Introduction
Fibromyalgia is a chronic and often highly debilitating condition, with an estimated prevalence of 0.4–9.3% worldwide (Queiroz, Reference Queiroz2013), depending on diagnostic criteria. The disorder is characterised by multiple somatic symptoms, including widespread musculoskeletal pain, hyperalgesia, stiffness/numbness of the limbs, persistent fatigue, and headaches (CDC, 2017; Clauw, Reference Clauw2014). Diagnosis according to the most recent American College of Rheumatology criteria is based on the presence of generalised widespread pain throughout the body for at least 3 months and the absence of other neurological disorders to explain the underlying symptoms (Wolfe et al., Reference Wolfe, Clauw, Fitzcharles, Goldenberg, Hauser, Katz and Walitt2016). Individuals with a fibromyalgia diagnosis often experience difficulties with their psychological and cognitive functioning; sleep and memory disturbances, anxiety, and depression are not uncommon and may have a dramatic impact on quality of life (Dell'Osso et al., Reference Dell'Osso, Carmassi, Consoli, Conversano, Ramacciotti, Musetti and Bazzichi2011).
Fibromyalgia is often classified as a functional disorder, a term that refers to a group of disorders previously designated as (psycho)somatic or somatoform in nature, owing to the absence of a clear physical (structural) pathology (Barsky & Borus, Reference Barsky and Borus1999; Keynejad et al., Reference Keynejad, Frodl, Kanaan, Pariante, Reuber and Nicholson2019; Nater, Fischer, & Ehlert, Reference Nater, Fischer and Ehlert2011). Most models of the disorder propose a biopsychosocial aetiology, whereby physiological, psychological, and social factors contribute to illness onset (Low & Schweinhardt, Reference Low and Schweinhardt2012; McLean et al., Reference McLean, Williams, Stein, Harris, Lyden, Whalen and Baraniuk2006; Van Houdenhove & Egle, Reference Van Houdenhove and Egle2004). As such, fibromyalgia has been conceptualised as a ‘centralised pain state’ that can be triggered and maintained by stressful life events (Clauw, Reference Clauw2014), which can include biological factors, such as infections and physical injuries, as well as psychosocial stressors (e.g. relationship/workplace difficulties) and psychologically traumatic experiences (e.g. childhood maltreatment or assault). The integrative biopsychosocial model of fibromyalgia proposes that dysfunction within the hypothalamic pituitary adrenal (HPA) axis, the primary biological stress system, may play a key role in the disorder (Van Houdenhove & Egle, Reference Van Houdenhove and Egle2004). In brief, the HPA axis regulates the release of cortisol through a negative feedback loop. Prolonged or extreme exposure to stressors, however, can cause habituation of the stress response and lead to abnormal basal cortisol levels, amplifying painful sensations and sensitivity to stress. Fibromyalgia has consistently been linked with HPA axis dysregulation (Schmidt-Wilcke & Clauw, Reference Schmidt-Wilcke and Clauw2011), although it is unclear whether fibromyalgia is characterised by hypo- or hypercortisolism (Crofford et al., Reference Crofford, Young, Engleberg, Korszun, Brucksch, McClure and Demitrack2004; Fries, Hesse, Hellhammer, & Hellhammer, Reference Fries, Hesse, Hellhammer and Hellhammer2005). Despite this uncertainty, the observed impairments in HPA axis functioning suggest that individual-environment interactions in life, such as stressful life events, may have a direct impact on physical health (Nater et al., Reference Nater, Fischer and Ehlert2011).
Stressors may act as precipitating factors in the onset of physical health disorders (e.g. strokes), mental health disorders (e.g. depression), and functional somatic syndrome disorders, such as fibromyalgia. For example, meta-analytic reviews have found that individuals exposed to psychological trauma are 2.7 times more likely to develop functional somatic syndromes later in life (Afari et al., Reference Afari, Ahumada, Wright, Mostoufi, Golnari, Reis and Cuneo2014), whereas a history of sexual abuse is significantly associated with a lifetime diagnosis of numerous somatic disorders, including non-specific chronic pain (Paras et al., Reference Paras, Murad, Chen, Goranson, Sattler, Colbenson and Zirakzadeh2009). In addition, lifetime stressors are more commonly reported by patients with functional neurological disorder (Ludwig et al., Reference Ludwig, Pasman, Nicholson, Aybek, David, Tuck and Stone2018) and chronic fatigue syndrome (Hatcher & House, Reference Hatcher and House2003) than by healthy controls.
Extant evidence for the aetiological significance of stressors in adult fibromyalgia is equivocal. A small cross-sectional study (n = 50) found that 40% of fibromyalgia patients reported no exposure to stressors prior to disorder onset (Gonzalez, Baptista, Branco, & Ferreira, Reference Gonzalez, Baptista, Branco and Ferreira2013); however, as the study utilised the Life Events Checklist (Gray, Litz, Hsu, & Lombardo, Reference Gray, Litz, Hsu and Lombardo2004), which focuses on traumatic events relevant to posttraumatic stress disorder, chronic stressors occurring in the context of daily life may have been missed. Indeed, semi-structured interviews conducted by trained researchers are considered to be a more precise and reliable method for ascertaining stressful life events than self-report checklists (Wethington, Brown, & Kessler, Reference Wethington, Brown, Kessler, Cohen, Kessler and Gordon1995), in part, because the former are not restricted to a set of specific pre-defined events. Additionally, a longitudinal cohort study examining the impact of the 9/11 terrorist attacks on female New York/New Jersey residents found no association between exposure to the event and subsequent development of fibromyalgia (Raphael, Natelson, Janal, & Nayak, Reference Raphael, Natelson, Janal and Nayak2002). The focus on such a rare, unique event, and the inclusion of only female participants, limits the generalisability of these findings; however, the results are consistent with studies showing that prolonged exposure to stressors, rather than exposure to a singular traumatic event, is more likely to lead to appearance of functional symptoms (Hatcher & House, Reference Hatcher and House2003; Ludwig et al., Reference Ludwig, Pasman, Nicholson, Aybek, David, Tuck and Stone2018; Nater et al., Reference Nater, Fischer and Ehlert2011). Indeed, studies of fibromyalgia patients suggest a dose–response relationship between stress and illness severity, where the number of lifetime stressors has been associated with worsening of physical symptoms (Filippon, Bassani, Aguiar, & Ceitlin, Reference Filippon, Bassani, Aguiar and Ceitlin2013; Loevinger, Reference Loevinger2012). Evidence, as supported by a critical review of 42 studies, suggests that fibromyalgia and other functional somatic syndromes are associated with interpersonal abuse (Romans & Cohen, Reference Romans and Cohen2008). Furthermore, a systematic review with meta-analysis determined fibromyalgia was significantly associated with self-reported sexual and physical abuse in childhood and adulthood, but not with emotional abuse (Hauser, Kosseva, Uceyler, Klose, & Sommer, Reference Hauser, Kosseva, Uceyler, Klose and Sommer2011). On the contrary, another meta-analytic review found that both chronic fatigue syndrome and fibromyalgia were associated with childhood stressors, such as physical and emotional neglect, as well as all three types of abuse (i.e. physical, sexual, and emotional) (Borsini, Hepgul, Mondelli, Chalder, & Pariante, Reference Borsini, Hepgul, Mondelli, Chalder and Pariante2014).
