Interventions to prevent the development of psychopathology in childhood have been impeded by difficulties surrounding valid and reliable ascertainment of early symptoms. While behavioural disorders resembling syndromal psychopathology do emerge in childhood, troubled behaviour is often evanescent, and children regularly move into and back out of diagnostic behavioural categories (Reference Offord, Boyle and RacineOfford et al, 1992). As a consequence, investigators have sought pre-syndromal, neurobiological indicators that might predict more effectively and reliably the onset of childhood behavioural morbidities. Such indicators have included variations in cognitive and attentional processes (Reference Fernandes, Keller and Giese-DavisFernandes et al, 1999), indices of cardiovascular function (Reference Pine, Wasserman and MillerPine et al, 1998), central neural processes (Reference Davidson, Davidson and HugdahlDavidson, 1995) and biologically based susceptibilities to stressors (Reference Boyce, Chesney and Alkon-LeonardBoyce et al, 1995a ).
Reactivity within sympathetic or para-sympathetic branches of the autonomic nervous system has been targeted as a measure of stress susceptibility because of its role in mobilising biological resources during ‘fight or flight’ responses to threatening environmental events (Reference TurnerTurner, 1989). Both adults (Reference Cacioppo, Berntson and MalarkeyCacioppo et al, 1998) and children (Reference Allen and MatthewsAllen & Matthews, 1997) show broad individual differences in autonomic reactivity, which have been associated with a variety of disorders, including internalising and externalising psychopathology (Reference KaganKagan, 1994; Reference Raine, Venables and MednickRaine et al, 1997), psychological and physical symptoms (Reference Gannon, Banks and SheltonGannon et al, 1989; Reference Boyce, Chesney and Alkon-LeonardBoyce et al, 1995a ) and risk-taking behaviour (Reference Liang, Jemerin and TschannLiang et al, 1995). Given the possible linkages of autonomic reactivity to psychopathology, the purpose of this study was to examine a new reactivity assessment protocol for children aged 4-8 years and to seek measures of autonomic response that could identify children with early signs of developmental psychopathology.
METHOD
Subjects
A sample of 122 children (73 girls and 49 boys) aged 6-7 years and their parents and teachers was recruited from one of two cohorts (totaln=500) in the longitudinal Wisconsin Study of Families and Work (Reference Hyde, Klein and EssexHyde et al, 1995). Eligibility for the current study was based on families' participation in prior waves of data collection, residence within geographic proximity of the project offices and the presence of a target child in a local school. A group of 203 families in the Wisconsin Study's first cohort met these criteria and was further refined using the mental health symptom sub-scales of the MacArthur Health and Behaviour Questionnaire (HBQ). The HBQ is a 140-item, parent and teacher report instrument assessing mental and physical health and social and academic functioning in middle childhood. The instrument has been shown to have strong psychometric properties, including test-retest reliability, internal consistency and empirical validity in discriminating groups of children 4-8 years old with and without signs of early psychopathology (Reference Ablow, Measelle and KraemerAblow et al, 1999). The sample of 122 children was assembled using HBQ scores at 5 years of age to achieve approximately equal representation of children with high and low reported symptoms and, among those high in symptoms, near parity between internalising or externalising behavioural profiles. Of the 122 families who were identified and agreed to participate, 120 completed all components of the study.
To examine the discriminant validity of the reactivity protocol, children were divided into groups with ‘low’ or ‘high’ symptoms using maternal and teacher reports on behaviour. Two composite scores were examined: ‘internalising problems’, comprising depression, over-anxious symptoms and separation anxiety, and ‘externalising problems/attention deficits’, comprising oppositional defiant and conduct problems, overt hostility, inattention and impulsivity. A designation of ‘low symptoms’ was given if both mother and teacher rated the child in the lower 80% on both scores; ‘high internalising’ or ‘high externalising’ if either rated the child in the upper 20%; and ‘high both’ if either reporter rated in the top 20% on both.
