Introduction
Brain tumors represent a diverse group of neoplasms. They can be primary, originating from various cells within the central nervous system (CNS), or secondary, having metastasized from a systemic malignancy outside of the CNS. Furthermore, they can be either benign or malignant. The most common primary malignant brain tumor is glioblastoma (GBM). GBM is an aggressive grade IV glioma in the tumor family that originates from astrocytes, a type of glial cell. Glioma types include astrocytomas and oligodendrogliomas. There are risk factors that have been conclusively shown to affect risk of gliomas, such as ionizing radiation and genetic syndromes, Reference Schwartzbaum, Fisher, Aldape and Wrensch1 and there are a number of potential causative factors that require further investigation, with traumatic brain injury (TBI) being one such factor.
TBI was first proposed as a potential risk factor for the development of glioma by Virchow in 1864. Reference Pérez-Díaz, Cabello, Lobato, Rivas and Cabrera2 Since then, the notion that TBI predisposes an individual to developing an intracranial tumor, both benign and malignant, has been a topic of debate and controversy for many years. Opinions were based largely on case reports, until Parker and Kernohan “reopened the discussion” with the first case–control study of 431 glioma cases in 1931. Reference Parker and Kernohan3 Following this, numerous case reports and observational studies have been published, but the opinions regarding the idea of TBI increasing risk of gliomas remain quite polarized. Because there are significant pathogenic and medicolegal implications of asserting whether a glioma developed secondary to trauma, it became necessary to delineate criteria as to what constituted a causal relationship between TBI and subsequent development of brain tumor. In 1974, Zulch reviewed the literature and put forth the following conditions for a causal relationship Reference Zulch and Mennel4 :
-
1. The patient must have been in good health before suffering the head injury.
-
2. The blow must be severe enough to cause brain contusion and a secondary reparative process.
-
3. The location of the impact and the tumor should correspond exactly one to the other.
-
4. There should be a time interval between trauma and the appearance of the tumor of at least 1 year, a longer latent period increasing the likelihood of a causal relationship.
-
5. The presence of the tumor must be proved histologically.
-
6. Trauma should consist of an external force.
Manuelidis then delineated three additional criteria for a causal relationship in 1978 Reference Manuelidis5 :
-
1. The traumatized brain must also be proved histologically.
-
2. Bleeding, scars, and edema secondary to the presence of the tumor must be clearly differentiated from that caused by trauma.
-
3. Tumor tissue should be in direct continuity with the traumatic scar, not merely in its vicinity or separated by a narrow zone of healthy or slightly altered brain tissue.
With defined criteria, authors were held to these standards when publishing case reports. As the seventh criteria suggests, the only way to reliably distinguish post-traumatic glioma from previously normal brain parenchyma is to have histologic confirmation of the absence of tumor at the time of TBI. Traumatized brain is not routinely sent for histological examination, so this criterion is not feasible to adhere to. With the advent of contrasted CT and MRI neuroimaging techniques, Moorthy et al. suggested the addition of radiologic criteria. Reference Moorthy and Rajshekhar6 The authors recommended that neuroimaging should be used in the following three instances: (1) at the time of injury to demonstrate significant TBI, (2) shortly after resolution of TBI to demonstrate no evidence of intracranial mass, and (3) at the time of brain tumor diagnosis to demonstrate tumor development at the exact site of prior TBI.
The existing literature primarily consists of anecdotal case reports, as well as several case–control studies and a few cohort studies. While many of these studies have reviewed aspects of the pertinent literature, there has not been a comprehensive review encompassing all of both case reports and observational studies. This review includes the literature published from 1978 to present, as 1978 was the year that Manuelidis finalized the criteria necessary to demonstrate a causal relationship between TBI and glioma.
Materials and Methods
In order to obtain a comprehensive pool of studies that investigated and reported the potential association between TBI and subsequent development of glioma, PubMed was systematically searched using the following terms: concussion, head trauma, neurotrauma, brain injury, TBI, post-traumatic, brain tumor, brain malignancy, brain neoplasm, intracranial tumor, intracranial malignancy, intracranial neoplasm, astrocytoma, oligodendroglioma, glioma, and GBM multiforme. Additionally, each key word was cross-referenced with each other. The search included studies that (1) were published from 1978 to January 2022, (2) were written in English, (3) were conducted in adults, and (4) reported or investigated the potential relationship between TBI and gliomas. Brain tumors other than gliomas, such brain metastases and meningiomas, were excluded from this review.