Although there have been previous systematic reviews examining the link between stressors and fibromyalgia, these have focused on specific exposures (i.e. physical, sexual, or emotional abuse; Hauser et al., Reference Hauser, Hoffmann, Wolfe, Worthing, Stahl, Rothenberg and Walitt2015) or specific time-periods (i.e. exposures occurring in childhood; Borsini et al., Reference Borsini, Hepgul, Mondelli, Chalder and Pariante2014); moreover, only one included a meta-analysis. As such, the extent to which the association between stressor exposure and fibromyalgia is consistent across stressor types and time-periods is unclear. We therefore aimed to systematically assess the relationship between all types of stressors described in the literature to-date (from physical injuries through to emotional neglect), with no restriction placed on the time of exposure. By performing a series of meta-analyses, we aimed to quantify and compare associations between specific exposures and fibromyalgia. In doing so, we hope to provide patients and clinicians with a more complete picture of the full range of exposures (both psychosocial and physical) that might contribute to this complex disorder. Moreover, by performing a robust assessment of study quality/risk of bias, we aimed to highlight methodological issues common to this field which may serve as a useful guide for future studies.
Method
Search strategy
This review was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009; see online Supplementary Table S1 for a detailed item report). The protocol for this review was prepared by a team of researchers in the fields of functional disorders (TC and TN) and a stressful life events expert (TH). Our search strategy was guided by a previous review (Ludwig et al., Reference Ludwig, Pasman, Nicholson, Aybek, David, Tuck and Stone2018) in that our search terms were deliberately broad so as to maximise the identification of relevant papers. Electronic databases (Embase, Medline, PsycINFO, PubMed, and Web of Science, through January 2018) were searched by the lead author (NK) for the following terms: (‘fibromyalgia’ OR ‘chronic pain’) AND (‘abuse’ OR ‘life event’ OR ‘stress’ OR ‘trauma’) AND (‘control’ OR ‘controlled’ OR ‘case-control’). Medical Subject Headings (MeSH terms) were also included in order to obtain all possible results. References of relevant articles and reviews were manually inspected for other potentially relevant articles.
Eligibility criteria
Eligible studies were case-control studies available in English as full publications in a peer-reviewed scientific journal. Studies were included regardless of their sample size or date of publication. Studies were required to have assessed at least one type of stressor and include a control group. Persons over the age of 18 years, diagnosed with fibromyalgia according to any criteria, were included. Studies with no control group, dissertations, and conference abstracts were excluded.
Data extraction
Data extraction was performed independently by two authors (NK and RE); a third author (AEC) checked all data used to compute effect sizes. Any discrepancies were discussed and resolved following a joint review of the primary publication. The following variables were extracted for all eligible studies: year of publication, sample size, source of fibromyalgia cases (e.g. community, tertiary treatment centre), control group type (e.g. healthy controls, other patient group), psychosocial stress measure, time-period of stressor assessment, total number of participants providing useable data in fibromyalgia cases and controls, and number exposed to stressor per group.
Appraisal of methodological quality
Study quality was assessed using a modified version of the Newcastle-Ottawa Scale for Quality Assessment of case-control studies (Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014). The original scale includes eight items, each awarded a score of 1 point, except for the comparability category (worth 2 points), yielding a maximum of 10 points. For the purposes of this review, we modified the scale to include an extra category evaluating the method of fibromyalgia diagnosis and added a third item within the comparability category, where sex, psychiatric comorbidity, and disease duration were chosen as potential confounders. Using the modified scale, items could be awarded 1–3 points, with a maximum score of 19 points (see online Supplementary Tables S2 and S3 for a detailed description of our scoring strategy). Studies which included hospital patients as a control group type were not assessed on the ‘Definition of controls’ item and could receive a maximum score of 18. Thus, individual scores were standardised to obtain a comparable percentage and study quality was categorised as follows: low (0–25%), moderate (26–50%), good (51–75%), and excellent (76–100%). Study quality was independently assessed by two authors (NK and RE). A third author (TC) was consulted to resolve any inconsistencies.
Statistical analysis
For meta-analyses, we computed odds ratios (ORs) with 95% confidence intervals (CIs) for binary exposures (exposed to stressor v. not exposed) for all studies where these data were reported. As not all studies accounted for potential confounders in analyses, we used raw data from each study (number of individuals with stress exposure/number of individuals without stress exposure) to compute ORs rather than using author-reported ORs derived from analyses adjusted for potential confounders. A continuity correction of 0.5 was applied to cells with zero counts (Friedrich, Adhikari, & Beyene, Reference Friedrich, Adhikari and Beyene2007). Only one eligible study (Bayram & Erol, Reference Bayram and Erol2014) provided continuous data only (scores on a measure of childhood trauma); authors of the original study were contacted to provide binary data, but, as this was not provided, this study was not included in meta-analyses.Footnote 1Footnote ‡ All other studies provided binary data only or a combination of continuous and binary data (where the latter only was included in meta-analyses). Thresholds used to define stressor exposure varied across studies, with some using a single questionnaire item to determine exposure (Anderberg, Marteinsdottir, Theorell, & von Knorring, Reference Anderberg, Marteinsdottir, Theorell and von Knorring2000) and others using cut-off scores on multi-item questionnaires (Hellou et al., Reference Hellou, Hauser, Brenner, Buskila, Jacob, Elkayam and Ablin2017). As such, the severity of stressor exposure varied considerably.