Design and procedures
In the summer of 1998, visits were conducted in the homes of the 120 study children, and several components of the MacArthur Assessment Battery (further details available from the author upon request) were used to examine constructs salient to children's mental and behavioural health. The MacArthur Assessment Battery is an integrated assessment measure that addresses biological, neurological, psychosocial and contextual aspects of development in middle childhood. During a 15-minute component of the 4-hour assessment, children's autonomic reactivity to standardised stressors was tested using a standardised protocol completed in a van stationed outside the family's home. Children were monitored using thoracic and precordial electrodes, a circumthoracic band transducer of respiratory activity, a cardiac monitor and an impedance cardiograph.
Reactivity protocol
Reactivity has been defined as “the deviation of a physiological response parameter from a comparison or control value that results from an individual's response to a discrete, environmental stimulus” (Reference Matthews, Matthews, Weiss and DetreMatthews, 1986). Four physiological response parameters were employed in the study protocol: heart rate, mean arterial pressure, respiratory sinus arrhythmia (a measure of parasympathetic influence on heart rate variability) and pre-ejection period (an index of sympathetic activation of the heart). Heart rate was ascertained from interbeat interval data acquired using an electrocardiograph (ECG) digitised at 500 Hz, edited for artefacts and analysed with the detection algorithm of Berntson et al (Reference Berntson, Quigley and Jang1990). Mean arterial pressure (MAP) was measured with the monitor at the point of maximal oscillatory amplitude (Reference Park and MenardPark & Menard, 1987). Respiratory sinus arrhythmia (RSA), as described in the work of Porges (Reference Porges1995) and Cacioppo et al (Reference Cacioppo, Berntson and Binkley1994a ), was derived from spectral analyses of interbeat interval data within the respiratory cycle-associated, high-frequency (0.15-0.50 Hz) band of the heart rate power spectrum. The natural logarithm of the variance in high-frequency heart period was calculated to estimate parasympathetic activity. The pre-ejection period (PEP), the duration of isovolumetric ventricular contraction, was measured with impedance cardiography as the 70-100 ms interval between the onset of electromechanical systole (indicated by the ECG Q-wave) and left ventricular ejection (indicated by the B point of the thoracic impedance signal). As described by Cacioppo et al (Reference Cacioppo, Uchino and Berntson1994b ), thoracic impedance (Z 0) and its first derivative (dZ/dt) were measured as resistance to a constant 4 mA, 100 kHz alternating current, using electrodes placed at the apex and base of the child's thorax. Impedance data were ensemble averaged in 1-minute intervals, and each waveform was verified or edited prior to analysis. Autonomic reactivity was thus assessed as accelerations in heart rate, increases in MAP, diminution in RSA (reflecting para-sympathetic withdrawal) or shortening of PEP (reflecting a sympathetic effect on cardiac chronotropism).
The discrete, environmental stimuli used in the reactivity protocol were administered by an unfamiliar female researcher. They included:
-
(a) a structured child interview modified from the Gesell School Readiness Screening Test (Reference Carlson, Keyser and SweetlandCarlson, 1985);
-
(b) digit span recitation modified from the Kaufman Assessment Battery for Children (Reference Kaufman and KaufmanKaufman & Kaufman, 1983);
-
(c) two or three drops of lemon juice placed on the child's tongue;
-
(d) two emotion-evoking videotapes, chosen to elicit fear and sadness (Reference Eisenberg, Fabes and BustamanteEisenberg et al, 1988).
The four epochs were selected to represent social, cognitive, physical and emotional challenges for children aged 4-8 years and were presented to each child in the same sequence. Before and after the set of four challenges, children were read a 3-minute calming story to procure resting physiological measures, and between the second and third challenges a 1-minute period of quiet inactivity (the ‘recovery’ epoch) was imposed to examine short-term recovery in physiological parameters. The total time for completion of the protocol varied between 20 minutes and 30 minutes, including the time required for attachment, application and testing of monitoring equipment.