All studies, with the exception of a few which were limited to the abstracts, were reviewed. For the case reports, variables of interest included patient demographics (age, gender), mechanism of injury (blunt injury by object, fall, motor vehicle accident, penetrating injury by object), type of brain injury (contusion, skull fracture, penetrating craniocerebral injury, intracerebral hemorrhage, subarachnoid hemorrhage), surgical intervention at the time of TBI, latency between TBI and glioma diagnosis, location of TBI/glioma, and type of glioma. For the observational studies, variables of interest included the study type, number of cases, TBI definition, tumor type(s), and findings. There were many early studies that were published in German, which are not reviewed here but are referenced by Stendel et al. Reference Stendel, Théallier-Jankó, Höll and Brock7 This review did not require Institutional Review Board approval.
Results
Case Reports
We identified 19 case reports describing 25 patients who were diagnosed with gliomas after TBI (Table 1). Reference Pérez-Díaz, Cabello, Lobato, Rivas and Cabrera2,Reference Moorthy and Rajshekhar6–Reference Juškys and Chomanskis23 About 77% of the patients were male. At the time of initial TBI, the average age of the patients was 41 (ranging from 17 to 66). At the time of glioma diagnosis, the average age of the patients was 55 (ranging from 29 to 68). The average latency period between TBI and glioma diagnosis was 15 years (ranging from 2 to 48 years). The TBIs included contusions, skull fractures, bleeds, and penetrating craniocerebral injuries. We also recorded the presence or absence of penetrating craniocerebral injury and surgical intervention, as these processes