Nearly all studies provided data on multiple forms of stressors, with many assessing each stressor across multiple time-periods. To avoid violating the independence of observations assumption, only one set of data from each study was included in a single analysis. This was addressed using the following procedures: (i) for stressors that were reported in at least three studies, separate meta-analyses were performed for each stressor type; (ii) where studies provided data for the same stressor across multiple time-periods, we used a hierarchy to select a time-period for inclusion in the main analysis (described below); (iii) for studies that provided multiple items relating to the same form of stressor (e.g. for childhood sexual abuse, molestation, and rape), where no overall exposure variable was provided, we selected the item that was most comparable to the definitions used in other studies; and (iv) for studies that examined exposure to multiple individual stressors, authors were contacted and requested to provide information on the number of individuals exposed to at least one stressful life event per group (Olivieri, Solitar, & Dubois, Reference Olivieri, Solitar and Dubois2012; Varinen, Kosunen, Mattila, Koskela, & Sumanen, Reference Varinen, Kosunen, Mattila, Koskela and Sumanen2017), with data subsequently obtained for one study (Varinen et al., Reference Varinen, Kosunen, Mattila, Koskela and Sumanen2017).
Meta-analyses were conducted in Stata version 15 using the ‘metan’ command. Given that group characteristics and recruitment methods for both the fibromyalgia and control groups varied considerably across studies, random-effects models were employed for all analyses with inverse weighting applied (DerSimonian & Laird, Reference DerSimonian and Laird1986). In the primary analyses, we used the following hierarchy to select effect sizes for inclusion in models when studies reported exposure to the same stressor at multiple time-periods: (i) lifetime exposure was selected for studies that reported lifetime, adult, and childhood exposure; (ii) adult exposure was selected for studies that included both adult and child time-periods (but not lifetime); and (iii) childhood exposure was included for studies that reported this time-period only. This procedure ensured that we maximised the number of studies contributing to each stressor type analysis. We then performed sensitivity analyses where we included childhood exposure for studies reporting adult and childhood only. Statistical significance was set at p < 0.05 (two-tailed) for all analyses.
Statistically significant heterogeneity was determined via the Cochran Q statistic. We additionally derived the I 2 statistic (estimating the percentage of the variability in ORs due to heterogeneity) categorised as likely ‘unimportant’ (0–40%), ‘moderate’ (30–60%), ‘substantial’ (50–90%), or ‘considerable’ (75–100%) depending on the magnitude/direction of effects and statistical significance of heterogeneity (Higgins & Green, Reference Higgins and Green2008). As our outcome measure was binary, and we anticipated substantial heterogeneity, small sample bias (aka publication bias) was assessed visually by means of a funnel plot (Sterne et al., Reference Sterne, Sutton, Ioannidis, Terrin, Jones, Lau and Higgins2011). To determine the influence of study characteristics (year of publication and standardised NOS score), univariable meta-regression analyses were performed using the ‘metareg’ command. As year of publication was not normally distributed, we derived a categorical variable for these analyses (pre-2005 v. 2005 or later). Funnel plots and meta-regression analyses were only performed for exposures for which 10 or more effect sizes were available.
Results
Literature search
A total of 2112records were identified through the database searches and other sources (see Fig. 1). After initial screening, 34 full-text articles were assessed for eligibility. Of those, six studies were excluded because they did not examine our outcome of interest, six were excluded due to a lack of control group, and two were excluded for not having a clearly defined fibromyalgia group. Twenty case-control studies were therefore eligible for inclusion in the literature review (Aaron et al., Reference Aaron, Bradley, Alarcon, Triana-Alexander, Alexander, Martin and Alberts1997; Al-Allaf et al., Reference Al-Allaf, Dunbar, Hallum, Nosratzadeh, Templeton and Pullar2002; Alexander et al., Reference Alexander, Bradley, Alarcon, Triana-Alexander, Aaron, Alberts and Stewart1998; Anderberg et al., Reference Anderberg, Marteinsdottir, Theorell and von Knorring2000; Bayram & Erol, Reference Bayram and Erol2014; Boisset-Pioro, Esdaile, & Fitzcharles, Reference Boisset-Pioro, Esdaile and Fitzcharles1995; Broderick & Ross, Reference Broderick and Ross2005; Carpenter et al., Reference Carpenter, Hugler, Enzenauer, Des Rosier, Kirk and Brehm1998; Castro et al., Reference Castro, Barrantes, Tuna, Cabrera, Garcia, Recinos and Garcia-Kutzbach2005; Ciccone, Elliott, Chandler, Nayak, & Raphael, Reference Ciccone, Elliott, Chandler, Nayak and Raphael2005; Haviland, Morton, Oda, & Fraser, Reference Haviland, Morton, Oda and Fraser2010; Hellou et al., Reference Hellou, Hauser, Brenner, Buskila, Jacob, Elkayam and Ablin2017; Imbierowicz & Egle, Reference Imbierowicz and Egle2003; Naring, van Lankveld, & Geenen, Reference Naring, van Lankveld and Geenen2007; Olivieri et al., Reference Olivieri, Solitar and Dubois2012; Ruiz-Perez et al., Reference Ruiz-Perez, Plazaola-Castano, Caliz-Caliz, Rodriguez-Calvo, Garcia-Sanchez, Ferrer-Gonzalez and Lopez-Chicheri Garcia2009; Smith et al., Reference Smith, Papp, Tooley, Montague, Robinson and Cosper2010; Taylor, Trotter, & Csuka, Reference Taylor, Trotter and Csuka1995; Varinen et al., Reference Varinen, Kosunen, Mattila, Koskela and Sumanen2017; Walker et al., Reference Walker, Keegan, Gardner, Sullivan, Bernstein and Katon1997).
