In vivo digestion of proteins is a complex process depending on several factors such as (a) the amount and composition of the digestive enzymes, (b) the pH, (c) the transit time in the various parts of the gastrointestinal tract and (d) the overall composition of the food ingested(Reference Dressman, Amidon and Reppas1–Reference Ekmekcioglu3).
Human digestion of milk and milk-derived products may release peptides of different sizes available for paracellular or transcellular uptake and thus enter the systemic circulation and exert biological effects(Reference Gardner4, Reference Bernasconi, Fritsche and Corthesy5). Several bioactive peptides have in the recent years been identified after in vitro enzymic digestion of milk proteins. Most of these studies have applied commercial digestive enzymes often of porcine origin(Reference Hernández-Ledesma, Quirós and Amigo6–Reference Kim, Ki and Khan10). Although commercial porcine enzymes provide an easy-to-use option to study in vitro digestion, their validity in mimicking physiological digestion might be questioned. Human digestive juices consist of a variety of enzymes, inhibitors and bile salts that collectively contribute to the digestion of macromolecules(Reference Whitcomb and Lowe11, Reference Roberts12).
In vitro gastric digestion assays have frequently been performed at pH 1–2(Reference Ekmekcioglu3, Reference Schmelzer, Schops and Reynell8, Reference Kim, Ki and Khan10, Reference Thomas, Aalbers and Bannon13). However, gastric pH varies from 1·0 to 3·5 in the fasting state of healthy adult individuals(Reference Fallingborg14) and increases to a median of 6·7 during a meal(Reference Dressman, Berardi and Dermentzoglou15). The type of food ingested(Reference Moreno2) will also influence the gastric pH. In cases with short transit times (liquid food) or with high buffering capacity of the food, the pH may never decrease to the levels used in most assays. Furthermore, patients suffering from gastro-oesophageal reflux disease are frequently treated with proton pump inhibitors (PPI) leading to steady-state gastric pH values of>4 for 24 h to diminish oesophageal injury(Reference Hunt, Armstrong and James16). The activity of the gastric proteases (pepsins) is highly influenced by pH, with decreased activity above pH 4·5(Reference Roberts12). Low activity of the pepsins delays gastric protein digestion, resulting in an increased amount of intact protein reaching the intestine. During duodenal digestion, the pH is normally about 6·2–6·7(Reference Ekmekcioglu3, Reference Kalantzi, Goumas and Kalioras17); however, pH values up to 7–8 are recorded both in in vivo and in vitro studies(Reference Russell, Berardi and Barnett18, Reference McCloy, Greenberg and Baron19).
We investigated whether digestion of caprine whey proteins would produce bioactive peptides. To do this, an in vitro method that would mimic the events taking place during human gastrointestinal digestion was needed. Consequently, the aim of the present study was to compare the degradation of caprine whey proteins digested with either human digestive juices or with commercial porcine digestive enzymes. In addition, the effect of different gastric pH values, 2, 4 or 6, on the gastroduodenal degradation of the proteins was studied.
Whey proteins consist primarily of β-lactoglobulin (β-LG), α-lactalbumin (α-LA), lactoferrin (LF), serum albumin (SA) and immunoglobulins. The main protein is β-LG, making up about 50–60 % of the protein fraction of both caprine and bovine whey(Reference Miranda, Mahe and Leroux20, Reference Park, Park and Haenlein21). Whey proteins are considered high-quality proteins due to their elevated content of essential amino acids and are used to increase muscle gain in athletes(Reference Ha and Zemel22) as well as for the elderly(Reference Katsanos, Chinkes and Paddon-Jones23). Moreover, whey proteins are rich sources of bioactive peptides(Reference Pihlanto-Leppala24–Reference Hernández-Ledesma, Recio and Amigo27). The β-LG peptide produced during the in vitro digestion of caprine whey proteins was compared with bovine β-LG peptides obtained with the same enzymes at gastric pH 2.
Materials and methods
Materials
Whey protein concentrate from goats' milk (caprine whey protein concentrate; WPCG) was produced from sweet, cheese whey with a protein content of 81 %(Reference Almaas, Holm and Langsrud28). Bovine β-LG was purchased from Arla Foods (95 %, variant A+B; Arla Foods, Videbæk, Denmark; PSDI-2400). Pepsin A (77 163, 655 U/mg) was isolated from porcine stomach mucosa (Fluka BioChemika, Buchs, Switzerland) and Corolase PP (Ch 6946; 350 proteolyticU/mg) derived from pig pancreas glands, a mixture of trypsin, chymotrypsin amylase, lipase and several amino- and carboxypeptidases (Röhm GmbH, Darmstadt, Germany), was used. Hb from bovine blood was provided by Sigma (St Louis, MO, USA) and Hammarstein casein was purchased from Merck Co. (Darmstadt, Germany). All reagents used for HPLC and MS were of HPLC grade.