significantly disrupt the brain parenchyma. Eight patients had neither a penetrating craniocerebral injury nor surgical intervention at the time of injury, and 15 patients had either penetrating craniocerebral injury, surgical intervention at the time of injury, or both. This was unable to be determined for two patients due to review restricted to abstract only or because it was not reported. The location of the initial TBI and subsequent glioma formation occurred in both hemispheres (64% in left hemisphere) and in all cerebral lobes (most commonly in frontal lobes, 50%). Most of the patients, 66%, were diagnosed histologically with GBMs. Most of the others were diagnosed with anaplastic astrocytomas, and three patients had low-grade gliomas (two grade II oligodendrogliomas and 1 grade II astrocytoma that subsequently transformed into a grade III anaplastic astrocytoma on repeat biopsy).
Table 1: Case reports

The bold font specifies studies that were not available for full review; “–” indicates that the information is not available from the abstracts.
Observational Studies
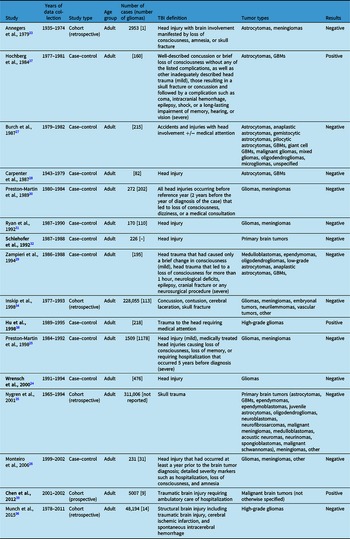
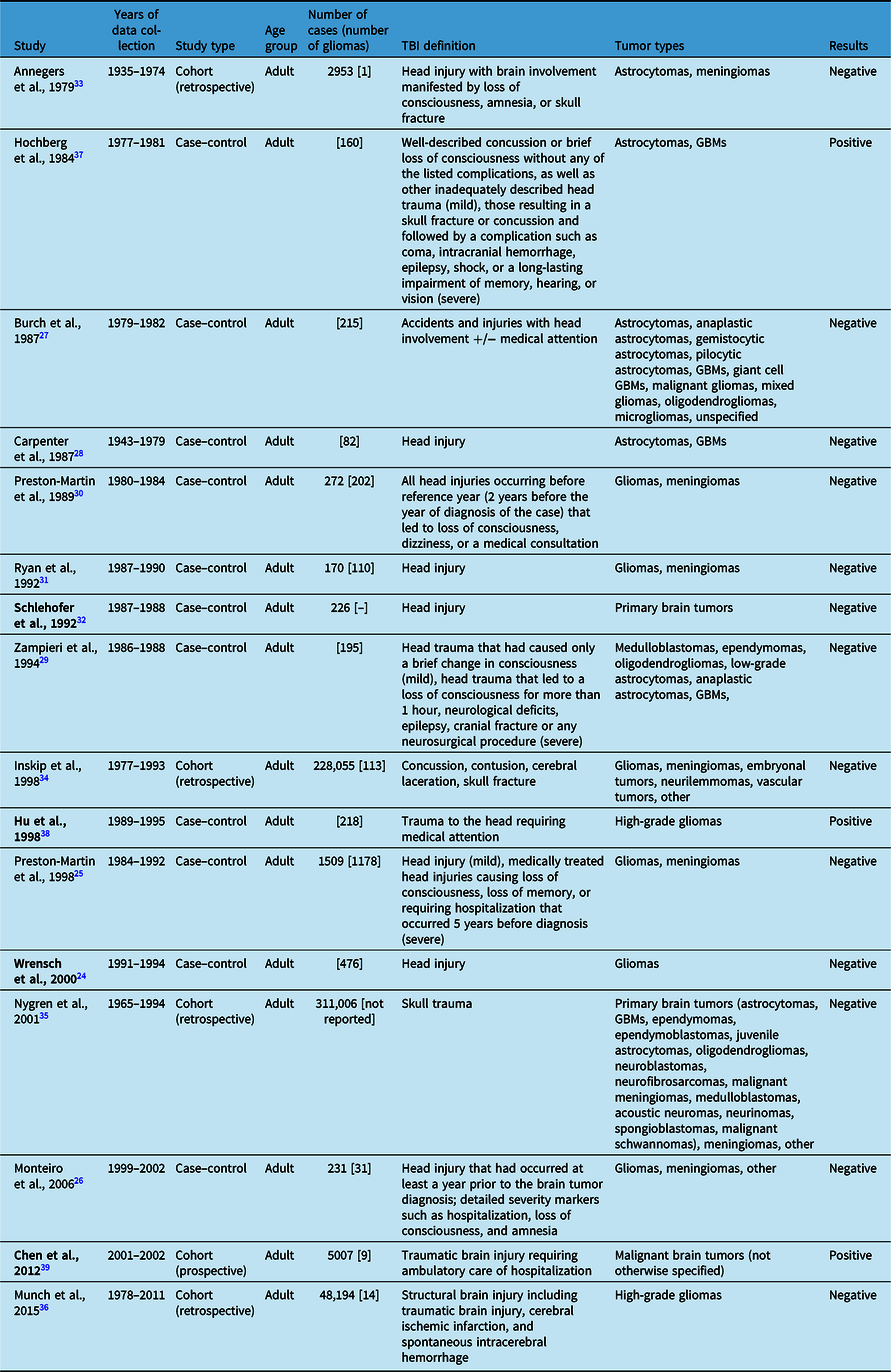
We identified 16 observational studies that sought to investigate the relationship between TBI and malignant brain tumors: 11 case–control studies and 5 cohort studies (Table 2). Many of these studies examined numerous risk factors and the development of various types of malignant brain tumors, but our discussion focused on the findings relating specifically to TBI and gliomas.
Table 2: Observational studies
The bold font specifies studies that were not available for full review; “–” indicates that the information is not available from the abstracts.