Reactivity scoring
Although physiological reactivity has most often been represented by change (Δ) scores or standardised residual scores, Boyce et al (Reference Boyce, Alkon and Tschann1995b ) have suggested that a more illuminating picture of individual response patterns might be derived from a multi-dimensional view of a child's reactivity profile. Such a profile would encompass multiple measures capturing several dimensions of a child's psycho-biological responses over time, rather than a singular measure reflecting only the magnitude of such responses. ‘Intensity’ was defined as the mean of the response parameter for the four challenging task epochs. ‘Recovery’, defined as the number recall measure minus the recovery measure, reflected attenuation in response over 1 minute of rest. ‘Slope’ was calculated as the coefficient of a single child's physiological measures regressed on time/epoch and it indicated the tendency of a given physiological measure to upregulate or downregulate over the course of the protocol. ‘Variability’ was indexed as the standard deviation of the four task values, with diminished variability reflecting greater reactivity. In this study, these four dimension scores and a Δ score were calculated for each of the four physiological variables, heart rate, MAP, RSA and PEP. The only exception was for MAP, which was not measured during the recovery epoch and thus had no recovery score calculated. In our previous work with middle-childhood subjects, the Δ and standardised residual scores have been almost perfectly correlated.
Statistics
Data analyses were conducted in a sequence of five steps. First, frequency distributions were examined, and intracorrelations among dimension scores were computed to determine the degree of independence or redundancy among the multiple measures. Second, gender effects on reactivity measures were evaluated using t-tests for male-female differences in means. Third, because some of the physiological variables were non-normally distributed, non-parametric Kruskal—Wallis one-way analyses of variance were used to assess differences in reactivity between the four symptom groups. Because these analyses offer insufficient insight into the magnitude of the observed associations, Cohen's δ, which expresses group differences in terms of standard deviations, was also calculated as an index of effect size. (Cohen's δ is calculated as δ=(Meancase — Meancontrol)/s.d.pooled. Thus, a δ of 0.10 indicates that the two means differ by a tenth of a standard deviation, a δ of 0.50 that the means differ by half of a standard deviation and a δ of 2.0 that they differ by two standard deviations.) Where large differences existed in subgroup sizes, effect size estimates were derived first from Mann—Whitney (Wilcoxon) tests and were then converted, for purposes of comparison, to an approximation of Cohen's δ. Fourth, results of the Kruskal—Wallis analyses were confirmed using a signal detection model identifying which physiological reactivity variables, and which cut-off points within those variables' range of values, discriminated between symptom groups with the greatest possible efficiency. This method employs a quality receiver operator characteristic (QROC) approach to maximising the selected variables' sensitivity and specificity in discriminating between groups (see Reference KraemerKraemer, 1992). Finally, to render symptom group differences more visible, radar plots were created to display comparisons among group-specific reactivity in the intensity, recovery, slope and variability dimensions.
RESULTS
Table 1 shows classifications by symptom group for boys, girls and the entire sample. As expected, boys and girls were not equally distributed among the four groups, with boys disproportionately represented among high externalisers and girls among the high internalisers. Although substantial variability in autonomic reactivity scores was found for both boys and girls, gender differences in reactivity scores were notable primarily for their scarcity. When only the low symptom group was examined, no significant differences in autonomic reactivity scores were found, indicating that gender was not a confounder in comparisons of high and low symptom children. Intracorrelations of dimension scores within and across physiological variables (heart rate, MAP, RSA and PEP) were universally modest in magnitude, with the single exception of heart rate—RSA relations. These results indicated that autonomic reactivity dimension scores were, as expected and desired, largely orthogonal.