Aspiration of human gastric and duodenal juices
To follow up and extend previous work on in vitro digestion of caprine whey(Reference Almaas, Holm and Langsrud28–Reference Eriksen, Vegarud and Langsrud30) the gastric and duodenal juices employed were obtained from the same individual (healthy male, no medication). The pepsin and total proteolytic activities of these juices were within the normal range observed in twelve individuals (men and women, preliminary results). The proteolytic enzymes were obtained in the activated state by collecting human gastric and duodenal juices according to Holm et al. (Reference Holm, Krogdahl and Hanssen31). In brief, a three-lumen tube (Maxter Catheters, Marseille, France) enabled both simultaneous instillation of stimulation solution in the duodenum, and aspiration of gastric and duodenal juices. The stimulation solution (70 g/l sucrose, 1·8 g/l NaCl, 3·2 g/l l-phenylalanine and 2·3 g/l l-valine in water) was instilled close to the papilla of Vater (100 ml/h) to stimulate the production of pancreatic enzymes and the human duodenal juices was aspirated some 18 cm distally. Aspirates were collected on ice, centrifuged (4500 g for 10 min) to remove mucous and cell debris before being frozen in samples and stored at − 20°C before use.
Enzymic activities of gastric and duodenal enzymes
Porcine pepsin A and human gastric juices were analysed for pepsin activity at pH 3·0 with Hb as substrate as described by Sánchez-Chiang et al. (Reference Sánchez-Chiang, Cisternas and Ponce32). Corolase PP and human duodenal juices were analysed for total proteolytic activity at pH 8·0 with casein as substrate, as described by Krogdahl & Holm(Reference Krogdahl and Holm33). In brief, the enzymes were incubated with substrate for 10 min at 37°C and the reactions were stopped by the addition of TCA. After an overnight sedimentation at 4°C the samples were centrifuged for 10 min at 3000 g and 4°C. The enzymic activities were measured as the difference in absorbance at 280 nm of the TCA soluble fractions. One unit of enzyme activity (U) was defined as the amount (ml or mg) of enzyme giving a difference in absorbance of 1·0 at 280 nm in 10 min at 37°C. The enzyme assays were run in triplicate and performed three or more times.
In vitro digestion of caprine whey proteins and bovine β-lactoglobulin
A two-step in vitro protein digestion assay (Fig. 1) was performed in duplicate at 37°C to simulate digestion in the stomach and duodenum as described by Almaas et al. (Reference Almaas, Holm and Langsrud28). Either human digestive juices or porcine enzymes were used at similar enzymic activities. In the first step, simulating gastric digestion, the pH was adjusted to 2, 4 or 6 with 2 m-HCl and 5 U of porcine pepsin A or human gastric juices was added per g protein in the solution. The second step simulating duodenal digestion was performed by adjustment to pH 8 with 2 m-NaOH adding 16 U of either Corolase PP or human duodenal juices per g protein in the solution. Both steps lasted 30 min and samples were extracted at the start, during step 1 (at 10, 20 and 30 min) and during step 2 (at 15 and 30 min). Samples were transferred directly on ice and frozen within 5 min to stop the enzymic reactions. For comparison, bovine β-LG was digested in duplicate only at gastric pH 2 at the same conditions as described above.

Fig. 1 Illustration of the in vitro digestion assay performed in two steps using either human or porcine gastrointestinal enzymes(Reference Almaas, Holm and Langsrud28). Whey protein concentrate from caprine milk (WPCG) or bovine β-lactoglobulin (β-LG) were digested at 37°C in two 30 min steps to simulate the digestion in the stomach and the duodenum. Samples were extracted 10, 20 and 30 min after the addition of human gastric juice (HGJ) or porcine pepsin, and after 15 and 30 min following the addition of human duodenal juice (HDJ) or Corolase PP. Step 1 was perfomed at pH 2, 4 or 6 for WPCG, and at pH 2 for β-LG.