There were 13 observational studies that found no association between TBI and glioma formation, with 9 being case–control studies and 4 cohort studies. Of the case–control studies, only one focused solely on the risk of having had a head injury prior to diagnosis with glioma. Wrensch et al. surveyed 476 adults with newly diagnosed gliomas about their history of head injury. Reference Wrensch, Miike, Lee and Neuhaus24 Compared to controls, the odds ratio (OR) for having had a head injury in glioma cases was 1.3 (CI 1.0–1.7), and the OR for having had a head injury which required medical attention was 1.1 (CI 0.8–1.4). Thus, head injury with or without medical attention was not an important contributor to the risk of glioma formation. Two other studies investigated head injuries as a risk factor, but they included meningiomas in addition to gliomas. Preston-Martin et al. investigated whether there was increased risk of tumor formation after head injuries, including sports-related head injuries, in 1509 adults. Reference Preston-Martin, Pogoda and Schlehofer25 Specific to gliomas in men, they found that the risk for ever having experienced any head injury was 1.2 (CI 0.9–1.5) and severe head injury was 1.1 (CI 0.7–1.6). This is one of two studies that investigated sports-related head injuries, and interestingly, there was a trend toward an inverse association between sports participation 5 or more years before diagnosis and risk of glioma formation. The other study was conducted by Monteiro et al. of 231 adults. Reference Monteiro, Pereira, Koifman and Koifman26 For the glioma cases, they found the risk of having experienced head injury that occurred at least a year prior to brain tumor diagnosis was positive but not statistically significant (OR 1.30, CI 0.71–2.35).
Three case–control studies examined the association of a myriad of risk factors, including brain injury and glioma formation. Burch et al. investigated 215 adults diagnosed with various types of gliomas. Reference Burch, Craib, Choi, Miller, Risch and Howe27 More cases than controls reported accidents or injuries with head involvement (relative risk 2.51, p < 0.0001), but there was not a significant difference when exposure was limited to those that required medical attention (RR 1.20, p = 0.65). The authors suggested that this discrepancy was likely due to recall bias, given that the significance disappeared when restricted to injuries requiring medical attention, which are thought to be less prone to recall bias. Carpenter et al. also focused primarily on gliomas, including astrocytomas and GBMs, and their association with prior head injury. Reference Carpenter, Flanders, Frome, Cole and Fry28 They identified 82 patients but did not find an association between reported prior head injury and brain tumor (OR 0.9, CI 0.2–4.2); for gliomas specifically, the OR was 1.4 (CI 0.3–7.2). A study by Zampieri et al. included 195 patients diagnosed with various types of gliomas and asked about their history of mild or severe head trauma, but there was not a significant association (OR, CI 0.3–1.4). Reference Zampieri, Meneghini and Grigoletto29
Three case–control studies investigated head injury among a number of other risk factors in patients with primary brain tumors not limited to gliomas. Preston-Martin et al. interviewed 272 men with a glioma or meningioma about history of head injury occurring 2 years prior to their tumor diagnosis that led to loss of consciousness, dizziness, or a medical consultation. Reference Preston-Martin, Mack and Henderson30 While they found a significant association for meningiomas, this risk was not significant for glioma development no matter the latency period or number of injuries (OR 0.8, CI 0.5–1.3). Ryan et al. also investigated adults with a diagnosis of glioma or meningioma and inquired about their history of head injury. Reference Ryan, Lee, North and McMichael31 The risk for having had a prior head injury in those with gliomas was not significant at 1.07 (CI 0.45–2.56). Lastly, Schlehofer et al. conducted a population-based study in Germany to investigate several risk factors for the development of primary brain tumors and found no significant association with head injury. Reference Schlehofer, Blettner, Becker, Martinsohn and Wahrendorf32
