Introduction
Patients with Parkinson’s disease (PD) are burdened not only with the motor symptoms of the disease which include rest tremor, bradykinesia, rigidity, and postural instability but also with a plethora of nonmotor symptoms (NMS). Reference Titova, Qamar and Chaudhuri1 Psychosis is one of the debilitating NMS of PD which markedly worsens the quality of life and is linked to malignant long-term outcomes. Reference Merola, Romagnolo and Dwivedi2 Psychosis in PD usually manifests with well-formed visual hallucinations (VH) or minor hallucinations (MH) such as illusions and a false sense of presence or passage. Reference Ffytche, Creese and Politis3,Reference Lenka, Pagonabarraga, Pal, Bejr-Kasem and Kulisvesky4 Hallucinations of the nonvisual modalities (auditory, olfactory, tactile) and delusions are relatively uncommon and when present, usually coexist with VH and MH. Reference Ffytche, Creese and Politis3,Reference Lenka, Pagonabarraga, Pal, Bejr-Kasem and Kulisvesky4 Early identification and prompt management of PD patients with psychosis (PD-P) are crucial as psychosis is associated with significantly higher rates of nursing home placement, caregiver distress, healthcare expenditure, and mortality in PD patients. Reference Forsaa, Larsen, Wentzel-Larsen and Alves5,Reference Fredericks, Norton, Atchison, Schoenhaus and Pill6 Although the precise mechanism of the emergence of psychosis in PD has remained elusive, several associations have been elucidated in the previous studies, some of which are with older age, rapid eye movement sleep (REM) behavior disorder (RBD), depression, and cognitive dysfunction. Reference Ffytche, Pereira, Ballard, Chaudhuri, Weintraub and Aarsland7,Reference Lenka, George and Arumugham8
The spectrum of cognitive dysfunction in PD ranges from mild cognitive impairment to dementia. Reference Simon-Gozalbo, Rodriguez-Blazquez, Forjaz and Martinez-Martin9 Previous studies have reported that PD-P have a greater risk of developing cognitive impairment and PD patients with cognitive impairment have a greater risk of having psychosis, thus highlighting a close association of psychosis and cognitive impairment in PD. Reference Lenka, Hegde, Jhunjhunwala and Pal10 Several studies have compared the cognitive parameters of PD-P and PD patients with no psychosis (PD-NP) and documented impairments across all the cognitive domains in the former, more prominent in the executive domain. Reference Lenka, Hegde, Arumugham and Pal11 However, the criteria used for the diagnosis of PD-P were not uniform across the previous studies. The majority of the studies on this topic were based on small sample sizes and were published prior to the availability of the diagnostic criteria proposed by the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke (NIMH-NINDS criteria). Reference Ravina, Marder and Fernandez12 Moreover, the cognitive correlate of MH in PD was never the theme of the previous studies. Hence, the current study aims to explore the cognitive correlates of MH and VH in PD in a larger cohort of patients who were diagnosed using the well-accepted NINDS-NIMH criteria.
Methods
Subject Recruitment and Clinical Assessment
This study was part of a large project aimed at identifying the biomarkers of psychosis in PD which was conducted at the National Institute of Mental Health and Neurosciences, Bangalore, India. Fifty-one PD-NP, 37 PD-P (with VH and/or MH), and 50 healthy controls who were matched for age, gender, years of education, and disease duration participated in this cross-sectional study. The Institute Ethics Committee had approved the study, and all the subjects provided written informed consent for their participation. The patients were recruited consecutively in the general Neurology clinic and Movement Disorders clinic of the institute. Diagnosis of PD was done as per the United Kingdom brain bank criteria, Reference Hughes, Daniel, Kilford and Lees13 and the diagnosis of PD-P was based on the NINDS-NIMH criteria. Reference Ravina, Marder and Fernandez12 We aimed to include the patients who experienced hallucinations only in the visual domain, that is, well-formed VH and/or MH. As mentioned above, this study was a component of a large project in which PD-psychosis (PD-P) was diagnosed as per the NINDS-NIMH criteria. However, for this study, we excluded those patients who had nonvisual symptoms (delusion, auditory hallucinations), yet fulfilled the NINDS-NIMH criteria (via well-formed VH and MH). The main reason to recruit only VH/MH for this study was to have homogeneity in terms of psychotic symptoms.