Visualisation of protein degradation by SDS-PAGE and ImageQuantTL analysis
The degradation profiles of the whey proteins were assayed by SDS-PAGE using the Bio-Rad Mini-PROTEAN® 3 Cell system (Bio-Rad Laboratories Ltd, Hemel Hempstead, Herts, UK). Each sample was run at least four times. Standard Laemmli reagents(Reference Laemmli34) and protocols were used to visualise protein degradation on 15 % polyacrylamide separating gels. Hydrolysates were mixed with 2 × SDS sample buffer (0·125 m-2-amino-2-hydroxymethyl-propane-1,3-diol-HCl, pH 6·8, 4 % SDS, 20 % glycerol, 2 % 2-mercaptoethanol, 0·03 mm-bromphenol blue) and boiled for 5 min before application to the gel. A low-molecular-weight marker (LMW-SDS Marker Kit; GE Healthcare, Little Chalfont, Bucks, UK) was used. Electrophoresis was performed at constant 200 V for 50 min and Coomassie Brilliant Blue R-250 was used to visualise the separated proteins. To evaluate the degree of protein degradation, the ImageQuantTL 7.0 software (GE Healthcare Bio-Sciences, Uppsala, Sweden) was used. The individual protein bands in the undigested WPCG were set to 100 % and the percentage remaining was calculated based on the average of at least four gels.
In-gel digestion of caprine whey protein concentrate proteins
Whey proteins were separated on a 15 % SDS-PAGE gel. Each band (1–6; Fig. 2(a)) was excised out of the gel, and subjected to in-gel reduction, alkylation, and tryptic digestion(Reference Shevchenko, Wilm and Vorm35). In brief, 10 mm-dithiothreitol in 100 mm-ammonium bicarbonate (Ambic) was added to reduce the cystines (56°C; 45 min). Cysteine alkylation was carried out in the dark in a solution of 55 mm-iodoacetamide in 100 mm-Ambic (room temperature; 30 min). In-gel digestion was performed using 6 ng/μl trypsin (V511A; Promega Corp., Madison, WI, USA) in 50 mm-Ambic containing 5 mm-CaCl2 (37°C, overnight). Before the mass spectrometer analyses, the trypsinated proteins were desalted and concentrated using C18 ZipTips (OMIX; Varian, Inc., Palo Alto, CA, USA) according to the manufacturer's instructions. Elution was performed with 2 μl 0·1 % formic acid in 60 % acetonitrile and diluted in 8 μl 0·1 % formic acid.
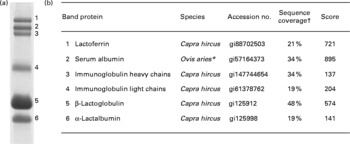
Fig. 2 Proteins identified in the caprine whey protein concentrate. (a) Proteins separated as individual bands by SDS-PAGE. (b) Identification of the protein band by nano-LC MS/MS. * All identified peptides were also present in serum albumin from Capra hircus; the mascot software did, however, not list this species. † Percentage of protein sequence covered by the identified peptides.
Prefractionation of digested proteins using size exclusion chromatography
Size exclusion chromatography was used to improve the separation of peptides and remove undigested proteins in the hydrolysates before mass spectrometric analyses. Freeze-dried samples of digested caprine whey proteins and bovine β-LG were dissolved to a concentration of 4 mg/ml (w/v) in a 100 mm-sodium acetate buffer mixed containing 30 % (v/v) acetonitrile and 0·1 % (v/v) trifluoroacetic acid at pH 5·5 (mobile phase). The samples were sterile filtered (0·22 μm Millex® GP filter, Millipore Express® polyethersulfone (PES) membrane; Millipore Corp., Billerica, MA, USA) and samples of 50 μl were added at a flow rate of 0·5 ml/min. Fractionation was carried out on a Tricorn™ Superdex Peptide 10/300 GL gel filtration column (GE Healthcare) with a linear separation range from 100 to 7000 Da. Both duplicates from the in vitro digestion assays were run seven or more times and the resulting peaks were collected and freeze dried.
Desalting and concentration of peptides
Freeze-dried peptide fractions (peaks) prepared by size exclusion chromatography were dissolved in 0·1 % (v/v) formic acid. The samples were desalted and concentrated using self-made columns consisting of C18 column material (3M Empore C18 extraction disks; 3M Bioanalytical Technologies, St Paul, MN, USA) inserted into Eppendorf GELoader micropipette tips (Eppendorf, Hamburg, Germany). The peptides were eluted using 2 μl 70 % acetonitrile–0·1 % formic acid (v/v).