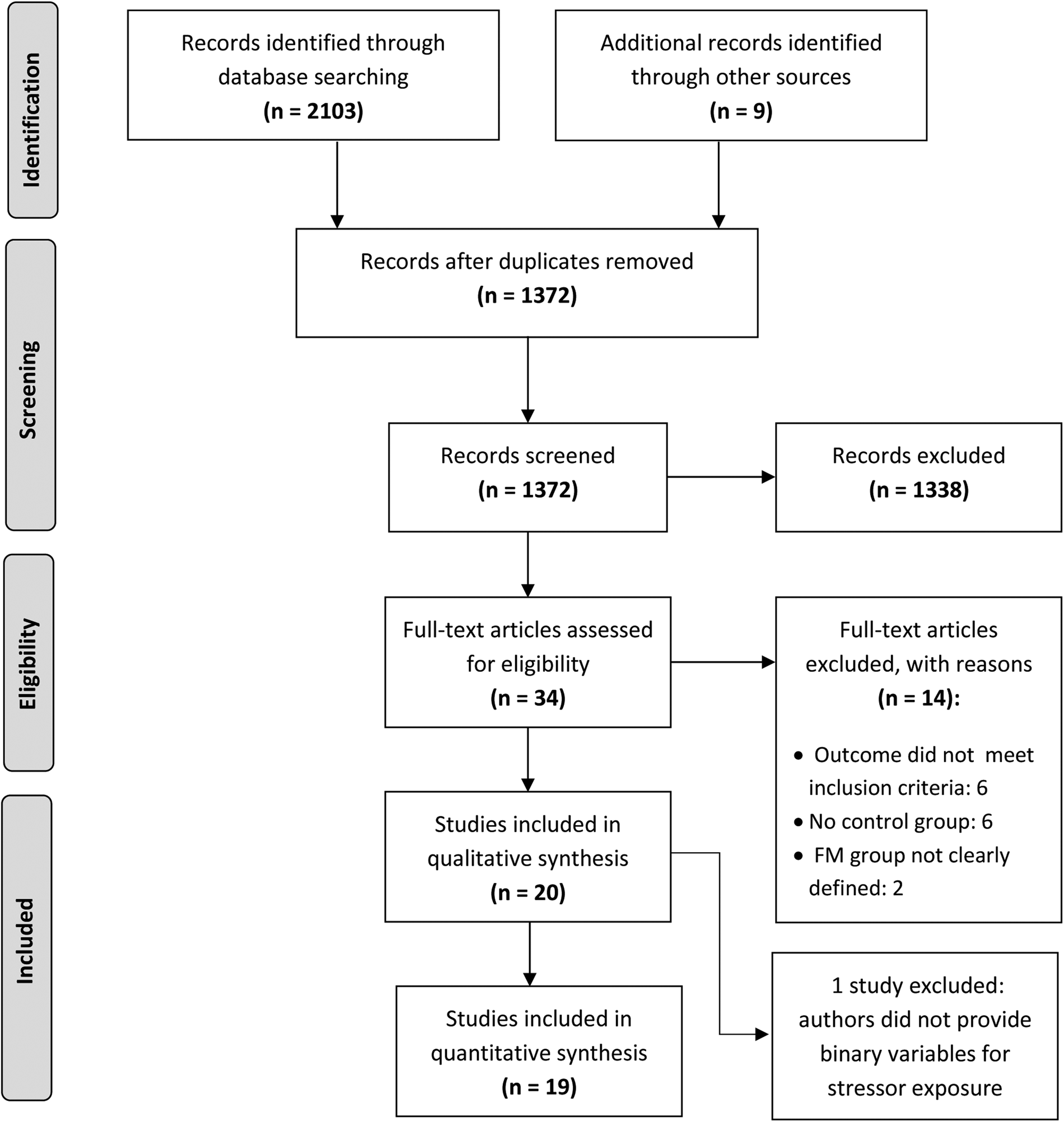
Fig. 1. Systematic search process.
Description of study characteristics
Study characteristics are presented in Table 1. The majority were conducted in North America (n = 11; 55%) or Europe (n = 7; 35%). Most (n = 17; 85%) recruited fibromyalgia patients from secondary and tertiary care practices, two recruited from the general population, and one did not specify a recruitment site. With regards to fibromyalgia diagnosis, 13 (65%) studies employed American College of Rheumatology criteria, four (20%) used physician diagnosis (without specifying the criteria used), two (10%) relied on self-reported diagnosis, and one (5%) used a tender point count. There was substantial variation in the type of control group used, ranging from hospital-based controls with other medical issues (n = 10; 50%), healthy individuals recruited from the community (n = 7; 35%), or a combination of both (n = 2; 10%). Notably, two studies (Aaron et al., Reference Aaron, Bradley, Alarcon, Triana-Alexander, Alexander, Martin and Alberts1997; Alexander et al., Reference Alexander, Bradley, Alarcon, Triana-Alexander, Aaron, Alberts and Stewart1998) utilised a fibromyalgia non-patient control group (i.e. people who met fibromyalgia diagnostic criteria but never sought medical attention for their symptoms) and a healthy control group; however, for the purposes of this review, only the healthy control group was included in analyses. Types of stressors assessed by different studies are presented in Table 1. Studies assessed stressors across multiple time periods (e.g. childhood, adulthood, or in the lifetime as a combined measure of stressors in childhood and adulthood), whereas two studies (10%) (Al-Allaf et al., Reference Al-Allaf, Dunbar, Hallum, Nosratzadeh, Templeton and Pullar2002; Anderberg et al., Reference Anderberg, Marteinsdottir, Theorell and von Knorring2000) included a timeframe of inquiry with specific reference to fibromyalgia onset (e.g. 6 months prior to symptom onset and at onset).
