Current energy-storage technologies rely heavily on lithium-ion batteries. And for good reason: they offer high capacities, relatively long cycle lives, and well-established manufacturing processes. However, Li-ion batteries raise concerns over flammability and require rare and expensive materials. While the storage potential of lithium-ion chemistries is largely tapped out, automobiles and portable electronics will require storage capacities and fire-safety assurances well beyond present-day capabilities. Scientists and engineers are investigating new electrode and electrolyte alternatives in an effort to overcome this challenge. Zinc—an inexpensive and abundant metal—has long been used as an anode in primary alkaline batteries. Unfortunately, zinc permits dendrites to grow on electrode surfaces under typical battery recharging conditions. These tiny needle-like structures may reach >100 µm in length and short-circuit the battery—but with a whimper (loss of voltage) rather than a bang (flames and sparks).
To address this problem, researchers from the US Naval Research Laboratory (NRL) developed a three-dimensional (3D) Zn sponge electrode architecture and, along with collaborators from start-up company EnZinc, Inc., have incorporated it into a rechargeable battery design. The research effort, led by NRL’s Joseph F. Parker, Debra R. Rolison, and Jeffrey W. Long, produced the 3D structure from Zn powder and benign ingredients, and then oxidized the surface to envelop the form factor in a ZnO shell with a fused Zn metal core. The researchers implemented the Zn sponge anode in a nickel-zinc (Ni-3D Zn) system, which uses a rechargeable nickel hydroxide (NiOOH) cathode. This configuration yielded long cycle lifetimes, energy densities that met or exceeded performance levels of lithium-ion batteries, while relying on inexpensive and nonflammable materials. The researchers published their work in a recent issue of Science (doi:10.1126/science.aak9991).
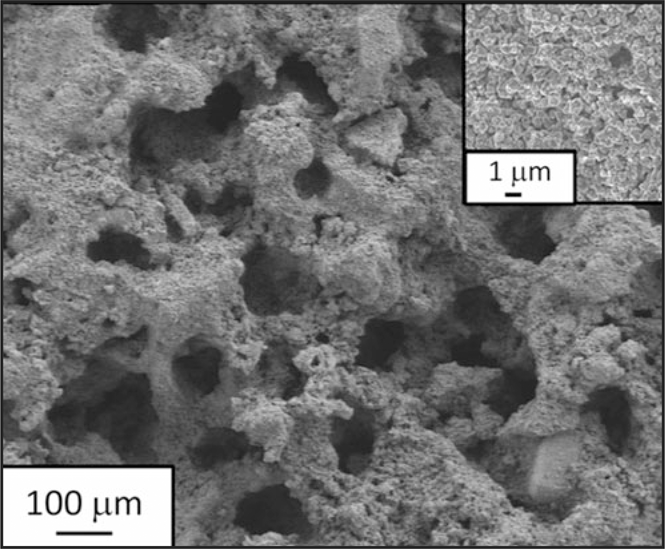
Scanning electron microscope image of a Zn anode after 54,000 cycles. The material retained its ordered architecture and shows no dendrites. Credit: Science Magazine (AAAS).
In describing the significance of this research, Rolison says, “In battery research, a common emphasis is on new materials; we instead turned to an old material but brought it into the 3D architectural realm. The aperiodic sponge with its co-continuity of solid zinc to wire electrons and void to plumb electrolyte and dissolved intermediates throughout the entire electrode volume improves electrochemical and chemical reaction uniformity during charge/discharge cycling. The architecture physically suppresses formation of separator-piercing dendrites, which enables unprecedented cycle life and deep utilization of the zinc.”
The research team relied on an aqueous mixture of potassium and lithium hydroxides as the electrolyte, along with insoluble calcium hydroxide that was deposited on specific surface locations of the metallic Zn sponge. The Ni-3D Zn cells were cycled in primary (single-use) and secondary (multiple charge/discharge cycles) configurations. A full discharge of the battery allowed 91% of the zinc sponge to oxidize and yielded 1.2 kilowatt-hours of energy per kilogram of the anode material. When the cells cycled repeatedly (reversibly tapping 40% of the total amount of zinc present in the battery), the energy densities reached single-cell Li-ion capacity for over 100 cycles. EnZinc implemented the NRL results into their multicell stack design and found that weight and volumetric advantages accrue for Ni-3D Zn; the new technology minimizes the added weight/volume of system components necessary to manage safety concerns with Li-ion stacks.
The sponge-like structure of the anodes demonstrated a vital microstructural advantage: repeated cycling, including both high and low power loads, did not allow for significant dendrite growth. The anodes endured 54,000 charge/discharge cycles using a duty cycle typical of start–stop batteries, which requires high power pulses, but low utilization of zinc per cycle. The Ni-3D Zn performance demonstrates that it can viably compete against advanced lead-acid batteries in microhybrid electric vehicles.
“By demonstrating extreme capacity in single-use mode, cyclability to high Zn-normalized depths-of-discharge, and tens-of-thousands of short-duration duty cycles,” Parker says, “we can now offer a technologically relevant alternative to fire-prone Li-ion batteries in multiple fields-of-use.”
A novel battery configuration is only as good as its large-scale production viability. To that end, the Ni-3D Zn approach presents important advantages because its raw materials are abundant and its balance of plant requirements (e.g., thermal management, electronic controls, and structural protection) is minimal. Furthermore, the battery can be effectively scaled up from small electronics (<500 Wh) to full electric vehicles (24 kWh). When compared to a lithium-ion battery of a Nissan Leaf automobile, the Ni-3D Zn system is projected to offer similar energy density over a longer lifetime, with greater safety, and at lower cost.
Outside researchers have taken notice. As US Army Research Laboratory (ARL) scientist Oleg Borodin points out, “The 3D sponge architecture enabled a remarkable Ni-3D Zn battery performance at high depth of discharge and high current densities (high power) without dendrite formation, making it a serious contender to some of the currently used lithium-ion packs due to significantly simplified thermal and electronic management systems.” ARL’s Kang Xu also commented: “For military applications, ‘water’ is really the direction to go. The recent revival of aqueous battery research, including PNNL [Pacific Northwest National Laboratory] and NRL’s Zn chemistries and ARL’s electrolyte, show encouraging results that enable an aqueous battery chemistry with energy densities and cycle life close to that of Li-ion, but with much higher safety.”
While it is too early to predict the path of this novel battery technology through the energy storage and electric vehicle markets, the Ni-3D Zn breakthrough has already made positive forward progress. The NRL signed a technology licensing deal with co-developer EnZinc, Inc. earlier this year. This agreement paves the way for initial prototyping and eventual market entry, and could mark the emergence of an important new player on the battery scene.