Nano-LC MS of peptides
Desalted and concentrated mixtures of peptides were diluted in 10 μl 1 % (v/v) formic acid before they were loaded onto a nanoACQUITY™ Ultra Performance LC® (Waters Corp., Milford, MA, USA), containing a 3 μm Symmetry® C18 Trap column (180 μm × 22 mm; Waters) in front of a 3 μm Atlantis™ C18 analytical column (100 μm × 100 mm; Waters). Peptides were separated with a gradient of 5–90 % (v/v) acetonitrile, 0·1 % (v/v) formic acid, with a flow of 0·4 μl/min eluted to a Q-TOF Ultima Global mass spectrometer (Micromass/Waters) and subjected to data-dependent tandem MS analysis. Peak lists were generated by the ProteinLynx Global server software (version 2.1), and the resulting pkl files were searched against the National Center for Biotechnology Information (NCBI) non-redundant protein sequence databases using the MASCOT search engine (http://www.matrixscience.com). Peptide mass tolerances used in the search were 100 parts per million (ppm), and fragment mass tolerance was 0·1 Da. The taxonomy used in the search was mammalia. Data were acquired over a mass:charge ratio of 400–1500 Da, detecting peptides with two or three charges. Thus, only peptides with mass above 800 and below 4500 Da were subjected to collision-induced fragmentation and further processing.
Statistical analysis
Statistical evaluation of the effect of enzyme source (porcine or human) and pH (2, 4 and 6) on protein degradation was performed on four or more SDS-PAGE gel runs per sample using Minitab 15 (Minitab Inc., State College, PA, USA). The experiment was designed as a 2 × 3 unbalanced factorial design, and a two-way ANOVA (general linear model) was used to analyse the results with Tukey's test for pairwise comparisons. The percentage remaining of each intact protein band after 10, 20 and 30 min gastric digestion or after 45 or 60 min gastric and duodenal digestion was compared at the P < 0·05 level. Bartlett's and Levene's tests were used to test for equal variances after transforming the data by the square root. The tests found unequal variances only for the means of the Ig heavy chains (IgHC). Nevertheless, these results were included in the further analysis.
Results
Identification of proteins in the caprine whey protein concentrate
The major proteins in the caprine whey as visualised by SDS-PAGE were identified by in-gel trypsination followed by nano-LC MS/MS analysis (Fig. 2). Bands 1–6 were identified as the following proteins (molecular weight in decreasing order): LF, SA, IgHC, Ig light chains (IgLC), β-LG and α-LA.
Effect of pH and enzyme source on the digestion of whey proteins
The amounts (%) of intact whey proteins remaining after gastric and duodenal digestion are shown in Table 1. Gastric pH had a significant effect on the proteolysis of all the proteins (P < 0·001) except for β-LG (P = 0·385), with pH 2 resulting in the most extensive degradation. Furthermore, based on the amount of intact proteins, the porcine pepsin was overall more efficient than the human gastric juice at degrading the whey proteins. Digestion at gastric pH 4 followed by duodenal digestion resulted in lower degradation of all the proteins with both porcine and human enzymes. However, the porcine pepsin and pancreatic enzymes seemed to be more efficient than the human digestive juices. At gastric pH 6, very low degradation of all proteins was observed for both types of gastric enzymes (approximately 80–100 % intact protein), with the consequence of reduced duodenal digestion.
Table 1 Amount of intact protein remaining (%) after digestion of caprine whey proteins with either human digestive juices or porcine enzymes
(Mean values and standard deviations)

HGJ, human gastric juice; HDJ, human duodenal juice; CPP, Corolase PP; LF, lactoferrin; SA, serum albumin; IgHC, Ig heavy chains; IgLC, Ig light chains; β-LG, β-lactoglobulin; α-LA, α-lactalbumin.
Mean value was significantly different from that when using human digestive juices at the same pH: * P < 0·05, ** P < 0·01.
† Gastric digestion was with either HGJ or porcine pepsin A performed at pH 2, 4 or 6. Average numbers are based on four SDS-PAGE gels or more.
‡ Gastric digestion was followed by duodenal digestion at pH 8 using either HDJ or porcine pancreatic enzymes (CPP). Average numbers are based on four SDS-PAGE gels or more.
The degradation profiles of the digested whey proteins at gastric pH 2 are shown in Fig. 3. Fig. 3 illustrates differences in the degradation of the minor proteins LF, SA and Ig, and the main proteins, β-LG and α-LA, in whey by human and porcine enzymes. Porcine pepsin A digested the LF, SA and IgHC and IgLC efficiently, leaving only 2, 5, 6 and 28 % intact proteins, respectively. In comparison, significantly more intact protein was detected (P < 0·0001) when human gastric juice was used, with 19, 32, 72 and 67 % of the LF, SA and IgHC and IgLC remaining, respectively. However, digestion with human gastric and duodenal juices resulted in a rapid degradation of the LF, SA, IgHC and IgLC (1, 3, 5 and 25 % intact protein).