Table 1. Case-control studies of stressors in patients with fibromyalgia and controls
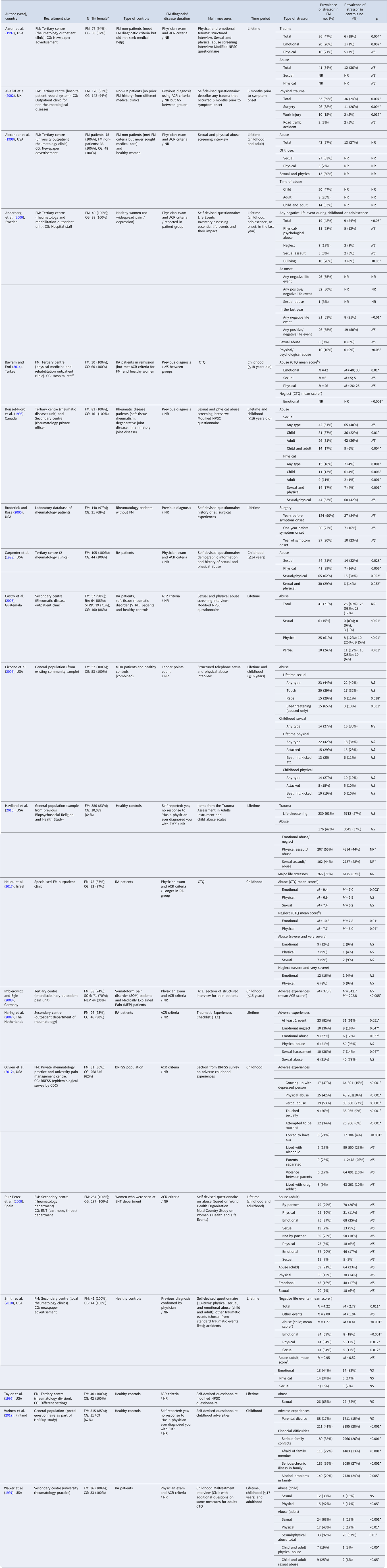
FM, fibromyalgia; CG: control group; NS, not significant; NR, not reported; ACR, American College of Rheumatology; RA, rheumatoid arthritis; STRD, soft tissue rheumatic disorder; MEP, medically explained pain; MDD, major depressive disorder; CTQ, Childhood Trauma Questionnaire; ACE, adverse childhood experiences; NPSC, National Population Survey of Canada; BRFSS, Behavioral Risk Factors Surveillance System.
Notes. The table refers to each stressor the way it was originally referred to in each study in order to avoid confusion.
a This column indicates the number and percentage (compared to the total number of participants) of female participants for the FM and control groups.
b Mean scores are provided instead of prevalence rates wherever these were not reported within the original study data.
A ‘*’ sign was used to indicate a result of statistical significance.
Quality appraisal
Twelve studies (60%) were deemed to be of ‘moderate’ quality (26–50%), with a further eight (40%) characterised as ‘good’ quality (see Table 2). No studies were of ‘low’ or ‘excellent’ quality. Within the subcategories of the quality assessment, methods used to ascertain exposure to stressor required the most improvement; 15 (75%) studies received only 1 of 3 possible points for using self-devised questionnaires, while only one study received maximum points for using a blinded interview (Imbierowicz & Egle, Reference Imbierowicz and Egle2003). Similarly, only one study received 2/2 for the definition of exposure category (Naring et al., Reference Naring, van Lankveld and Geenen2007), where prior exposure to stressors was validated by an independent source (e.g. primary records). Most studies received low scores in the comparability category due to the predominance of female participants: 11 studies (55%) included only female participants, and of the nine studies that included both sexes, only one reported rates of previous stressors for male and female participants separately. Furthermore, psychiatric comorbidities and fibromyalgia disease duration were included as covariates in the statistical analyses of only a few studies, which could have resulted in an over-estimation of the number of stressors associated with adult fibromyalgia. Additional weaknesses included: (i) recruitment of controls from hospital settings only or from different populations, (ii) using a self-reported fibromyalgia diagnosis, and (iii) using various criteria to ascertain fibromyalgia diagnosis.
Table 2. Quality assessment of case-control studies based on adapted version of NOS (Wells et al., Reference Wells, Shea, O'Connell, Peterson, Welch, Losos and Tugwell2014)
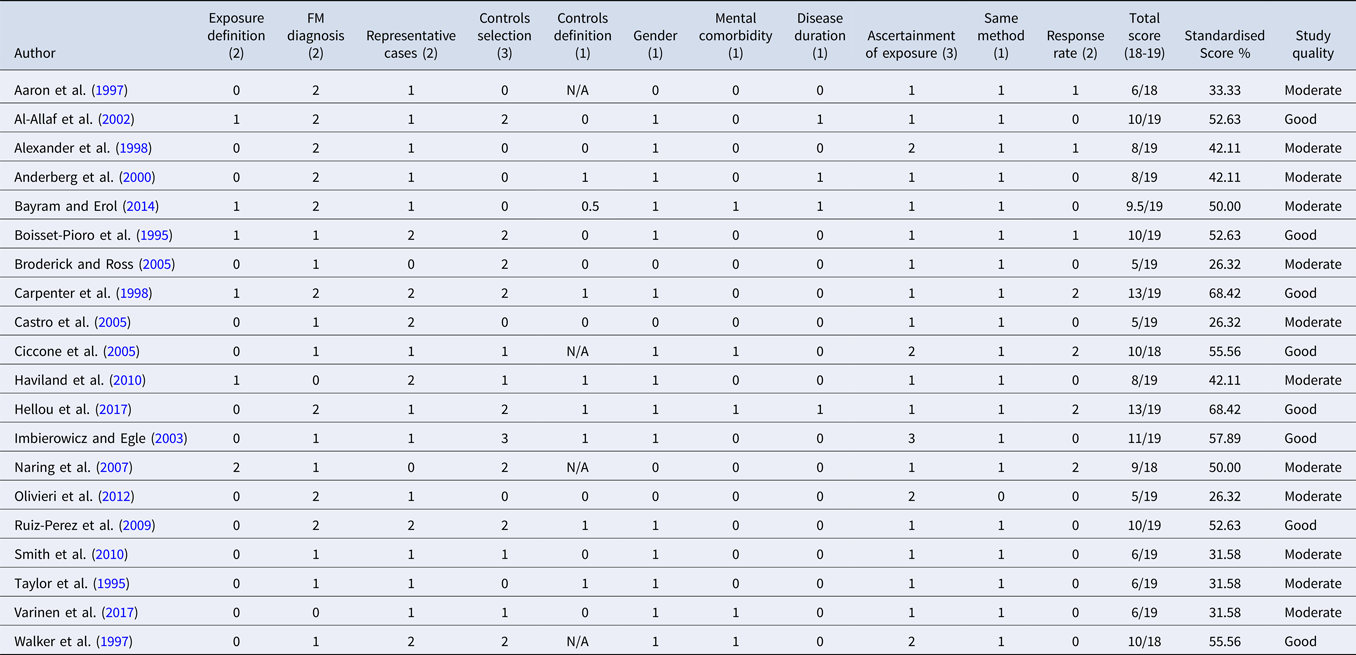
Notes. FM, fibromyalgia. For each item, number in parantheses indicates maximum score available;studies which included hospital patients as a control group type were not assessed on the ‘Definition of controls’ item and could receive a maximum score of 18 points. Individual scores were standardised to obtain a comparable percentage and categorised as follows: low quality (0–25%), moderate quality (26–50%), good quality (51–75%), and excellent quality (76–100%).
