INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major global health problem, because it is an important cause of death and disability. Furthermore, there has been an increase in both the prevalence and mortality associated with COPD, even in industrialized countries. In 1990 COPD was the sixth most common cause of death worldwide and it is estimated that it could become the third commonest cause of death by 2020 [Reference Barnes1].
COPD is defined by fixed airflow limitation. The decline observed in the forced expiratory volume in 1 second (FEV1) is one of the most important prognostic markers of this condition [Reference Barnes1]. However, COPD can no longer be considered a disease restricted to the pulmonary tract, because recent data indicate that it is often associated with a wide variety of systemic consequences, including the presence of systemic inflammation [Reference Stone and Nici2]. Furthermore, it has been increasingly recognized that patients with COPD usually have a number of comorbidities, some of which can have a definite impact on disease progression. Indeed, a recent study demonstrated that patients with COPD and hepatitis C virus (HCV) infection have an accelerated decline of FEV1 compared to patients without such infection [Reference Kanazawa, Hirata and Yoshikawa3].
HCV infection leads to chronic hepatitis in the majority of acutely infected patients. The World Health Organization estimates that 3% of the world's population (~170 million people) are chronically infected with HCV. Many infected individuals are asymptomatic, therfore the early diagnosis of this condition greatly depends on the screening of individuals with known risk factors [Reference Strader4].
Considering that chronic hepatitis C is a potentially curable disease and that the treatment of this infection could reduce FEV1 decline in patients with COPD, it is conceivable that HCV screening would have to be conducted for all COPD patients. Based on this assumption, the aim of our study is to determine the prevalence of HCV infection in a sample of COPD patients, and to compare the clinical and functional characteristics between HCV-positive and HCV-negative patients.
METHODS
Study subjects
Our study included consecutive outpatients with COPD from a general, tertiary-care, university-affiliated hospital with 750 beds, located in the city of Porto Alegre, Rio Grande do Sul State, in the south of Brazil. The patients were enrolled between January and December 2007. All outpatients from our hospital with COPD were invited to participate. COPD was confirmed by a ratio of FEV1 to forced vital capacity (FVC) of <0·7, measured 20 min after the administration of salbutamol. COPD severity was classified according to global initiative for chronic obstructive lung disease (GOLD) criteria [Reference Rabe5]. The control group was composed of 16 138 blood donors who attended our hospital during the same period of the study (January–December 2007). Written informed consent was obtained from each patient prior to participation. The study was approved by the local ethics committee.
Study design
This is a cross-sectional study to determine the prevalence of HCV infection in patients with COPD. The sample size was calculated based on known prevalences of HCV infection in the world (3%), in Brazil (1·2–2%), and in Rio Grande do Sul State (0·7%) [Reference Alter6, 7]. Using a power of 80% and significance at 5%, with a maximal acceptable difference of 2% (prevalence between 0% and 3%), and considering 10% of patients to be lost to follow-up, we calculated that 106 patients would be needed.
Clinical characteristics and pulmonary function tests
The patients were interviewed after the medical visit, and the medical records were also reviewed. The following data were collected: smoking habits, comorbidities, current medications, blood transfusions in the past, frequency of emergency department visits and hospitalizations in the past 2 years, emergency department and in-hospital length of stay, drug use, and long-term domiciliary oxygen therapy. The degree of dyspnoea was measured by the Modified Medical Research Council (MMRC) Dyspnoea Scale [Reference Mahler and Wells8]. Body mass index (BMI) was calculated for all patients. Similarly, the BODE (body mass index, airflow obstruction, dyspnoea, and exercise capacity) index was calculated as described previously [Reference Celli9]. Arterial blood gas (ABG) analysis and echocardiogram were performed for some patients, as indicated. The 6-min walking test with real-time biotelemetry was carried out in a 27-m corridor under supervision of trained personnel (either a nurse or a physician). The use of telemetry allowed for the heart rate and pulse oximetry to be precisely and constantly monitored throughout the 6-min walking test.
Pulmonary function tests [spirometry, pletismography, and diffusing capacity of the lung for carbon monoxide (D LCO)] were performed using a computerized spirometer (Jäeger, Germany), according to American Thoracic Society/European Respiratory Society guidelines [Reference Miller10–Reference Macintyre12], with previously published reference values [Reference Crapo, Morris and Gardner13–Reference Crapo and Morris15].