Unified Parkinson’s Disease Rating Scale (UPDRS section-III) was used to assess the severity of the motor symptoms, and the Hoehn and Yahr (H&Y) scale was used to estimate the stage of PD. Hamilton Anxiety rating scale (HAM-A) and Hamilton depression rating scale (HAM-D) were used to document symptoms of anxiety and depression, respectively. Assessment of the cognition was done in the best-ON state through a battery of neuropsychological tests described below. Table 1 summarizes the key demographic and clinical characteristics.
Table 1: Demographic and clinical details of the subjects

AAO = age at onset; DA = dopamine agonist; H&Y = Hoehn and Yahr; HAM-A = Hamilton rating scale for anxiety; HAM-D = Hamilton rating scale for depression; LEDD = Levodopa equivalent dose per day; MAO-BI = monoamine oxidase B inhibitor; PD = Parkinson’s disease; PD = PD patients with psychosis; PD-NP = PD patients with no psychosis; RBD = rapid eye movement sleep behavior disorder; THP = trihexyphenidyl hydrochloride; UPDRS = unified Parkinson’s disease rating scale.
Neuropsychological Assessment
Mini-Mental Status Examination (MMSE) was used as a screening tool for cognitive impairment. As part of the initial study protocol, only the subjects with MMSE score >25 were recruited. Later, we used the Montreal cognitive assessment (MoCA) test for the assessment of the overall cognitive status. Functions of several key cognitive domains such as executive function, visuospatial functions, memory, language, and attention were evaluated. The standard neuropsychological tests used for the cognitive assessment were color trial test (CT part A-mental speed, CT part B-focused attention and executive function), forward and backward digit span test (attention), stroop test (executive function), Rey’s auditory and verbal learning test (RAVLT, for memory and learning capabilities), complex figure test (CFT, for visual memory and visuospatial function), corsi block tapping test (visuospatial function), animal naming test (category fluency and language). Details regarding the methods of doing the aforementioned tests are provided in the supplementary section of this article. Reference Cullum14
Statistics
Statistical computing was done by Prism v9.0, GraphPad, LLC, San Diego, CA, USA. For comparison of two continuous variables, either student t-test (for normally distributed data) or Mann–Whitney U test (when data were not distributed normally) were used. For comparison of categorical variables, chi-square test was employed. For comparison of means of continuous variables of more than two groups, analysis of variance was used (parametric and nonparametric based on the normality or non-normality of data). Bonferroni correction was done for the multiple comparisons. A p-value <0.05 was considered statistically significant.
Results
Of the 37 PD-P patients, 25 had isolated MH (21 with isolated presence hallucinations, 2 with isolated illusions, 2 with a combination of presence hallucinations and illusions). Six patients had isolated VH, 6 had a combination of VH and MH (all with presence hallucinations). Thirty-two patients had an into the psychotic symptoms, and 5 did not have insight (all had VH).
Based on the protocol of the project, the groups were comparable for age, gender, education, and duration of PD. There were no statistically significant differences in the UPDRS-III score during the OFF state, H&Y stage, HAM-A score, HAM-D score, and total levodopa equivalent dose per day (LEDD). A higher proportion of patients in the PD-P group (51.3% vs. 25.5%, p = 0.02) had symptoms of REM RBD (Table 1).
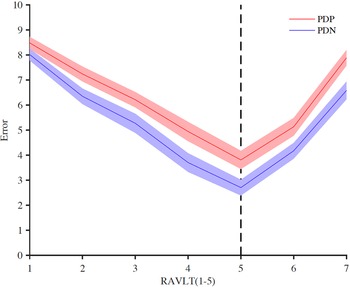
PD-P and PD-NP groups had significant differences in the performances in tests assessing executive, memory, and visuospatial cognitive domains. Of the two tests that were used for assessing executive function, that is, CT-B and Stroop test, PD-P patients had comparatively poor performance in CT-B. Visuospatial function assessed by two separate tests, that is, CFT and Corsi block tapping, and the PD-P group performed poorly compared to PD-NP in both these tests. Similarly, both learning and recall (IR and DR) assessments done through RAVLT were impaired in the PD-P group compared to the PD-NP. The number of errors as demonstrated in the Figure 1 was consistently higher in the PD-P group across all the trials in the RAVLT. On the digit span test, only the forward span was different between the two groups (PD-P < PD-NP). Table 2 summarizes the results of the cognitive assessments.