Fig. 3 SDS-PAGE (15 %) protein profiles of caprine whey protein concentrate (WPCG) digested with either human gastroduodenal juices or porcine digestive enzymes. Step 1: gastric digestion at pH 2. Step 2: duodenal digestion at pH 8. In both gels: lane 1, low-molecular-weight (LMW) marker; lane 2, undigested WPCG; lanes 3–5, WPCG digested with gastric enzymes (pepsin A or human gastric juice (HGJ)) for 10, 20 and 30 min; lanes 6 and 7, WPCG digested with gastric enzymes for 30 min followed by duodenal enzymes (porcine pancreatic enzymes or human duodenal juice (HDJ)) for 15 min (total time 45 min) and 30 min (total time 60 min). The molecular weights (MW) of the standards (STD) (LMW, in kDa) are marked at the side of the gel showing the digestion of WPCG with human enzymes. The framed areas show the proteins in whey where the most pronounced difference in enzyme source was observed. Arrows indicate the main bands before digestion: lactoferrin (LF); serum albumin (SA); Ig heavy chains (IgHC); Ig light chains (IgLC); β-lactoglobulin (β-LG); α-lactalbumin (α-LA).
The main whey proteins, β-LG and α-LA, seemed very resistant to gastric degradation with human gastric juices, whereas the porcine pepsin was significantly more efficient, leaving 90 % β-LG intact (P = 0·0253) and 66 % α-LA intact (P < 0·0001). The following duodenal digestion with the porcine pancreatic enzymes resulted in 56 and 50 % protein remaining, respectively, compared with a significantly higher amount observed with human gastroduodenal enzymes, 74 % β-LG (P = 0·0285) and 81 % α-LA (P = 0·0003).
Special attention was paid to the degradation of LF, an antibacterial protein in whey, with time (Fig. 4). Within the 30 min gastric digestion at pH 2, the porcine pepsin A was significantly more efficient (P < 0·0001) compared with human gastric juice and only 6 % intact LF remained after 10 min. Human gastric juices resulted in a more gradual degradation, with approximately 36 % LF remaining after 10 min and 19 % remaining after 30 min. Gastric pH 4 resulted in a markedly delayed degradation, and the digestion was overall very low at gastric pH 6. The difference in the amount of LF remaining after duodenal digestion with either porcine or human enzymes was not significant (P>0·05).

Fig. 4 Degradation profiles of lactoferrin after digestion of caprine whey protein concentrate (WPCG) with either human (H) or porcine (P) enzymes. Samples were extracted after simulated gastric digestion for 10, 20 and 30 min at pH 2, 4 or 6 followed by simulated duodenal digestion for 15 and 30 min. (■), 0 min; (![]() ), 10 min; (▧), 20 min; (□), 30 min; (
), 10 min; (▧), 20 min; (□), 30 min; (![]() ), 45 min; (
), 45 min; (![]() ), 60 min. Values are means, with standard deviations represented by vertical bars. Significant differences (P < 0·0001) were obtained only at pH 2 for WPCG digested with human (pH 2 H) compared with porcine enzymes (pH 2 P).
), 60 min. Values are means, with standard deviations represented by vertical bars. Significant differences (P < 0·0001) were obtained only at pH 2 for WPCG digested with human (pH 2 H) compared with porcine enzymes (pH 2 P).
Peptides identified after in vitro gastroduodenal digestion
Nano-LC MS/MS was used to identify the peptides produced by in vitro digestion after separating out peptides in the range of 100–7000 Da using gel filtration. Peptides from all the major proteins in the caprine whey including fragments of β-casein and κ-casein glycomacropeptide were identified (data not shown). Fig. 5 highlights the origin of peptides within the β-LG primary sequence when caprine whey was digested with human or porcine gastrointestinal enzymes using a gastric pH of 2. Two replicates were run and the peptides identified in both replicates were included in the tabulation. An overview of the β-LG peptides identified after digestion of caprine whey using human and porcine enzymes (gastric pH 2, 4 and 6) are given in Supplemental Appendix 1A and B, respectively. Bovine β-LG was digested at gastric pH 2 as a comparison with the digested β-LG in the caprine whey, and the peptides produced were identified. The sequence coverage was above 70 % and almost identical areas in the β-LG primary sequence were covered irrespective of the enzyme source as seen in Fig. 6. More peptides were found when pure bovine β-LG was digested compared with the β-LG in the digested caprine whey (Supplemental Appendix 1C).