Table 1 Classification of sample by gender and symptom group

| Low symptoms | High internalising | High externalising | High both | |
|---|---|---|---|---|
| Boys (n=47) | 24 (36%) | 3 (14%) | 12 (71%) | 8 (57%) |
| Girls (n=72) | 42 (64%) | 19 (86%) | 5 (29%) | 6 (43%) |
| Total (n=119) | 66 (100%) | 22 (100%) | 17 (100%) | 14 (100%) |
Reactivity in the four symptom groups
The first analyses of discriminant validity, using Kruskal—Wallis tests for group differences in autonomic reactivity scores, are summarised in Table 2. Means and standard deviations of scores for the four symptom groups are presented along with effect sizes where group differences were identified. Effect sizes of approximately 0.20 are considered small, 0.50 moderate and 0.80 large (Reference KraemerKraemer, 1992). Several symptom group differences were identified that met criteria for both statistical significance and moderate to large effect magnitudes. The RSA intensity score, for example, robustly discriminated high internalising children from low symptom children. Specifically, internalisers were significantly more RSA reactive (that is, showed greater parasympathetic withdrawal under challenge), as reflected in lower RSA intensity scores. Further, the RSA and PEP Δ scores discriminated high externalising children from those with low symptoms, with externalisers showing significantly less reactive scores (that is, higher RSA/PEP Δ scores under challenge). A moderate to large and significant effect was also found for the PEP Δ score in discriminating children with both internalising and externalising symptoms from low symptom children. Trends of borderline significance and moderate effect magnitude were also observed for RSA variability (higher, indicating less reactivity, among externalising children) and MAP Δ scores (lower, indicating less reactivity, among children with both internalising and externalising symptoms). These results indicated that autonomic reactivity scores discriminated children with internalising and externalising symptoms from controls, that RSA and PEP were more effective in making such discriminations than measures of heart rate and MAP, and that internalising and externalising children showed contrasting patterns of autonomic arousal under challenge.
Table 2 Kruskal—Wallis analyses and effect size estimates for autonomic reactivity scores by symptom group (group differences with both statistical significance and moderate or greater effect sizes shown in bold)

| Low symptoms | High internalising | High externalising | High both | χ 2 | Effect size estimates1 | |||
|---|---|---|---|---|---|---|---|---|
| Low symptoms v. internalisers | Low symptoms v. externalisers | Low symptoms v. high both | ||||||
| RSA | ||||||||
| Intensity | 6.13 (1.01) | 5.51 (0.84) | 6.05 (0.82) | 5.88 (1.11) | 7.87 * | -0.75 | -0.06 | -0.31 |
| Variability | 0.56 (0.28) | 0.55 (0.28) | 0.77 (0.31) | 0.62 (0.25) | 6.37 2 | -0.04 | 0.69 | 0.33 |
| Recovery | -0.46 (0.60) | -0.02 (0.72) | -0.42 (0.54) | -0.51 (0.51) | 5.23 | 0.24 | 0.58 | 0.58 |
| Slope | 0.34 (0.56) | 0.42 (0.54) | 0.55 (0.50) | 0.63 (0.31) | 5.50 | 0.59 | 0.09 | -0.10 |
| Difference | 0.02 (0.40) | 0.00 (0.29) | 0.30 (0.22) | -0.09 (0.36) | 13.11 ** | -0.15 | 1.03 | -0.37 |
| PEP | ||||||||
| Intensity | 83.16 (6.63) | 84.90 (6.46) | 84.34 (6.27) | 83.59 (6.47) | 1.68 | 0.30 | 0.28 | 0.03 |
| Variability | 1.85 (0.93) | 1.67 (0.67) | 1.87 (0.95) | 2.39 (1.37) | 1.64 | -0.14 | 0.00 | 0.32 |
| Recovery | 0.60 (1.93) | -0.19 (1.97) | 0.49 (2.80) | 0.14 (2.84) | 2.23 | 0.12 | 0.44 | 0.29 |
| Slope | 0.29 (0.57) | 0.34 (0.64) | 0.55 (0.33) | 0.40 (0.63) | 1.99 | -0.42 | 0.06 | -0.09 |
| Difference | 0.77 (9.35) | 1.03 (8.12) | 1.81 (8.17) | 2.01 (7.16) | 7.91 * | 0.04 | 0.56 | 0.79 |
| Heart rate | ||||||||
| Intensity | 91.92 (9.35) | 96.25 (8.12) | 95.31 (8.17) | 92.44 (7.16) | 4.29 | 0.51 | 0.31 | 0.08 |
| Variability | 5.28 (2.00) | 5.10 (2.09) | 6.03 (2.18) | 5.50 (0.97) | 2.28 | -0.01 | 0.36 | 0.30 |
| Recovery | 2.00 (3.79) | 0.22 (3.76) | 2.25 (4.58) | 0.83 (3.70) | 3.57 | -0.12 | -0.42 | -0.16 |
| Slope | -0.54 (0.28) | -0.55 (0.36) | -0.60 (0.39) | -0.55 (0.38) | 1.64 | -0.59 | -0.02 | -0.21 |
