Introduction
Unearthed and undisturbed sediment sequences hold substantial potential as unique stratigraphic archives that contain vital information on archaeological, climatological and palaeoecological contexts. The stored sediments and soil samples from older excavations, however, are often at risk due to changes (e.g. micromorphological structural damage and collapsed pollen) that can occur during post-excavation storage (Blake et al. Reference Blake, Goulding, Mott and Poulton2000). The archived organic resource as a valuable future research potential contradicts the reduction in financial investment in museum and archive repositories that is leading to dwindling resources, facilities and in-house skills (Ray et al. Reference Ray, Shepherd, Flinn, Ander and Laperdix2013). It is therefore crucial that these storage challenges are met by technical solutions—both to secure present sediment cores and to enable an increase in contextual environmental research. This project aims to develop a preservation strategy for the long-term storage of archaeostratigraphic sediment cores that accommodates future research and, additionally, reduces energy consumption.
Prevailing procedures
Archaeological excavations and palaeoclimate field studies are often costly, long-term and complex endeavours. One strategy is to extract sediment cores to accommodate the requirements of present and future in-depth analyses (Figure 1). This method, however, leaves excavation leaders, museums and universities with large quantities of sediment cores for storage (Harrison et al. Reference Harrison, Bartolini, DeSilvey, Holtorf, Lyons, Macdonald, May, Morgan and Penrose2016). As air-dried sediments will lose important physical properties, the prevailing procedure is to store sediment cores in cold stores. As an example, air-filled palynomorphs (microscopic plant and animal structures) tend to collapse under uncontrolled dehydration in ambient conditions (Fægri et al. Reference Fægri, Kaland and Krzywinski1989). Long-term storage in cold stores also results in the slow drying of the sediment and/or fungal growth on and inside the cores, as well as changes in biochemistry, bacteriology and bulk carbon properties (Clark & Hirsch Reference Clark and Hirsch2008). The latter especially can bias radiocarbon dates, and NPP (non-pollen palynomorph) and sedaDNA (sedimentary ancient DNA) analysis. As a result, comparative studies have revealed that freezing is more suitable for long-term storage of environmental samples (Sun et al. Reference Sun, Cai, Chang and Bhatti2015). Freezing is, however, not sufficient to preserve all organic properties, even with fast nitrogen-freezing (Thieme et al. Reference Thieme, Graeber, Kaupenjohann and Siemens2016); when frozen uncontrolled drying will still occur. Therefore, new solutions are urgently needed.
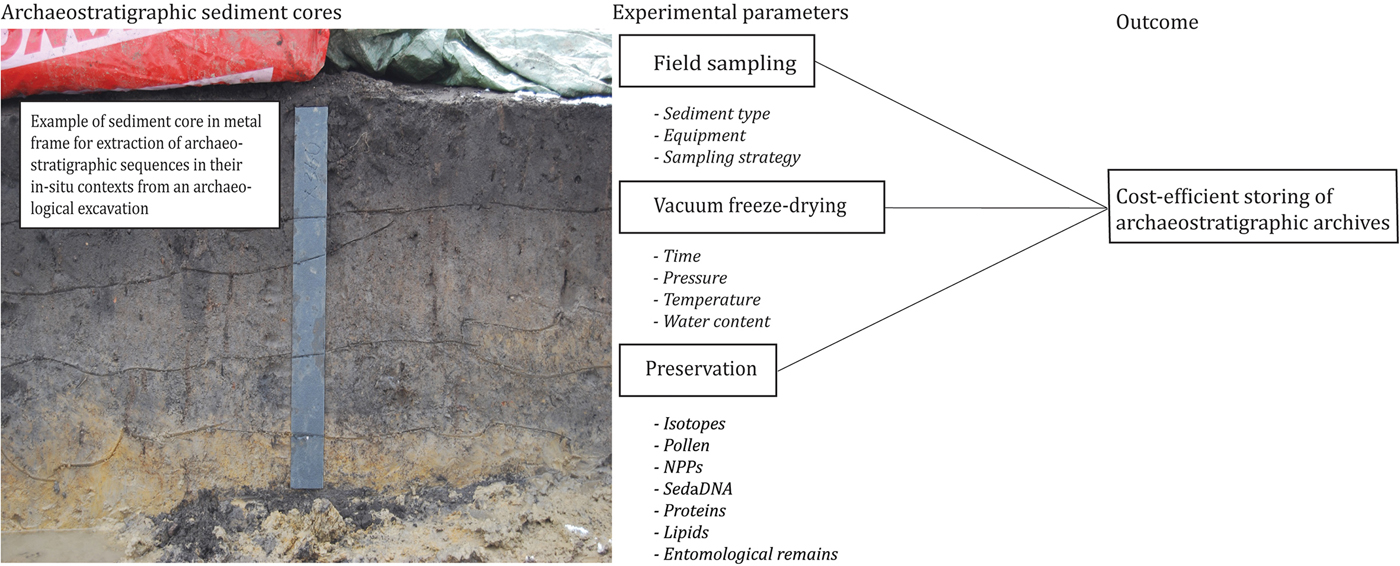
Figure 1. Overview of experimental parameters in the survey setup (photograph courtesy of Moesgaard Museum).
Vacuum freeze-drying of sediment cores
Palynological laboratories often vacuum freeze-dry small sediment samples to preserve the morphology and structure of palynomorphs, such as pollen (Tirlea et al. Reference Tirlea, Beaudoin and Vinebrooke2015). In preliminary studies, we have compared pollen preservation in soil samples from a vacuum freeze-dried sediment core with pollen preservation in fresh samples from the same sediment core and found a convincing equivalence. We therefore propose to introduce vacuum freeze-drying, under controlled conditions, of smaller and larger sediment cores in order to preserve several proxies, such as palynomorph morphology, sedaDNA and NPPs for long-term storage (Figure 2). As a prerequisite, the treatment will prevent slow drying and cracking of the sediment surfaces, microbial alterations of organic properties and/or fungal growth on the core surfaces.

Figure 2. The large freeze-dryer at the Moesgaard Museum is ideal for the processing of sediment cores under controlled conditions such as time, pressure, water content and temperature, as well as energy consumption (photograph courtesy of the Moesgaard Museum).
We will explore the possibilities of a controlled vacuum freeze-drying of sediment cores and conduct experiments using:
i) Highly flexible software, in terms of semi-automatic or fully manually controlled.
ii) Wireless temperature probes.
iii) Experimenting with a range of pressures, freezing temperatures and time intervals.
iiii) Different sediment types, such as gyttja, peat and sand.
Our analytical work will include a review of the North European storage facilities, long-term costs of energy consumption and the future need for archaeostratigraphical archives. The outcome of this project will facilitate future climate, palaeoecological and archaeological research, and will have the potential to be implemented on a worldwide scale. The preliminary studies are supported by Moesgaard Museum, and further funding will be applied for during the subsequent international collaboration.
Acknowledgements
The project is based at the Moesgaard Museum, Denmark, and is executed in collaboration with Aarhus University, the Danish Technological Institute and the University of Reading, UK.