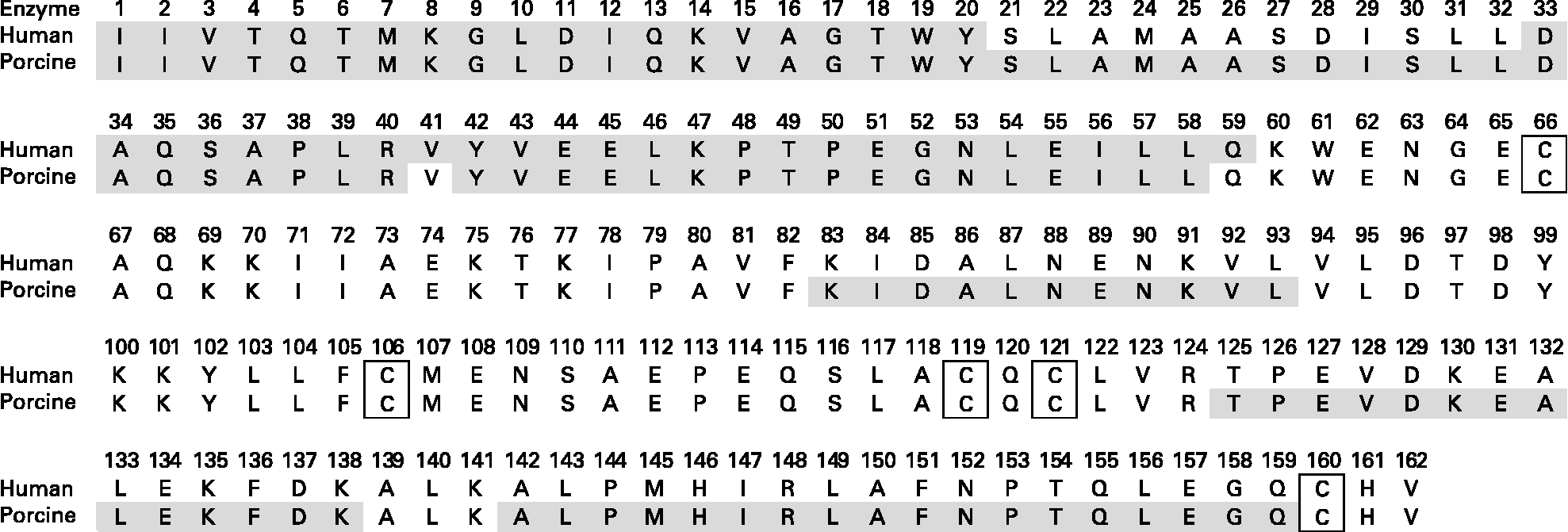
Fig. 5 Primary structure of caprine β-lactoglobulin. Grey sections denote the location of peptides identified by MS/MS after a two-step in vitro digestion assay. Step 1: gastric digestion at pH 2. Step 2: duodenal digestion at pH 8. Disulfide bridges are located between Cys66 and Cys160 and between Cys106 and Cys119/121.
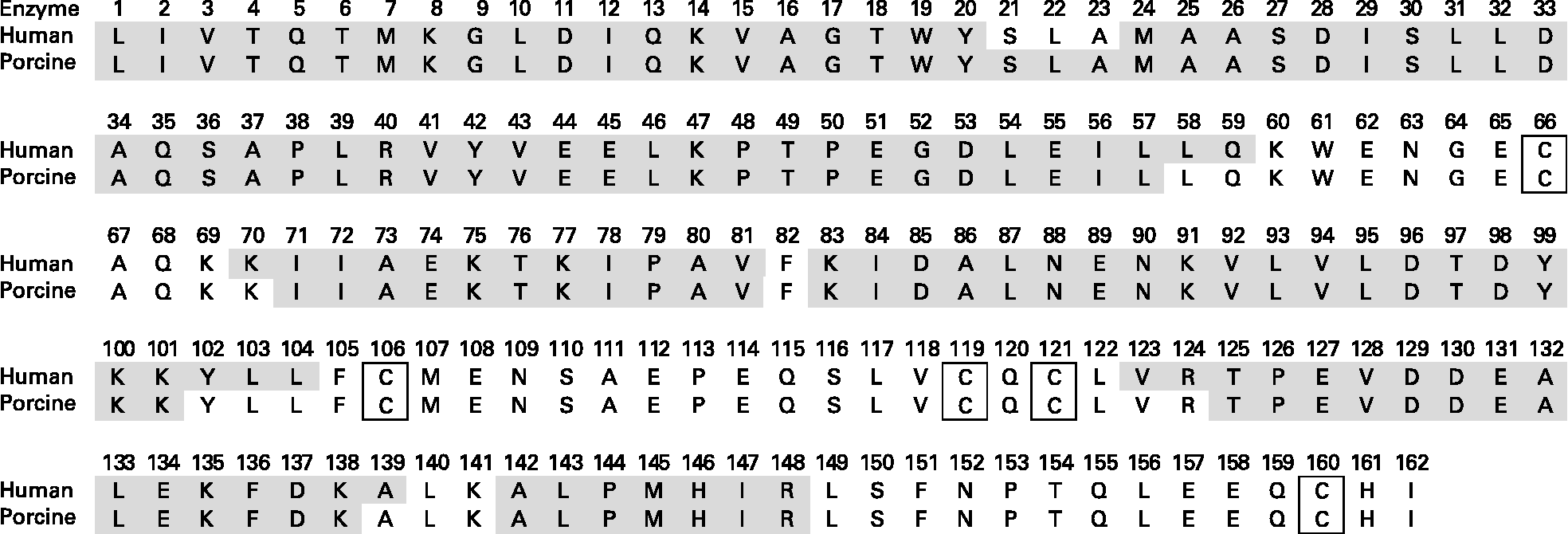
Fig. 6 Primary structure of bovine β-lactoglobulin. Grey sections denote the location of peptides identified by MS/MS after a two-step in vitro digestion assay. Step 1: gastric digestion at pH 2. Step 2: duodenal digestion at pH 8. Disulfide bridges are located between Cys66 and Cys160 and between Cys106 and Cys119/121.