| Difference | -0.05 (2.26) | 0.91 (2.08) | -0.51 (2.76) | 0.25 (1.92) | 3.24 | 0.47 | -0.14 | 0.10 |
| MAP | ||||||||
| Intensity | 74.32 (5.55) | 76.33 (4.94) | 74.53 (4.65) | 74.23 (6.35) | 2.70 | 0.38 | 0.06 | -0.16 |
| Variability | 5.10 (2.50) | 5.15 (2.18) | 4.90 (1.75) | 4.50 (2.54) | 1.21 | 0.10 | 0.01 | -0.26 |
| Slope | -0.15 (0.58) | -0.02 (0.53) | 0.11 (0.65) | -0.05 (0.58) | 2.49 | 0.27 | 0.39 | 0.19 |
| Difference | 2.20 (4.05) | 2.89 (3.38) | 2.42 (4.40) | -0.52 (3.74) | 6.36 2 | 0.15 | 0.07 | -0.72 |
A second approach to assessing the discriminant validity of autonomic reactivity scores, using a signal detection model, both confirmed and extended results from the Kruskal—Wallis analyses (further details available from the author upon request). For both internalising and externalising symptoms, an optimally efficient algorithm was developed using reactivity scores to identify children showing significant behaviour problems. In these analyses, children with high internalising problems were maximally and significantly discriminated by three autonomic dimension scores, each reflecting higher reactivity relative to asymptomatic peers: (a) RSA intensity ≤ 6.3; (b) RSA recovery ≥ 0.50; and (c) RSA Δ score ≤ 0.05. Eighty-three per cent of children meeting all three of these criteria were high internalisers, while only 15% of those meeting none of the criteria were high internalisers. Children with high externalising problems, on the other hand, were maximally and significantly discriminated by two dimensions scores, both reflecting low reactivity: (a) PEP score ≥ 1.25; and (b) RSA slope ≥ 0.50. Fifty-three per cent of children meeting both criteria were high externalisers v. 15% of those meeting neither. Finally, the signal detection algorithm for children with both internalising and externalising behaviour problems contained only a single criterion, a PEP variability score of 3.20 or more, reflecting low sympathetic reactivity. Seventy-one per cent of children meeting this criterion were in the ‘high both’ symptom group, compared with 15% of those not meeting the criterion.
Visual representations of differences in reactivity
Figures 1 and 2 summarise and integrate the discriminant validity analyses of autonomic reactivity scores. Figure 1 displays four-dimensional radar plots in which standardised scores for each reactivity dimension (intensity, recovery, slope and variability) are plotted for children with and without internalising and externalising symptoms. Tick marks on each of the four axes define three points on a standardised scale for that dimension: −1 (at the origin), 0 and +1. Further, scores have been arranged, and in some cases reversed, so that increasing distances from the origin universally indicate higher reactivity. As expected, data points from the majority, asymptomatic children lie at approximately the mean (a standardised score of 0) for each reactivity dimension. Internalising children exhibited exaggerated reactivity in two dimensions, intensity and recovery, but only for the parasympathetic (RSA) component of autonomic response. In contrast, externalising children showed diminished reactivity in the two other dimensions, variability and slope, for both the parasympathetic and sympathetic (PEP) components. Children with internalising and externalising disorders were thus distinguishable from each other and from children without behavioural difficulties by three distinct aspects of their autonomic reactivity to challenge: ‘magnitude’ (that is, increased or decreased reactivity relative to controls), ‘dimension’ (that is, effects in the intensity/recovery v. variability/slope dimensions) and ‘branch’ (that is, involvement of the para-sympathetic and/or sympathetic branches of the autonomic nervous system).

Fig. 1 Standardised scores for each reactivity dimension are plotted for children with and without internalising and externalising symptoms. Left-hand plots: high internalising children (squares, internalisers; diamonds, non-internalisers). Right-hand plots: high externalising children (squares, externalisers; diamonds, non-externalisers). PEP, pre-ejection period; RSA, respiratory sinus arrhythmia.