Figure 1: Graphical demonstration of the number of errors during Rey’s auditory and verbal learning test (RAVLT) in PD-P and PD-NP groups. Points 1 to 5 in the X-axis represent the five learning trials whereas points 6 and 7 represent immediate and delayed recall, respectively. The error rate was calculated by subtracting the total number of words (15) by the number of correct recalls for each attempt.
Table 2: Comparison of the neuropsychological parameters

CFT = complex figure test; CT = color trails; DR = delayed recall; IR = immediate recall; LTPR = long-term percent recall; MoCA = Montreal cognitive assessment; PD = Parkinson’s disease; PD-NP = PD patients with no psychosis; PD-P = PD patients with psychosis; RAVLT = Rey auditory verbal learning test; TL = total learning.
Parameters that were significantly different between the two groups are boldfaced.
We further categorized the PD-P group into two – those with MH (PD-MH) and those with VH (PD-VH). Comparison of cognitive characteristics of three groups, that is, PD-MH, PD-VH, and PD-NP, was done (Supplementary Table 1). VH and MH groups differed only in the score of color trail part A (VH performed poorly). In CT-B, RAVLT (IR and DR), CFT (copy, IR, DR), and Corsi block tapping (backward), both PD-MH and PD-VH performed poorly compared to PD-NP but there was no difference between MH and VH. There was no difference among the three groups in the comparison of the remaining neuropsychological tests.
Discussion
In this study, we compared the cognitive characteristics of PD-P and PD-NP. The principal result of this study is multimodal cognitive dysfunction in the PD-P group. The affected domains are executive, memory, and visuospatial. PD-VH patients had poor performance in CT-A compared to PD-MH; otherwise, the two subgroups of PD-P were comparable across all other cognitive tests.
The PD-P group, as a whole, had significantly worse performance in the CT-B which reflects the frontal executive function (focused attention and set-shifting ability). Executive function which usually localizes the prefrontal cortex and its connections is central in orchestrating the goal-directed activities. Reference Diamond15 The executive functions are encompassed by certain core features which include inhibition (resisting temptations and resisting acting impulsively), interference control (selective attention and cognitive inhibition), working memory, and cognitive flexibility. Reference Diamond15 Several neuroimaging studies have identified structural and functional abnormalities in brain regions associated with the regulation of executive functions. Reference Lenka, Jhunjhunwala, Saini and Pal16 One of the common results from the previous functional imaging studies is increased activation of the frontal regions and reduced activation in the visual cortex in the PD-P in response to visual stimuli. Reference Goetz, Vaughan, Goldman and Stebbins17,Reference Stebbins, Goetz and Carrillo18 This has led to the speculation that the emergence of an aberrant top-to-down visual processing system dominating over the usual down-to-top system is perhaps key to the genesis of hallucinations in PD. Reference Lenka, Herath, Christopher and Pal19–Reference Weil and Reeves21 In this context, the possibility of a “cortical release” phenomenon has also been speculated in the past which could explain the abnormalities in the executive functions in PD-P group. Reference Collerton, Perry and McKeith22
We observed significant impairment in the visuospatial function (visuospatial working memory, visual memory) in the PD-P group. It is consistent with the previous reports of visuospatial dysfunction in the PD-P group. Reference Lenka, Hegde, Arumugham and Pal11 While the volumetric studies have elucidated gray matter volume reduction in the visuoperceptive regions in the parieto-occipital areas, Reference Lenka, Jhunjhunwala, Saini and Pal16,Reference Goldman, Stebbins and Dinh23 abnormalities in the white matter tracts which are probably associated with visuospatial functions were shown to have abnormalities in PD-P in one of our previous studies. Reference Lenka, Ingalhalikar and Shah24 Abnormalities in the CFT and Corsi block test noted in this study provide further consolidate the role of impaired visuospatial function and visual memory in the genesis of VH in PD. Abnormalities in the visuospatial domain, however, are not specific to PD-P, rather it is a cognitive abnormality that is observed in the context of VH related to other neurodegenerative conditions such as dementia with Lewy bodies Reference Hamilton, Salmon and Galasko25 and Alzheimer’s disease. Reference Quaranta, Vita and Bizzarro26
