicide absorption and translocation or to non–target site metabolism. Similar results were reported by Lim et al. (Reference Lim, Kim, Noh, Lim, Yook, Kim, Yi and Kim2021), who found sensitivity differences between E. crus-galli and E. phyllopogon populations from Korea to florpyrauxifen-benzyl that were related to either possible existence of CYP450s involved in the metabolism of the repeatedly used ALS- and ACCase-inhibiting herbicides or reduced herbicide absorption and translocation.
The 1X rate of the HPPD inhibitor tembotrione (corn herbicide) reduced the fresh weight of 7 and 10 E. phyllopogon populations by 81% to 86% and 90% to 100%, respectively, whereas the respective reduction due to 2X and 4X rates of tembotrione was 96% to 100% and 100%, respectively (Figure 3). These findings indicated clearly that the E. phyllopogon populations with either cross-resistance to ALS or multiple resistance to both ALS and ACCase inhibitors could be effectively controlled by tembotrione applied in corn cultivated as a rotational crop with rice.
Target-Site (ACCase and ALS Gene) Sequence Analysis
The ACCase gene fragment sequences of the four P14 and four P15 plants revealed a point mutation at codon 1781 that resulted in substitution of Ile (ATA) to Leu (TTA). All plants of both E. phyllopogon populations were heterozygous (Ile-1781-Leu), carrying the wild-type allele (Ile) and the mutant allele (Leu) (Figure 4). However, the four plants of the S population were homozygous for the wild-type allele at position Ile-1781. This ACCase mutation confirmed the previously determined whole-plant cross-resistance to ACCase inhibitors and is in agreement with the results reported by Amaro-Blanco et al. (Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021), who found the same point mutation (Ile-1781-Leu) in E. phyllopogon/early watergrass [Echinochloa oryzoides (Ard.) Fritsch] populations grown in rice production systems of Spain, which conferred cross-resistance to both aryloxyphenoxypropionate and cyclohexanedione herbicides. The 1781 along with the 1999 and 2041 amino acid residues are directly implicated in the binding of ACCase-inhibiting herbicides (Jang et al. Reference Jang, Marjanovic and Gornicki2013), but the Ile-1781-Leu is the most frequently identified field-evolved amino acid substitution conferring high-level resistance across all chemical classes of ACCase inhibitors (aryloxyphenoxypropionate and some cyclohexanedione and phenylpyrazoline herbicides) (Beckie and Tardif Reference Beckie and Tardif2012). Regarding the levels of ACCase resistance, they is greatly influenced by specific amino acid changes, the number of resistance-endowing mutant alleles and their initial frequency, the relative expression levels of resistant genes, the recessive or dominant allele interactions, the weed species, the plant growth stages, the herbicide(s), and the recommended field rates of ACCase inhibitors (Iwakami et al. Reference Iwakami, Uchino, Watanabe, Yamasue and Inamura2012; Kaundun Reference Kaundun2014). However, although resistance level is affected by several factors, the fitness cost of field-selected herbicide-resistant grass weed populations is not associated with the Leu-1781 ACCase mutant allele (Menchari et al. Reference Menchari, Chauvel, Darmency and Délye2008).

Figure 4. ACCase gene nucleotide sequence alignment of the susceptible (S), P14, and P15 Echinochloa phyllopogon populations using BioEdit v. 7.2.6 software. The codons refer to the standard of the Alopecurus myosuroides mRNA sequence of the ACCase gene (GenBank accession no. AJ310767.1). The observed point mutations are highlighted in bold and correspond to the Ile-1781 position of the E. phyllopogon ACCase gene. IUPAC-IUB nucleotide codes: ATA (Ile), WTA (ATA/TTA [Ile-1781-Leu]).