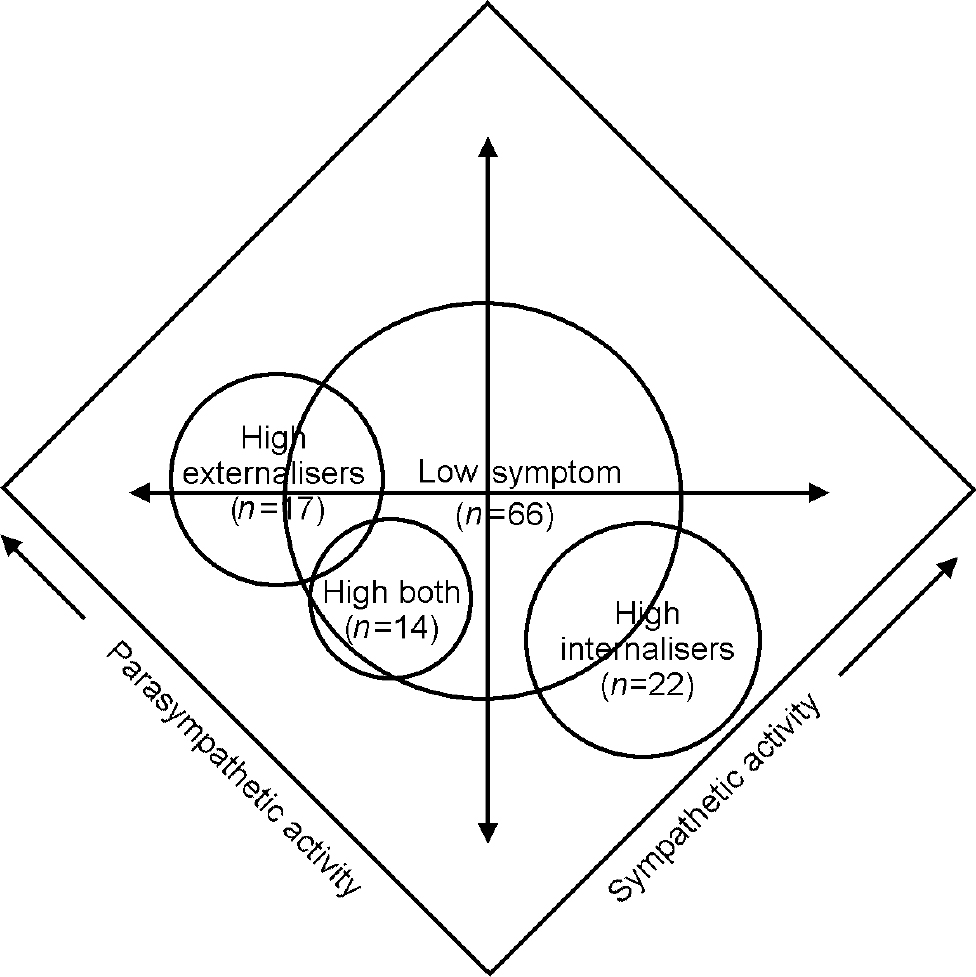
Fig. 2 High internalising, high externalising, high both and low symptom children within a two-dimensional autonomic space (adapted from Reference Berntson, Cacioppo and QuigleyBerntson et al, 1994).
Mapped within two-dimensional autonomic space as conceptualised by Berntsonet al (Reference Berntson, Cacioppo and Quigley1994) (Fig. 2), the autonomic response biases of the children in this study occupy discrete psychophysiological territories. The axes in Fig. 2 represent the proportional and independent activations of the sympathetic and parasympathetic branches of the autonomic nervous system. Maximal autonomic reactivity, which involves both sympathetic activation and para-sympathetic withdrawal, would be plotted into the far right corner of the autonomic space. Children from the study with internalising symptoms showed heightened reactivity in the form of parasympathetic withdrawal, resulting in a position adjacent to the lower right border of the autonomic map. Conversely, children with externalising symptoms showed diminished reactivity, in the form of both maintenance of parasympathetic tone and relative inactivation of the sympathetic circuitry, resulting in an autonomic position approaching the far left corner. The smaller number of children with both categories of symptomatic behaviour showed unusually low sympathetic activation alone, placing them in a mapped location between the internalisers and externalisers. As supported by the Kruskal—Wallis and signal detection analyses, each of these autonomic profiles was significantly and substantively different from the physiological response patterns of children for whom no behavioural symptoms were reported, who occupy the centre of the autonomic space.