We also noticed impairments in verbal learning and verbal memory in the PD-P group (Figure 1). It is unclear how abnormalities in this domain are directly related to hallucinations in the visual domain. Previous studies, albeit using different neuropsychological tests, have documented abnormalities in this domain in PD-P. Reference Factor, Scullin and Sollinger27,Reference Ramírez-Ruiz, Junqué, Martí, Valldeoriola and Toloso28 This indicates that the cognitive domains associated with VH in PD are not restricted to the frontal executive and visuospatial dysfunction and possibly include temporal and limbic lobes, areas where the most profound Lewy body pathology is associated with hallucinations. Reference Harding, Broe and Halliday29
It is now speculated that the psychotic phenomena in PD have a spectrum, that is, those begin with MH and subsequently if risk factors are not treated adequately, progress to well-formed and complex VH. Hence, it is conceivable that PD patients with a severe form of psychosis (VH) have greater cognitive dysfunction compared to those with MH. Hence, to explore the same, we compared the cognitive characteristics of the MH, VH, and PD-NP groups (Supplementary Table 1). This analysis showed that MH and VH differed significantly only on the score of CT-A (VH performed poorly) which is a measure of mental speed. While this needs to be replicated in larger samples of MH patients, assessment of mental speed can be a focus of future studies predicting the onset of MH in PD. In a recently published longitudinal study by Bejr-Kasem et al., Reference Bejr-Kasem, Sampedro, Marín-Lahoz, Martínez-Horta, Pagonabarraga and Kulisevsky30 there was no difference between the MH and non-MH groups in the formal cognitive assessment; however, the MH group had more subjective cognitive dysfunction. This highlights the requirement of long-term assessment of PD-MH patients to see if they gradually develop more cognitive dysfunction or not.
The cross-sectional design is one of the major limitations of this study. As PD is a progressive disease, it is possible that the patients who did not have psychosis at the time of recruitment would have developed such symptoms in the future. Hence, similar assessments in a longitudinal study would provide robust information regarding the cognitive correlates of PD-P. Although statistically insignificant, the LEDD was higher in the PD-P group. As adverse effects of the antiparkinsonian medications are not always dose-dependent, it is possible that the relatively higher LEDD might have an association with a higher incidence of psychosis. The PD-P group also had a higher number of patients on amantadine and trihexyphenidyl hydrochloride which are known to have an association with psychosis. The other confounding factor is a higher proportion of patients with RBD in the PD-P group. While we took very careful histories related to the nature of psychotic symptoms (only during wakefulness), it is theoretically possible that REM intrusion in some patients might have resulted in visual misperceptions. RAVLT protocol in this study did not include a recognition trial which may be considered another limitation. On the other hand, the study has several strengths which include well-matched groups, the use of the well-accepted NINDS-NIMH criteria for the diagnosis of PD-P, relatively large sample size, homogeneity in terms of psychotic symptoms (included only those with a visual spectrum of hallucinations-VH or MH), and comparison of MH and VH groups.
Conclusion
This study provides further evidence to suggest that psychosis in PD is associated with cognitive dysfunction involving executive, memory, and visuospatial functions. Cognitive assessments should be done for all patients with PD-P even if scores are high in screening tools such as MoCA. Additional longitudinal studies are warranted to further explore the association of cognitive dysfunction with psychosis in PD.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.507
Funding
This study is part of a project funded by the Indian Council of Medical research (ICMR). [ICMR/003/304/2013/00694]. Author PKP received honoraria for delivering lectures in Asia-Oceania Parkinson disease and movement disorders congress, Movement Disorders Congress, International Association of Parkinsonism and Related Disorders (IAPRD) Webinar, Regional Conferences of Movement Disorders Society. Author SH is a recipient of the Wellcome Trust-DBT India Alliance Intermediate Clinical Fellowship (IA/CPHI/17/1/503348).
Conflict of Interest
The authors declare that there are no conflicts of interest relevant to this work.
Author roles
(1) Research Project: A. Conception, B. Organization, C. Execution
(2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
A.L.: 1A, 1B, 1C, 2A
S.H: 1A, 1B, 1C, 2B
S.S.A: 1A, 2C, 3B
P.S: 1B, 1C, 2A
R.Y: 1A, 1B, 2B
P.K.P: 1A, 1B, 1C, 2B