The ALS gene fragment sequences of the same four ACCase sequenced P14 and four P15 plants revealed a point mutation in three out of the four sequenced plants of the P14 population at codon 574 that resulted in substitution of Trp (TGG) to Leu (TTA). The three ALS resistant plants were heterozygous, having the wild-type and the mutant alleles (Trp-574-Leu), while the remaining one plant of the P14 population and the four plants of the P15 population were homozygous for the wild-type allele (TTG) at codon 574 (Trp-574-Trp) (Figure 5). The heterozygous sequenced ALS resistant individual plants for the Trp-574-Leu allele are in agreement with results reported by Kaloumenos et al. (Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013) for two E. phyllopogon populations originating from the same location, which evolved broad-spectrum cross-resistance to the ALS-inhibiting herbicides penoxsulam, bispyribac-Na, imazamox, foramsulfuron, nicosulfuron, and rimsulfuron. These results strongly support the evidence for coexisting E. phyllopogon multiple target-site resistance to ACCase (Ile-1781-Leu) and ALS (Trp-574-Leu) inhibitors, which is reported for the first time. However, the absence of the Trp-574-Leu mutation in one plant and four plants of the ACCase target-site resistant P14 and P15 populations, respectively, indicates possible non-coexisting target-site multiple resistance to ACCase and ALS inhibitors, which has also been reported in E. crus-galli (Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013) and E. phyllopogon (Amaro-Blanco et al. Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021; Jia et al. Reference Jia, Won, Khaitov, Lee and Park2021). Regarding ALS resistance, Amaro-Blanco et al. (Reference Amaro-Blanco, Romano, Palmerin, Gordo, Palma-Bautista, De Prado and Osuna2021) also found E. phyllopogon populations in rice fields of Spain that harbored the three amino acid substitutions of Pro-197-Ser, Pro-197-Thr, and Ser-653-Asn in the ALS gene. Moreover, due to recurrent applications of ALS-inhibiting herbicides in many rice production systems in Korea, many Echinochloa populations have evolved cross-resistance to these herbicides (Lim et al. Reference Lim, Yook, Park, Yu, Kim, Lee and Kim2018). Furthermore, the point mutations of Ala-122-Asn and Trp-574-Leu in the ALS gene of E. crus-galli and E. phyllopogon, respectively, resulted in high resistance levels in many populations (Panozzo et al. Reference Panozzo, Mascanzoni, Scarabel, Milani, Dalazen, Merotto, Tranel and Sattin2021).

Figure 5. ALS gene nucleotide sequence alignment of the susceptible (S), P14, and P15 Echinochloa phyllopogon populations using BioEdit v. 7.2.6 software. The codons refer to the standard E. phyllopogon mRNA sequence for the ALS gene (GenBank accession no. AB636580.1). The observed point mutations are highlighted in bold and correspond to the Trp-574 position of the E. phyllopogon ALS gene. IUPAC-IUB nucleotide codes: TGG (Trp), TTG (Leu), TKG (TGG/TTG [Trp-574-Leu]).
Regarding the implication of heterozygous resistant plants for the evolution rate of the herbicide resistance, it is impossible to reliably predict this, as the target-site resistance mechanisms were not elucidated at the genetic and biochemical levels. In particular, it is not examined whether the detected specific target-site mutations confer resistance to herbicides due to reduced ability of a given herbicide to bind to the target protein, or due to increased expression of the target-site gene, or due to reduced function (activity) of the target-site enzyme. In addition, the inheritance, the genetic basis, and the frequency of the different resistance mechanisms in the populations are unknown. Furthermore, the ploidy of a species has been documented to affect the evolution of herbicide resistance, indicating that the presence of a mutation in only one genome confers a lower herbicide resistance level than the same mutation in both genomes of an allotetraploid species such as E. phyllopogon. In general, the evolution of weed resistance to herbicides in rice fields can occur rapidly and can spread effectively by both seed and pollen if the herbicide resistance–endowing alleles act in an additive to dominant fashion, meaning that the resistance allele could be selected even in heterozygous plants (Gaines et al. Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020).
Herbicide Efficacy against Echinochloa phyllopogon under Field Conditions
The P16 E. phyllopogon population evaluated in two field experiments established in a rice field of Serres (northern Greece) exhibited high levels of cross-resistance to all three chemically dissimilar ALS inhibitors: penoxsulam, imazamox, and bispyribac-Na (Table 2). More specifically, the application of the recommended (1X) field rate of the abovementioned ALS inhibitors provided 2%, 0%, and 4% weed control, respectively, whereas the respective level of control due to 2X rate was 4%, 10%, and 6%. Moreover, the ACCase inhibitor cyhalofop-butyl applied at 1X and 2X rates provided 19% and 45% weed control, while the respective field rates of the auxin herbicide quinclorac controlled E. phyllopogon plants by 77% and 97%. By contrast, the ACCase inhibitor profoxydim applied at 1X and 2X rates provided excellent control (99% to 100%) of this weed population. These findings on the response of P16 E. phyllopogon population to selected ALS, ACCase, and auxin-type herbicides applied under field conditions confirmed the results found by the rate–response pot assays and suggest the use of profoxydim for effective control of this E. phyllopogon population in the rice field studied. Similar results in Greece have been previously reported by Kaloumenos et al. (Reference Kaloumenos, Chatzilazaridou, Mylona, Polidoros and Eleftherohorinos2013), who found field-selected populations with high-level and broad-spectrum cross-resistance to the ALS inhibitors penoxsulam, imazamox, and bispyribac-Na used in rice.