In addition to the negative case–control studies above, there are four cohort studies that did not find an association between TBI and glioma. Annegers et al. performed one of the earliest observational studies, a retrospective cohort analysis of 2953 adult patients in Minnesota who suffered and survived a “head injury with brain involvement manifested by loss of consciousness, amnesia, or skull fracture.” Reference Annegers, Laws, Kurland and Grabow33 The patients were subsequently followed for a total of 29,859 person-years. They identified four patients who developed brain tumors, three of which were meningiomas and one of which was an astrocytoma. The one astrocytoma did not differ significantly from the expected number of astrocytomas, based on age-specific incidence rates for brain tumors in the local population per one of their earlier studies. Inskip et al. conducted another retrospective cohort study of 228,055 Danish adults who were hospitalized for concussion, contusion, skull fracture, or cerebral laceration. Reference Inskip, Mellemkjaer, Gridley and Olsen34 The patients were then followed for 8 years on average after injury and were evaluated for development of benign or malignant intracranial tumors. Gliomas, specifically, occurred at higher than expected rates during the first year after injury (standardized incidence ratio (SIR) 3.9, CI 2.7–5.5), but the authors suggested the likelihood for head trauma to have caused these tumors that grew rapidly enough to be diagnosed within 1 year was low. When excluding the first year after injury in accordance with the criteria proposed by Zulch and Manuelidis, the risk of developing a glioma was not significant (SIR 1.0, CI 0.8–1.2). Nygren et al. sought to explore the association between various diagnoses related to skull trauma and brain tumor development through a retrospective cohort study of 311,006 patients contributing to 3,225,317 person-years of follow-up in Sweden. Reference Nygren, Adami and Ye35 They identified a total of 281 cases and found that there was no association between TBI and overall risk of primary brain tumors (SIR 1.0, CI 0.9–1.2), nor in subgroup analyses of tumor type, age, year of follow-up, and severity of brain injury. Lastly, based on the hypothesis that any condition causing astrogliosis could lead to development of an astrocytic tumor, Munch et al. performed a retrospective cohort study of 404,812 adults in Denmark who had experienced any structural brain injury, including TBI (48,194), cerebral ischemic infarction, or spontaneous intracerebral hemorrhage. Reference Munch, Gørtz, Wohlfahrt and Melbye36 6152 patients ultimately developed a high-grade glioma, either a grade III anaplastic astrocytoma or GBM, 14 of which were after TBI. There was no significant association after a structural brain injury between 1 and 4 years post-injury (RR 1.14, CI 0.87–1.46), and surprisingly, there was a significant protective effect of structural brain injury after 5 years (RR 0.68, CI 0.49–0.90). For TBI only, the risk of glioma development was 1.99 between 1 and 4 years (CI 1.00–3.50) and 0.32 after 5 years or more (CI 0.10–0.75).
There were three studies that found positive associations between TBI and glioma, two of which were case–control studies. Hochberg et al. published a case–control study of 160 adults with high-grade gliomas in the Boston area. Reference Hochberg, Toniolo and Cole37 Data were collected by questionnaire and telephone interview, and patients were asked about whether they had sustained either a mild or severe head injury prior to tumor development. They found that there was an association between formation of high-grade glioma after head injury, but only in those who sustained a severe injury at age 15 or older (RR 10.6, CI 2.1–53.3). Hu et al. performed a case–control study of risk factors for 281 adults with high-grade gliomas in China. Reference Hu, Johnson and Mao38 They found that trauma to the head requiring medical attention was associated with increased risk of glioma (OR 4.09, CI 2.51–10.31).
There was one cohort study that found a positive association between TBI and development of malignant brain tumor. Furthermore, this is the only prospective cohort study to our knowledge. Chen et al. conducted this prospective cohort study of 5007 adults in Taiwan who had sustained a TBI requiring ambulatory care or hospitalization. Reference Chen, Keller, Kang and Lin39 The patients were then followed for 3 years after injury to assess the risk of developing a malignant brain tumor. Within 3 years of surveillance, patients were more likely to develop a malignant brain tumor than controls, with risk being 4.67 times greater (CI 1.84–11.83). There was also an association between brain injury severity and malignant brain tumor (p = 0.033).
Discussion
Since the idea was first proposed, TBI as a potential risk factor for glioma development has been a controversial topic, particularly when considering the medicolegal implications. In an effort to better elucidate this relationship, we reviewed the pertinent literature between 1978 and 2022 and identified 19 case reports (detailing 25 patient cases) and 16 observational studies (11 case–control studies, 5 cohort studies). To our knowledge, ours is the first comprehensive review of both case reports and observational studies.