HCV infection
All patients were tested for anti-HCV antibodies by a third-generation enzyme-linked immunosorbent assay (ELISA) (Ortho HCV 3.0, Ortho Clinical Diagnostics, USA). In patients with positive anti-HCV antibody test, serum HCV-RNA was performed by reverse transcription (RT–PCR) and nested polymerase chain reaction (PCR) with primers derived from the highly conserved 5′-untranslated region (NS-5′R) of the viral genome. HCV-positive patients were defined as anti-HCV and HCV-RNA positive. HCV genotyping was performed by restriction fragment length polymorphism (RFLP) analysis. Because patients were included consecutively, some patients had already been tested for anti-HCV antibodies. These patients were included in the study and data was collected from the time of diagnosis. In patients with a negative anti-HCV antibody test performed >6 months previously, a new test was requested.
Analysis
Data analysis was performed using SPSS version 14.0 (USA). Data were presented as number of cases, mean±standard deviation (s.d.), or median with interquartile range (IQR). Categorical comparisons were performed by χ2 test using Yates's correction if indicated or by Fisher's exact test. Continuous variables were compared using the t test or Mann–Whitney test. A two-sided P value <0·05 was considered significant for all analyses.
RESULTS
A total of 192 patients met the inclusion criteria. One patient refused to participate. Five patients gave signed, informed consent, but were lost to follow-up, and we did not obtain a blood sample for anti-HCV detection. Overall, 187 patients were included in the analysis. Demographic, clinical and functional characteristics of the study population are given in Table 1.
Table 1. Characteristics of patients with chronic obstructive pulmonary disease
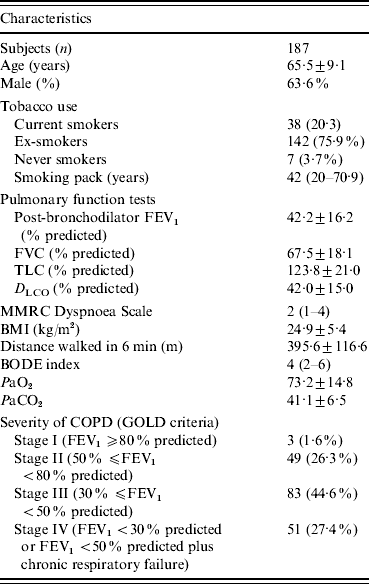
FEV1, Forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; D LCO, carbon monoxide diffusing capacity of the lung; MMRC, Modified Medical Research Council; BMI, body mass index; COPD, chronic obstructive pulmonary disease; BODE, body mass index, airflow obstruction, dyspnoea, and exercise capacity; GOLD, global initiative for chronic obstructive lung disease.
Data are presented as mean±s.d., n (%), or median (interquartile range).
The patients had a mean age of 65·5±9·1 years, and 119 (63·6%) were male. According to GOLD severity criteria, 134 (72%) patients were classified as GOLD stages III or IV. Table 1 shows the data relative to pulmonary function tests. The median of the BODE index was 4 (IQR 2-6). Comorbidities were present in 127 (67·9%) patients, and the most common were systemic arterial hypertension (n=65, 34·9%), diabetes mellitus (n=18, 9·6%), ischaemic heart disease (n=20, 10·7%), and heart failure (n=12, 6·4%).
Twenty (10·7%) patients had a positive anti-HCV antibody test. Of these patients, 14 had already been tested for anti-HCV antibodies, with a mean time of diagnosis of 4·4±1·8 years. The prevalence of anti-HCV positivity in COPD patients (10·7%, 95% CI 9·25–12·2) was significantly higher than in blood donors (0·41%, 95% CI 0·40–0·42) (OR 29·2, 95% CI 17·3–49·2, P<0·0001). Seventeen COPD patients who were anti-HCV positive were tested for HCV-RNA. Three patients were not tested (two were lost to follow-up and one died before a sample could be obtained). Five anti-HCV-positive patients were found to be HCV-RNA negative (but two of them had a previous positive HCV-RNA which became negative after interferon treatment) and 12 were HCV-RNA positive. Thus, the overall prevalence of HCV-positivity (anti-HCV- and HCV-RNA-positive patients) was 7·5% (14/187) (95% CI 6·52–8·48). HCV genotyping was performed for eight patients: genotype 1 (four patients), genotype 2 (two patients), and genotype 3 (two patients). Liver transaminase elevations were present in 10 of these patients. One patient had clinically evident cirrhosis and hepatocellular carcinoma. A history of injecting drug use was present in three patients. Co-infection with human immunodeficiency virus (HIV) was present in one patient and another had co-infection with chronic hepatitis B virus (HBV).