Meta-analysis
Table 1 presents the results of all statistical analyses conducted by study authors, as reported in the primary publications; as we only performed meta-analyses on binary data when more than three effect sizes were available, not all data provided in Table 1 were included in meta-analyses. All studies but one (Bayram & Erol, Reference Bayram and Erol2014) provided binary variables for stressor exposure (exposed v. not exposed). This study compared scores on the abuse subscales of the Childhood Trauma Questionnaire (Bernstein & Fink, Reference Bernstein and Fink1988) among fibromyalgia patients and healthy females and observed significantly higher scores on the emotional abuse subscale in fibromyalgia patients (p = 0.01), but no significant group differences on the sexual and physical abuse subscales.
Primary analyses
Three or more effect sizes were available for the following binary exposures: total abuse (n = 9), physical abuse (n = 12), sexual abuse (n = 14), emotional abuse (n = 5), other lifetime stressors (n = 3), and medical trauma (n = 3). In the primary analyses, significant associations with fibromyalgia status were observed for all six exposure types (see Fig 2; Table 3). Effect sizes were largest for physical abuse (OR 3.23, 95% CI 1.99–5.23) and total abuse (3.06, 1.71–5.46), intermediate for sexual abuse (2.65, 1.85–3.79), and somewhat smaller but not insignificant for medical trauma (1.80, 1.19–2.71), other lifetime stressors (1.70, 1.31–2.20), and emotional abuse (1.52, 1.27–1.81). Substantial heterogeneity (I 2 range: 54–81%) was observed for all exposures except for emotional abuse and medical trauma, with statistically significant heterogeneity observed for total abuse, physical abuse, and sexual abuse (p < 0.001).
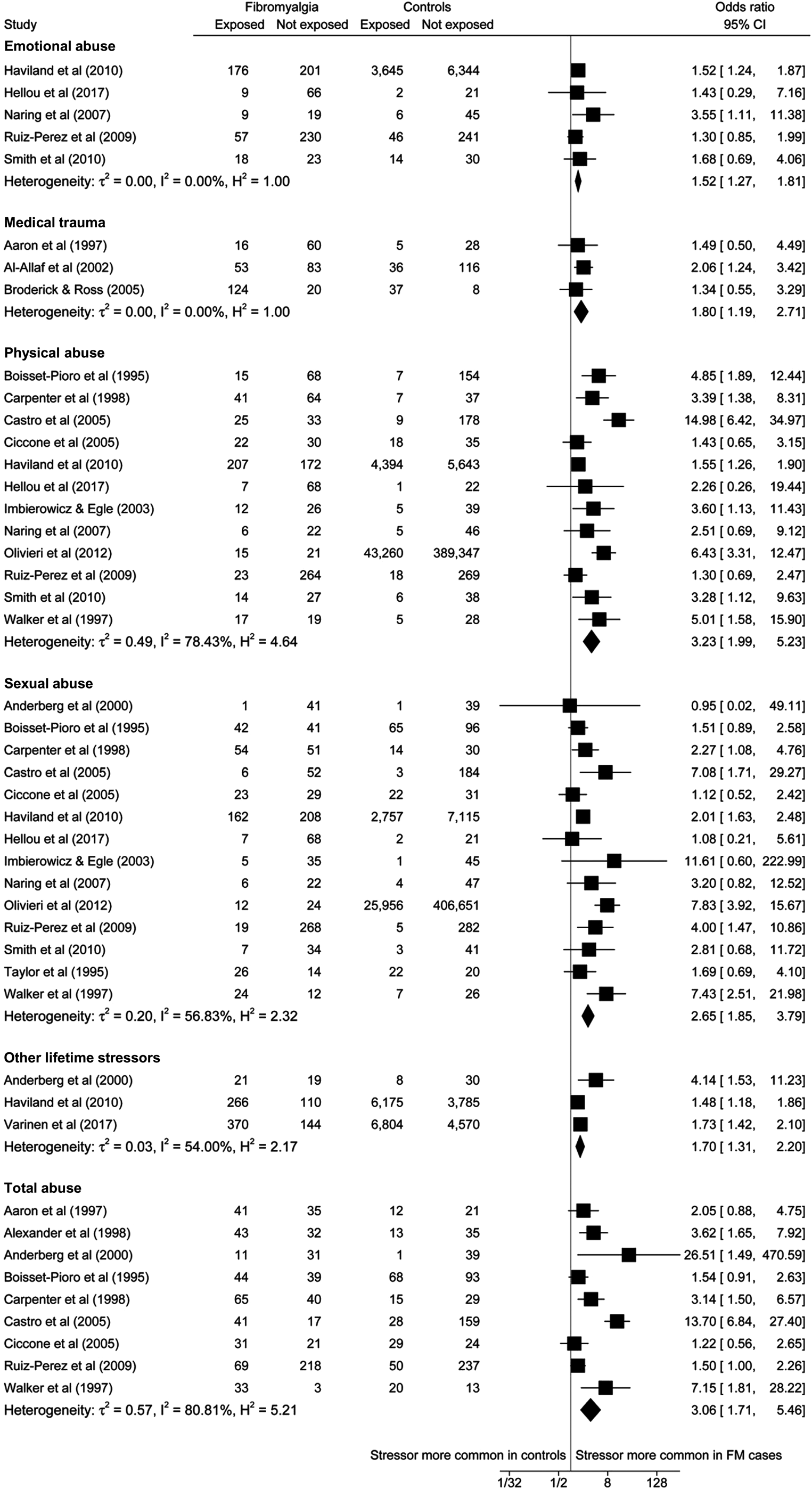
Fig. 2. Random effects meta-analyses examining associations between stress exposure and fibromyalgia.