Table 2. Chemical control of the P16 Echinochloa phyllopogon population in the field experiment conducted at the Monoklissia location, prefecture of Serres, northern Greece.
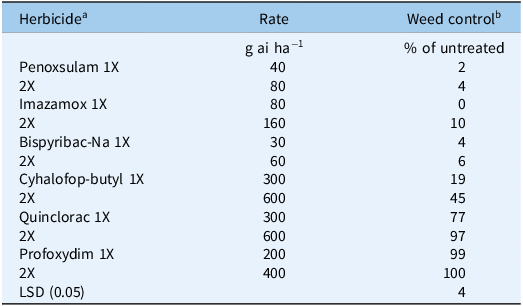
a Penoxsulam (triazolopyrimidine, acetolactate synthase [ALS] inhibitor); imazamox (imidazolinone, ALS inhibitor); bispyribac-Na (pyrimidynil benzoate, ALS inhibitor); cyhalofop-butyl (aryloxyphenoxy-propionate, acetyl-CoA carboxylase [ACCase] inhibitor); quinclorac (benzoate, auxin mimic); profoxydim (cyclohexanedione, ACCase inhibitor).
b Mean values are averaged across two experiments (4 + 4 replicate plots per treatment).
The pot and field experiments of this work indicated that the P1, P2, P5, P6, P7, P9, P11, P13, P14, P15, P16, and P17 putative resistant E. phyllopogon populations originating from rice monoculture fields of northern Greece were found with high-level and broad-spectrum cross-resistance to the ALS-inhibiting herbicides bispyribac-Na, imazamox, penoxsulam, and nicosulfuron + rimsulfuron. In addition, the P14 and P15 populations were found with multiple resistance to both ALS and ACCase inhibitors cycloxydim, cyhalofop-butyl, profoxydim, and quizalofop-P-ethyl, whereas the P1, P4, P7, and P10 populations were found to be multiple resistant to ALS inhibitors and quinclorac. Furthermore, the P3, P8, P9, and P12 E. phyllopogon populations were also multiple resistant to ALS and ACCase inhibitors and quinclorac, whereas the amplification and sequencing of the ACCase and ALS gene fragments revealed coexisting multiple target-site resistance to ALS (Trp to Leu substitution at codon 574 of the ALS enzyme) and ACCase (Ile to Leu substitution at codon 1781 of the ACCase enzyme) inhibitors in individual plants of P14 and P15 populations, which is reported for the first time. As a consequence of these findings and of the steadily increasing multiple herbicide resistance in Echinochloa populations globally (Rouse et al. Reference Rouse, Roma-Burgos, Norsworthy, Tseng, Starkey and Scott2018; Vulchi et al. Reference Vulchi, Guan, Clark and Brim-DeForest2024), the implementation of long-term, diversified, integrated weed management strategies is essential to reduce the frequency of repeated herbicide applications in rice crops and alleviate the intense selection pressure imposed on Echinochloa populations. The use of different herbicide modes of action in annual rotations, tank mixtures, and sequential applications are chemical options for effective control of Echinochloa species and significant reduction of selection pressure on this weed. However, great care should be taken into account for the implementation of imazamox and cycloxydim in Clearfield® and Provisia™ rice cultivars, respectively, as multiple resistance in Echinochloa populations growing in rice fields has already evolved. Finally, the utilization of harvest weed seed control (HWSC) systems and cleaning of harvesting and other agricultural machinery could contribute to minimizing further spread of multiple-resistant Echinochloa species to ALS- and ACCase-inhibiting herbicides.
Acknowledgments
We are grateful for the constructive comments by the anonymous reviewers that significantly contributed to the improvement of the article.
Funding statement
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.
Competing interests
The authors declare no conflicts of interest.