The characteristics of HCV-positive and HCV-negative patients are given in Table 2. We excluded the two patients with previous interferon treatment from this analysis, because they had a negative HCV-RNA since 2002. The pre-bronchodilator FEV1 (l s−1 and % predicted) was statistically lower in HCV-positive patients (0·79±0·22 l, 29·9±8·4%) than in HCV-negative patients (1·02±0·41 l, 36·7±14·6%) (P=0·05, P=0·042, respectively). The HCV-positive patients also had a post-bronchodilator FEV1 (% predicted) significantly lower (34·7±8·6%) than HCV-negative patients (42·7±16·5%) (P=0·011). The amount of cigarette smoking was higher in HCV-positive (median=44 pack years) than in HCV-negative patients (median=20 pack years), but this difference was not statistically significant (P=0·234). In HCV-negative patients, 69·4% (n=118) were in stages III or IV, and 30·6% (n=52) were in stages I or II, according to GOLD criteria. All HCV-positive patients, except one, were classified in stages III or IV. This difference was statistically significant (P=0·021). The mean distance walked in the 6-min test was not different between groups (HCV-negative patients: 394·3±116·3 m; HCV-positive patients: 412·1±124·1; P=0·612). The MMRC Dyspnoea Scale score was statistically higher in HCV-positive patients (median=4) than in HCV-negative patients (median=2) (P=0·023). BODE index was significantly higher in HCV-positive (median=6) than in HCV-negative (median=4) patients (P=0·027). The history of past blood transfusion was not different between groups (P=0·065). Injecting drug use was more common in HCV-positive than in HCV-negative patients (P=0·021). The number of emergency department visits and hospitalizations in the past 2 years were not statistically different between HCV-positive (IQR 0·00–1 and 0·00–2·8, respectively) and HCV-negative (IQR 0·00–1 and 0·00–1, respectively) patients (P=0·939, P=0·570, respectively).
Table 2. Characteristics of HCV-positive and HCV-negative patients
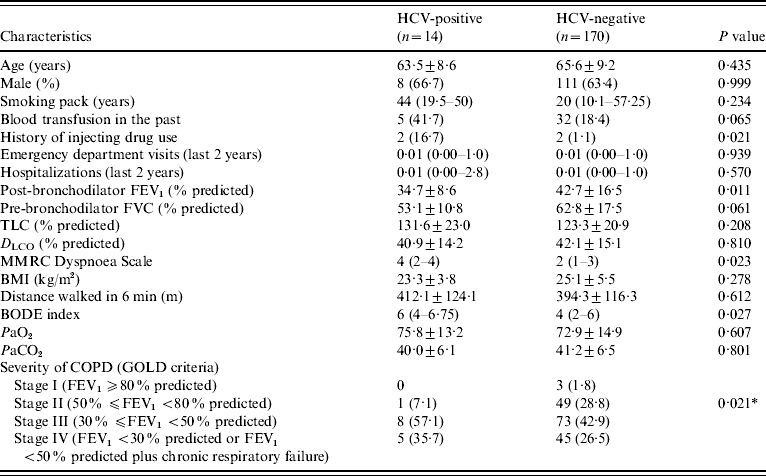
FEV1, Forced expiratory volume in 1 s; FVC, forced vital capacity; TLC, total lung capacity; D LCO, carbon monoxide diffusing capacity of the lung; MMRC, Modified Medical Research Council; BMI, body mass index; COPD, chronic obstructive pulmonary disease; BODE, body mass index, airflow obstruction, dyspnoea, and exercise capacity; GOLD, global initiative for chronic obstructive lung disease.
Data are presented as mean±s.d., n (%), or median (interquartile range).
* P for the comparison between stages I+II and stages III+IV.
DISCUSSION
In this study, we determined the prevalence of HCV infection in a sample of COPD patients. The anti-HCV antibody test was positive in 10·7% of patients. In 70·6% of these patients, HCV-RNA was positive. Then, the prevalence of HCV positivity (anti-HCV and HCV-RNA positive) was 7·5%.
The significantly high prevalence of HCV infection in COPD patients is a new finding. This prevalence is especially high, if compared to the prevalence in blood donors from our institution, and considerably higher than the overall prevalence in Brazil (1·2–2·0%) [7], and in the world (~3%) [Reference Alter6].