Discussion
There are many reports in the literature on the physiological function of bioactive peptides produced by in vitro digestion of whey proteins using commercial enzymes from animal sources. There are, however, relatively few comparable data on digestion using human gastrointestinal enzymes. Since gastric pH seems to vary considerably in humans according to age, diet, health condition, etc, three different pH values, 2, 4 and 6, representing common variations in gastric pH were included in the present study.
As a general trend, the presented results showed that commercial porcine enzymes digested the caprine whey proteins more rapidly than the human digestive juices used at similar enzymic activities. Especially during the gastric digestion all the whey proteins seemed to be more rapidly degraded by the porcine pepsin than by human gastric juice irrespective of gastric pH. Human gastric juice contains a combination of different pepsin isoforms. Pepsin A (3a, b, c and 1) constitutes about 90 %, whereas 10 % of the proteases in non-stimulated human gastric juice is found to be pepsin C (gastricsin)(Reference Jones, Balan and Jenkins36). The additional pepsin isoforms present in the human gastric juice may be less efficient at degrading the caprine whey proteins than porcine pepsin A alone or their activities may have different pH-optima.
Furthermore, Tang et al. (Reference Tang, Mills and Chiang37) demonstrated how the proteolytic rate of gastricsin and pepsin depends on the substrate used. To determine the enzymic activity of the human gastric juices and porcine pepsin A, the substrate employed was Hb. When using a complex substrate such as whey the various pepsin isoforms may have different affinities for whey proteins as compared with their affinity for Hb.
In general, we observed that the lower the pH during the gastric digestion, the more degraded were the proteins in both steps. At gastric pH 2 all the proteins were significantly more degraded with porcine pepsin compared with human gastric juices (P < 0·05) especially for the minor whey proteins. Further degradation with porcine pancreatic enzymes or human duodenal juices showed only minor difference in the amount of intact protein remaining. Gastric digestion at pH 4 resulted in a significantly lower degradation compared with pH 2 for LF, SA and the immunoglobulins (P < 0·05). At pH 6 most of the proteins resisted the gastric digestion and the duodenal digestion was even more delayed.
The most resistant protein seemed to be β-LG and α-LA which were hardly degraded during gastric digestion irrespective of pH. This is in agreement with Chatterton et al. (Reference Chatterton, Rasmussen and Heegaard38), who showed that α-LA and β-LG in bovine milk digested with human neonatal gastric juice were more resistant to degradation at pH above 4 compared with pH 2. Schmidt et al. (Reference Schmidt, Meijer and Slangen39) also showed a marked decrease in peptic degradation of α-LA at pH 4 compared with pH 2 and 3, whereas β-LG was hardly degraded with pepsin at all pH values. However, as the whey proteins are digested, the high-molecular-weight proteins such as LF and SA may be cleaved to peptide fragments that may add to the bands of the smaller proteins when running SDS-PAGE. This could make the quantification of the degradation of the low-molecular-weight proteins (β-LG and α-LA) somewhat inaccurate, and is probably the reason why values above 100 % were recorded for these proteins during digestion (Table 1).