Table 3. Results of random effects meta-analyses examining association of stressor exposure and fibromyalgia
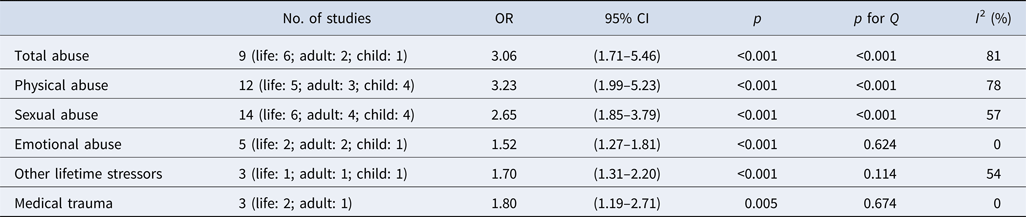
Notes. OR, odds ratio; CI, confidence interval; Q, Cochran's Q to detect statistically significant heterogeneity, p < 0.05 indicates significant heterogeneity; I 2, variation in OR attributable to heterogeneity.
Sensitivity analyses
We additionally performed sensitivity analyses where we included childhood exposure (as opposed to adult exposure) for studies that did not report lifetime exposure. Sensitivity analyses were performed for all exposures except for medical trauma (for which no studies examined exposure in childhood) and yielded a similar pattern of results to the primary analyses: physical abuse (3.10, 1.89–5.08), total abuse (2.65, 1.42–4.96), sexual abuse (2.16, 1.57–2.99), emotional abuse (1.87, 1.05–3.33), and other lifetime stressors (1.64, 1.38–1.95).
For exposures with 10 or more effect sizes (physical and sexual abuse), we examined small sample bias by means of funnel plots with pseudo 95% CIs and conducted meta-regression analyses to examine the effect of year of publication and study quality score. Both were performed on effect sizes derived from primary analyses only. Slight asymmetry was observed in the funnel plot for physical abuse; studies with lower precision (standard error of log-transformed OR < 0.5) all reported positive associations between fibromyalgia status and stressor exposure (see online Supplementary Figs. S4 and S5 for visuals on our assessment of small sample bias). For sexual abuse, however, there was no clear evidence of asymmetry. Publication year and quality score were not significantly associated with effect sizes for either physical abuse or sexual abuse in meta-regression analyses (p > 0.05 for all).
Discussion
This systematic review and meta-analysis of 20 case-control studies indicated significant associations between fibromyalgia diagnosis and several forms of psychosocial stress exposure. To our knowledge, this is the first review to summarise the existing case-control evidence on such a broad spectrum of stressors and adult fibromyalgia. Despite the limitations of the available literature, we were able to find substantial evidence for a significant association between exposure to stressors in the lifetime and fibromyalgia. Specifically, total abuse (as a combined measure of all abuse types) and physical abuse had the strongest association with fibromyalgia. Sexual abuse, emotional abuse, other lifetime stressors, and medical trauma were also respectively associated with fibromyalgia. These findings are consistent with previous reviews on stressful events and functional somatic syndrome disorders (Coppens et al., Reference Coppens, Van Wambeke, Morlion, Weltens, Giao Ly, Tack and Van Oudenhove2017; Filippon et al., Reference Filippon, Bassani, Aguiar and Ceitlin2013; Paras et al., Reference Paras, Murad, Chen, Goranson, Sattler, Colbenson and Zirakzadeh2009; Romans & Cohen, Reference Romans and Cohen2008), although it is important to understand these environmental risks in terms of their interacting role with other biological factors.
Our findings provide further support for the hypothesis that sustained exposure to environmental stressors likely leads a habituation to the stress response, thereby disrupting normal production of cortisol by the HPA axis. Although studies disagree on whether fibromyalgia is characterised by hypo- or hypercortisolism (Crofford et al., Reference Crofford, Young, Engleberg, Korszun, Brucksch, McClure and Demitrack2004; Fries et al., Reference Fries, Hesse, Hellhammer and Hellhammer2005), similar dysregulation patterns have been observed in other stress-related disorders and can reflect mechanistic heterogeneity within patient populations as well as a lack of definitive studies of these disorders. For example, dysregulated cortisol levels have been found in functional neurological disorder, chronic fatigue syndrome, and post-traumatic stress disorder patients (Fries et al., Reference Fries, Hesse, Hellhammer and Hellhammer2005; Keynejad et al., Reference Keynejad, Frodl, Kanaan, Pariante, Reuber and Nicholson2019; Yehuda et al., Reference Yehuda, Southwick, Nussbaum, Wahby, Giller and Mason1990). HPA axis hyperactivity and increased basal cortisol levels, on the contrary, have been observed in depressed and chronic pain patients, where higher levels were associated with a history of more severe childhood abuse (Nicolson, Davis, Kruszewski, & Zautra, Reference Nicolson, Davis, Kruszewski and Zautra2010). Similarly, in fibromyalgia patients, increased cortisol levels upon awakening were associated with a history of childhood sexual and physical abuse, suggesting a link between stressful life events and long-term changes in HPA axis functioning (Weissbecker, Floyd, Dedert, Salmon, & Sephton, Reference Weissbecker, Floyd, Dedert, Salmon and Sephton2006).