Gender differences
A remaining question was whether identified associations between autonomic reactivity and early psychopathology were confounded by unevenness in the gender distribution among the four symptom groups. Two findings suggested that this was not the case. First almost no gender differences were found in measures of sympathetic and parasympathetic reactivity, reducing substantially the likelihood of a confounded association with symptoms. Second, when mean reactivity scores were computed for the eight subgroups defined by gender and symptom, asymptomatic boys and girls were virtually identical. Even more convincingly, gender differences in the internalising and externalising groups were opposite in direction from those that could have confounded reactivity—symptom associations. Internalising boys, for example, had RSA intensity scores reflecting even greater reactivity than internalising girls, while externalising girls had RSA and PEP Δ scores indicating even lower reactivity than their externalising male counterparts. These findings support a conclusion that associations linking autonomic reactivity to behavioural symptoms were unconfounded by gender.
DISCUSSION
Taken together, results of this study suggest a pattern of autonomic dimorphism in the biological reactivity of children at risk for early internalising and externalising psychopathologies. Our observations comprised three core findings. First, we confirmed the feasibility of eliciting and measuring autonomic response differences in a 6— to 7-year age group in a field setting. Second, we found systematic differences in the patterns of autonomic reactivity among children with internalising and externalising behavioural symptoms compared with peers with no symptomatic behaviour. Children with behaviour suggesting developmental psychopathology revealed profiles of upregulated or downregulated physiological arousal derived from one or both branches of the autonomic nervous system. Third, different categories of behavioural difficulties were associated with distinctive profiles of autonomic reactivity. Specifically, children with internalising patterns of symptomatic behaviour revealed increased parasympathetic arousal within the intensity and recovery dimensions of their reactivity profiles, whereas children with externalising problems showed profiles of diminished sympathetic and parasympathetic arousal in the variability and slope dimensions. A plausible interpretation of this finding is that internalising children exhibit a psychobiological ‘fingerprint’ reflecting differences in the magnitude of restorative autonomic functions, while externalising children show a pattern of differences in the variability of more general autonomic responses. These observations together indicate that school-age children's risk for future psychopathology may be estimable using assessments of autonomic reactivity and that such assessments may suggest heightened risks for specific forms of mental disorder.
Commonalities with past research
Our findings are commensurate with those of several other research groups. The seminal work of Ekman and Levenson (Reference Ekman, Levenson and FriesenEkman et al, 1983), for example, suggested that discrete categories of experienced emotion were associated with specific patterns of autonomic reactivity in adult subjects. Other studies in adults have found that a high heart rate following a traumatic event was predictive of post-traumatic stress disorder (Reference Shalev, Sahar and FreedmanShalev et al, 1998) and that heart rate variability was inversely associated with affective and anxiety disorders (Reference Rechlin, Weis and SpitzerRechlin et al, 1994). Parasympathetic dysregulation is predictive of chronic behaviour problems in toddlers (Reference Porges, Doussard-Roosevelt and PortalesPorgeset al, 1996) and teenage boys (Reference Mezzacappa, Tremblay and KindlonMezzacappa et al, 1996), and resting heart rate in children is inversely associated with externalising psychopathology and positively associated with anxiety disorders (Reference Rogeness, Cepeda and MacedoRogeness et al, 1990; Reference RaineRaine, 1996). Other studies have observed increased heart rate reactivity among pubertal boys at familial risk for alcoholism (Reference Harden and PihlHarden & Pihl, 1995) and decreased heart rate reactivity and faster habituation to a reward-conditioned repetitive motor task in children with attention-deficit hyperactivity disorder (Reference laboni, Douglas and DittoIaboni et al, 1997). Pine et al (Reference Pine, Wasserman and Miller1998) found inverse correlations between measures of heart rate variability and externalising behaviour problems among the younger brothers of convicted delinquents. Children at risk for criminality, moreover, appear protected by high autonomic reactivity, suggesting that a predisposition toward an internalising biological response to stressors may prevent expression of disordered conduct (Reference Brennan, Raine and SchulsingerBrennan et al, 1997).