When studying the digestion of proteins, individual variations in gastric pH may be taken into consideration(Reference Dressman, Amidon and Reppas1). When food is ingested, the pH in the stomach tends to rise depending on the composition and quantity of the meal, but decreases gradually as HCl is secreted and the stomach empties(Reference Dressman, Amidon and Reppas1, Reference Kalantzi, Goumas and Kalioras17, Reference Chatterton, Rasmussen and Heegaard38). Individuals treated for gastrointestinal reflux disease with proton pump inhibitors (PPI) have a dramatically reduced secretion of HCl, and as a result the gastric pH is kept well above 4(Reference Hunt, Armstrong and James16, Reference Armstrong40). At this elevated pH, degradation of normal food proteins may be significantly impaired compared with the digestion observed at low gastric pH. This pH-related resistance to hydrolysis can affect the allergenicity of food proteins as epitopes can either remain stable or be degraded(Reference Monaci, Tregoat and van Hengel41). Increased gastric pH could also possibly result in the production of bioactive peptides further down the gastrointestinal tract due to delayed digestion in addition to delayed release of amino acids.
The reduced gastric digestion by human gastric juices compared with porcine pepsin was to some extent compensated for by the activity of human duodenal juices (Table 1). The presence of bile salts in the duodenal juice used may also affect the proteolytic activity. Gass et al. (Reference Gass, Vora and Hofmann42) demonstrated that the addition of a 10 mmol/l physiological bile acid mixture significantly enhanced the digestion of β-LG and BSA by trypsin and chymotrypsin. The concentration of bile salts in the fasting state varies widely among individuals, but is usually within the range of 2·6–6·4 mm(Reference Dressman, Amidon and Reppas1, Reference Kalantzi, Goumas and Kalioras17). Postprandial duodenal bile salt concentration has been shown to peak 30 min after ingestion of a test meal(Reference Dressman, Amidon and Reppas1, Reference Fausa43), reaching a mean of 14·5 mmol/l. The human duodenal juice used in the present study was collected in a semi-fasting state (secretion stimulated by amino acids and sucrose) and therefore probably much less bile salts are present compared with duodenal juice collected after administration of a meal. However, the yellow colour of the human duodenal juices (bilirubin) indicated the presence of bile salts that in increased amounts may enhance the proteolysis compared with the porcine pancreatic enzyme mixture (Corolase PP), which, according to the manufacturer, contains only traces of bile salts.
The peptide profile of β-LG was studied in more detail since this protein is known to be rather resistant to degradation by pepsin(Reference Mota, Ferreira and Oliveira44, Reference Pintado and Malcata45). The β-LG peptide profiles of the digested WPCG confirmed a more extensive degradation by porcine enzymes with a higher amount of peptides detected compared with the digestion with human enzymes.
The identified peptides released after digestion with human enzymes compared with the porcine enzymes differed to some extent. No peptides containing any of the cysteine groups after digestion of bovine β-LG was detected. Shorter peptides covering β-LG Cys160 were, on the other hand, identified in one of the replicates of the digested WPCG. Pecquet et al. (Reference Pecquet, Bovetto and Maynard46) identified a peptide consisting of fragment 61–69 linked to fragment 149–162 via the disulfide bond Cys66-Cys160. The digested β-LG may contain intact cyclic peptides of various lengths linked through Cys106-Cys119/121 and/or Cys66-Cys160. These peptides were, however, not identified, indicating that either larger intact cyclic peptides or fragments too small to be detected by our method were produced.
To conclude, the present study compared the in vitro gastrointestinal digestion of caprine whey proteins by using either human or porcine enzymes at different gastric pH values. Differences in the degradation profiles of the individual proteins indicated that perhaps human digestive enzymes should be preferred over pure commercial enzymes from other species when mimicking human digestion. Elevated gastric pH resulted in delayed protein digestion irrespective of the enzyme source. However, further studies are needed to establish if there is a clinical significance for this finding.
Acknowledgements
The present study was supported by the Norwegian University of Life Sciences.
We also greatly appreciated the excellent technical assistance offered by Toril Anne Grønset and Jack-Ansgar Bruun at The Tromsø University Proteomic Platform (TUPP) where all mass spectrometrical analyses were performed.
We wish to acknowledge Irene Comi for her helpful advice and laboratory assistance during HPLC work. The statistical expertise of Thomas Ulleberg was also greatly appreciated.
E. K. E. performed most of the experiments as well as writing the manuscript. H. H. donated his gastric and duodenal juices and assisted when performing the enzymic analyses. E. J. suggested the MS techniques to be used and helped with the interpretation of the acquired Mascot peptide files. R. A. performed the digestion of bovine β-LG, prefractionation and preparation for gel filtration. T. G. D. and M. J. participated in the research planning and interpretation of data. G. V. coordinated the research and designed the experiments. All authors contributed during the research planning, discussion of the results as well as during the revision of the manuscript and have approved the final version.
The authors report no conflict of interest.
Supplemental Appendix 1 is available online only at http://journals.cambridge.org/action/displayJournal?jid = bjn