We suggest the adoption of a multidisciplinary approach to the diagnosis and treatment of fibromyalgia – all patients should be routinely and sensitively assessed for past abuse, as well as pre-onset ongoing difficulties, and recent stressful life events. Treatment should target both psychological and physiological symptoms, while taking into consideration moderating factors, including mental health comorbidities. Given the association between lifetime stressors and fibromyalgia, the development and evaluation of an intervention which targets emotional regulation may improve patients' ability to appropriately appraise stressful situations and select appropriate coping strategies (Gross, Uusberg, & Uusberg, Reference Gross, Uusberg and Uusberg2019). For instance, assessing and reframing unhealthy affect is central to psychotherapeutic approaches such as cognitive-behavioural therapy. Thus, accounting for lifetime stressors during formulation and articulating their impact on physiological processes (e.g. HPA axis) and psychological processes (e.g. affect regulation) may be beneficial to the patient.
Limitations
As our study protocol was not publicly registered (e.g. via PROSPERO), our analysis strategy may have been unconsciously biased by the study findings. However, we consulted the PRISMA guidelines prior to study commencement and ensured our protocol was fully concordant (see online Supplementary Table S1); moreover, our analysis strategy was developed independently by one author (AEC) who was not involved in the search process or the initial data extraction. Our decision to exclude dissertations and conference abstracts is a potential limitation of the study. We were keen to only include studies that had undergone rigorous peer review (which is not always the case for these article types) but acknowledge that this may have contributed to publication bias. Importantly, the funnel plots provided no clear evidence of this. As a final issue, our meta-analyses were limited to stressors that were examined as binary variables (exposure v. no exposure) in three or more studies. As such, our analyses do not capture the full range of stressors examined in primary publications.
Our findings are also limited by the characteristics of the included studies, in particular, the prevailing use of questionnaire measures to ascertain stressor exposure allows for a less exhaustive and sensitive inquiry of stressful life events. Interview methods cover a wider range of events, yield more accurate rates of reported stressors, and allow for a more contextualised manner of inquiry that considers individual differences, e.g. in duration of impact, as well as abuse frequency and severity (Wethington et al., Reference Wethington, Brown, Kessler, Cohen, Kessler and Gordon1995). Moreover, studies rarely accounted for the duration of fibromyalgia when inquiring about stressors in adulthood and could have, therefore, included events that occurred following disease onset. We adopted a consistent approach for dealing with studies reporting stressors in more than one time-period: when child and adult stressor exposures were reported separately, we used the latter in primary analyses. Although this strategy may have increased the possibility that stressors could have occurred after fibromyalgia onset, adult exposures were more commonly reported, and we were keen to reduce heterogeneity in our primary analysis. Importantly, our sensitivity analyses using childhood exposures in place of adult exposures were consistent with our primary analyses. A further limitation pertains to the fact that many studies included ‘control’ groups with other medical issues: given that psychosocial stress is likely a common risk factor for many physical and psychiatric disorders, this may have underestimated the true association between fibromyalgia and stress exposure. Additional study limitations identified by the quality assessment were the variable methods used to ascertain fibromyalgia diagnosis (e.g. self-report, clinical diagnosis, and fulfilment of formal criteria) as there is evidence to suggest that these different approaches do not always converge (Katz, Wolfe, & Michaud, Reference Katz, Wolfe and Michaud2006), lack of operationalised definitions of stressors, variability in time-frames for stressor exposure (ranging from 6 months prior to fibromyalgia symptom onset, to the entire lifespan), lack of ascertainment of exposure by comparison with external records, and recruitment biases, as patients recruited from tertiary centres might have experienced a higher number of stressors and might present with psychiatric comorbidities. Finally, as we computed ORs from raw data (rather than using precomputed effect sizes reported in original studies) our analyses were not adjusted for covariates; as such, we cannot rule out the possibility that confounding factors (e.g. participant sex and psychiatric comorbidity) contributed to the associations we observed between stressors and fibromyalgia. As a related issue, the data extracted for meta-analyses were derived from disproportionately female populations (across both fibromyalgia and control groups, the majority were female). Although this limits the generalisability of findings to males, it is important to note the prevalence of this fibromyalgia is higher in women than men (Arout, Sofuoglu, Bastian, & Rosenheck, Reference Arout, Sofuoglu, Bastian and Rosenheck2018). Further studies, that purposively recruit male fibromyalgia patients and controls, are needed to establish whether the observed association with stressor exposure also holds for males.
Conclusions
In summary, this is the first systematic meta-analytic review to summarise the existing case-control evidence on a broad spectrum of stressors and adult fibromyalgia. Although this relationship has yet to be fully explored, we demonstrated a strong association between experiences of stressors, including three types of abuse and various other stressful events in the lifetime, and a fibromyalgia diagnosis in adulthood. Based on the reviewed literature, we propose those can act as precipitating factors, triggering the experience of fibromyalgia symptoms, as well as predisposing factors, which accumulate over time and exert a detrimental impact on biological stress regulation systems. The adoption of emotion-regulation interventions is proposed in order to enable patients to better understand past stressful events and prepare them to be better equipped in handling stressful situations in the future, which would help reduce the experience of fibromyalgia symptoms. We further recommend that future research employ more robust and higher-quality designs, as well as the use of interview ascertainment methods in order to corroborate and expand the findings of our review.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004547.
Acknowledgements
We would like to thank all authors who provided additional statistics for this review. TC acknowledges financial support from the Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry at King's College London. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author contributions
TC had the idea for the study. TH, TN, TC, NK, and RE contributed to the study conception and design. NK did the systematic literature search. NK and RE selected studies for inclusion and extracted data. AEC checked data for accuracy. NK and RE assessed the quality of the studies, TC was consulted to resolve any disagreements. AEC conducted the statistical analyses and produced the figures. NK wrote and edited the manuscript drafts; AEC wrote the results from the meta-analyses; RE wrote the abstract. All authors critically revised the manuscript and analyses for important intellectual content and approved the final draft.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflict of interest
None.