Neural substrates of autonomic reactivity
As noted by Pine et al (Reference Pine, Wasserman and Miller1998), abnormalities in brain monoamine systems, which have been implicated in cardiovascular functions and psychiatric conditions, could potentially account for associations between autonomic reactivity and psychopathology. Limbic structures such as the amygdala can affect autonomic regulation and have been implicated in psychiatric disorders involving emotion regulatory processes (Reference Kagan, Reznick and SnidmanKagan et al, 1988). Porges' ‘polyvagal theory’ suggests that the evolution of the autonomic nervous system, particularly the brain-stem regulatory centres of the vagus and related cranial nerves, produced a neuropsychological substrate for affective processes that are the basis for both social relations and certain psychopathological disorders (Reference Porges, Doussard-Roosevelt and StifterPorges et al, 1999). Although some evidence exists for the heritability of reactive phenotypes (Reference Higley, Thompson and ChampouxHigley et al, 1993; Reference Scarpa and RaineScarpa & Raine, 1997), stressful experiences, particularly early in development, also appear capable of altering individual response characteristics (Reference SchneiderSchneider, 1992; Reference Anisman, Zaharia and MeaneyAnisman et al, 1998).
As Kagan (Reference Kagan1997) has argued, progress in understanding and classifying psychopathology is likely to depend on the use of multiple and diverse criteria, almost undoubtedly including both behaviour and biology. It appears likely that all current diagnostic categories are heterogeneous with regard to their biological and experiential aetiologies, and that significant gains could be achieved by refining taxonomic distinctions through pursuit of a combined behavioural and biological approach. The significance of the findings reported here lies, at least in part, in observations that many forms of adult psychopathology are meaningfully linked to overt behavioural differences in childhood, and that biological response predispositions may constitute one aetiological ‘bridge’ between troubled child behaviour and adult disorders. Only the binocularity of a concurrently biological and contextual view of childhood risk may yield a clearer, more coherent path towards understanding and preventing developmental psychopathology.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Patterns of autonomic reactivity to standardised psychological stressors distinguish between school-age children with internalising and externalising behavioural symptoms and children without symptoms.
-
• Children at risk for internalising disorders show high autonomic reactivity, primarily in the parasympathetic branch, while children at risk for externalising problems show unusually low autonomic arousal, in both the parasympathetic and sympathetic branches.
-
• Combined with multiple-informant reports on child behaviour, assessments of autonomic reactivity could offer greater precision and validity in the estimation of childhood risk for developmental psychopathology.
LIMITATIONS
-
• Although drawn from a larger, longitudinal cohort, the study sample is only moderate in size and may not be representative of populations beyond that from which it was recruited.
-
• Because the study was cross-sectional in design, causality or even antecedence cannot be imputed from the observed associations between autonomic reactivity and psychopathological risk.
-
• The study addressed reactivity only within the sympathetic and parasympathetic branches of the autonomic nervous system. Further research is needed to understand the independent and interactive roles of other central and peripheral stress response circuits, such as the corticotrophin-releasing hormone system.
Acknowledgements
The authors gratefully acknowledge the assistance of Professors John T. Cacioppo (University of Chicago) and Gary G. Berntson (Ohio State University) for the development and generous provision of autonomic reactivity software employed in this study; Mr David Lozano (Ohio State University) for his technical and programming support; and Professor Jerome Kagan (Harvard University) for his invaluable conceptual contributions to the development of the autonomic reactivity protocol. The MacArthur Assessment Battery Working Group has relied upon the active involvement and insights of the following individuals: Jennifer C. Ablow, Abbey Alkon, Jeffrey M. Armstrong, W. Thomas Boyce, Marilyn J. Essex, Lauren H. Goldstein, Richard Harrington, Helena C. Kraemer, David J. Kupfer, Jeffrey R. Measelle, Charles Nelson, Jodi Quas, Nancy A. Smider and Laurence Steinberg.






eLetters
No eLetters have been published for this article.