One hypothesis to explain such high prevalence of HCV infection might be that patients with COPD are frequently exposed to risk factors for HCV acquisition during hospitalizations. Several authors related the possibility of nosocomial HCV transmission [Reference Tillman and Manns16–Reference Forns20]. However, we could not find a statistically significant difference in the number of hospitalizations between HCV-positive and HCV-negative patients. To clarify the role of nosocomial HCV transmission it would be necessary to conduct a prospective study with a control group composed of patients with a similar frequency of hospitalizations to that of COPD patients, e.g. patients with heart failure, but this was not done in our study.
Another possible explanation for the high number of HCV-infected patients in our study could be that HCV infection worked as a risk factor for development of COPD, or for disease severity, being associated with a worse prognosis in these patients resulting in a selection bias. Moreover, it is known that chronic HCV infection is associated with multiple extrahepatic manifestations, including pulmonary diseases. Indeed, it has been shown that chronic HCV infection can have both direct and indirect effects on pulmonary tissue. The direct effects on the lungs may present as worsening of lung function in some patients with asthma or COPD [Reference Moormann21]. The only prospective study [Reference Kanazawa, Hirata and Yoshikawa3] to evaluate the association between HCV and COPD included 30 HCV-positive and 29 HCV-negative patients, all with COPD. The annual rates of decline in FEV1 and D LCO were significantly higher in HCV-positive patients. The patients who responded to interferon treatment showed a slower decline of FEV1 than non-responders.
Some reports have suggested an important role for latent viral infections, particularly adenovirus and HIV, in the aetiology and/or progression of COPD. Investigators have hypothesized that chronic HCV infection may also increase the risk for development and/or exacerbation of COPD [Reference Retamales22, Reference Diaz23], through induction of chronic immune activation and inflammation [Reference Moormann21].
T lymphocytes are typically observed within the hepatic parenchyma in patients with chronic HCV infection, and seem to contribute to the pathological alterations associated with HCV [Reference Koziel24]. Smokers with COPD have an increased number of CD8+ T lymphocytes in central and peripheral airways in comparison with asymptomatic smokers. The number of CD8+ T lymphocytes is directly related to airway remodelling [Reference Saetta25], and has an inverse relationship with pulmonary function in COPD patients [Reference Fabbri26]. Studies in individuals chronically infected with HCV have showed an increased level of cytokines, especially interleukin-8 (IL-8), a chemokine that mediates several inflammatory pulmonary processes. IL-8 expression may inhibit the antiviral activity of interferon-γ, and can directly provoke bronchoconstriction. The HCV core protein can increase the IL-8 expression in pulmonary fibroblasts. COPD patients have an influx of neutrophils and increased local pulmonary IL-8 levels [Reference Moormann27]. Similarly, HCV-infected patients, even in the absence of pulmonary disease, have high levels of neutrophils in bronchoalveolar lavage [Reference Yamaguchi28, Reference Idilman29].
In the current study, HCV-positive patients had a more severe COPD compared to those that were HCV-negative. All were classified as having severe or very severe COPD according to GOLD criteria, they also had a significantly lower BODE index score. Most (70%) of our patients already had a previous diagnosis of HCV infection, with a mean time of diagnosis of 4·4±1·8 years. It is possible that chronic HCV infection could have contributed to the severity of pulmonary disease in these patients. However, one of the limitations of our study is that it is a cross-sectional study, which cannot provide evidence of a cause–effect relationship. Only prospective studies with long-term follow-up can establish the possible aetiological or prognostic role of HCV infection in COPD patients.
HCV infection was highly prevalent in COPD patients in our study, and this finding can justify the screening of this population. It is well-known that the screening of high-risk populations can lead to early detection of individuals at risk of progressive hepatic disease who can benefit from antiviral therapy [Reference Mallette, Flynn and Promrat30]. Moreover, successful HCV treatment might help to reduce the decline observed in FEV1 in this population. Nevertheless, additional studies are necessary to confirm the high prevalence of HCV in COPD, as well as its role in disease severity and progression.
Our results suggest a high prevalence of chronic HCV infection in patients with COPD in comparison with the prevalence in the health population, represented by the blood donors from our hospital. Further, HCV-positive patients had findings suggestive of a more severe disease. These results, if confirmed in larger prospective, randomized controlled trials, could contribute to a better understanding of the pathogenesis of both conditions, and could potentially improve the care of HCV-positive patients with COPD.
DECLARATION OF INTEREST
None.