The 19 case reports detailed a total of 25 patient cases in which a glioma formed at the exact site of prior TBI. Demographically, this was observed in a wide range of adult ages; of note, glioma formation after TBI has also been observed in children, but those studies were not reviewed here. Reference Howe, Burch, Chiarelli, Risch and Choi40–Reference Gurney, Preston-Martin, McDaniel, Mueller and Holly44 Cases were also observed in patients of both genders. Over 75% of the patients were male, which may represent a differential trend between genders or reflect the increased risk of TBI in males. According to the TBI Model System National Database Statistics from 2017, males accounted for 73% of TBIs reported. Reference Capizzi, Woo and Verduzco-Gutierrez45
Among the cases described, patients were diagnosed histologically with grade II, III, or IV gliomas, with 88% of the tumors being high-grade tumors; there were only three cases of low-grade gliomas after TBI, and one of the cases was reclassified as a high-grade glioma after a repeat biopsy, which may have reflected tumor progression or initial sampling error. Glioma formation occurred after a variety of TBI mechanisms, including contusions, skull fractures, hemorrhages, and penetrating craniocerebral injuries. One proposed explanation is that there may be neoplastic proliferation of “initiated” cells from disruption of the blood–brain barrier or cerebrovascular architecture by a penetrating foreign object or surgical instrumentation. Consistent with this proposed mechanism, 15 patients did have a penetrating craniocerebral injury and/or surgical intervention at the time of injury, but interestingly, glioma formation was reported in 8 patients that had not sustained either invasive event. The latency period between TBI and glioma diagnosis ranged widely from 2 to 48 years. According to the proposed guidelines for a causal relationship, there should be a time interval of at least 1 year, but given the aggressive nature of GBMs, it is reasonable that the number of cases reported in the literature may be underestimated through timing exclusion. Gliomas form in both hemispheres and all cerebral lobes. The most common location is the frontal lobe, which may correlate with the fact that the frontal lobe is particularly vulnerable to injury due to its large size and the bony prominences of the front of the skull. Reference Bigler46
Of the 16 observational studies identified, there were 11 case–control studies and 5 cohort studies, with conflicting negative and positive findings. There were 13 observational studies (9 case–controls, 4 cohorts) that found no association between TBI and glioma formation, while 3 observational studies (2 case–controls, 1 cohort) found such an association. These observational studies, and in turn our systemic review, have several limitations. First, these studies are limited by their retrospective nature, predisposing them to recall bias as many of these studies relied on patient recall on questionnaires; patients with brain tumors may be more likely to recall and report any head trauma than control patients. Furthermore, many studies used review of records to determine details of prior brain injury, which often did not clearly report mechanism, severity, and location of brain injury, further predisposing these studies to misclassification bias. This limitation of data collection makes it difficult to determine the extent to which the cases adhered to the Zulch and Manuelidis criteria, such as demonstrating that the location of injury impact and tumor corresponded. On a similar note, it is important to recognize the advent of CT and MRI neuroimaging around the same time that these criteria were being proposed and updated. However, it was not until 2004 that Moorthy et al. proposed radiologic criteria for demonstrating the absence of glioma at the time of injury and the development of glioma at the exact site of prior TBI. Some of the observational studies, although published in 1978 or later, used data that were collected in years prior and did not have neuroimaging to establish the absence of glioma at the time of TBI. The advancements in neuroimaging modalities over time may have also increased the likelihood of identifying neuropathology in later studies. Lastly, all observational studies have small sample sizes, largely due to the rarity of gliomas.
The lone prospective study found a positive association between TBI and glioma, a 4.67 times greater risk of developing a malignant brain tumor within 3 years of TBI, as well as an association between TBI severity and development of a malignant brain tumor. Reference Chen, Keller, Kang and Lin39 Despite the larger number of observational studies that found no association, this prospective cohort study had the highest level of evidence. Methodologically, they utilized a nation-wide registry, allowing for a large, population-based study (>98% of the Taiwanese population) and longitudinal follow-up. Furthermore, their registry requires biopsy and histological verification in order to make a definite diagnosis of brain malignancy. However, several limitations of this should be noted. First, this study was a population-based study in Taiwan, which may not allow the results to be generalizable. Second, there was no active surveillance during the follow-up period for the TBI and control groups; thus, there is the possibility of ascertainment bias, such that patients with persistent TBI-related symptoms may have received more neuroimaging during the follow-up period and thus were more likely to have brain malignancy detected. Third, the study used an arbitrary 3-year follow-up period; it is possible that their findings are underestimated given that malignant brain tumors may have a longer latency. Lastly, the authors report the diagnoses of “malignant brain tumor” but do not further specify histopathological or molecular classification which would be helpful to note.
Given these inconsistent findings and study limitations, additional research is required to better elucidate the relationship between TBI and development of glioma. The ideal study design would be a large, prospective cohort study in which TBI patients would be identified and followed for subsequent development of glioma. This would allow for accurate and precise documentation of the brain injury, and the observed incidence would be compared to the incidence of glioma in the general non-TBI population. Another approach would be to focus research efforts on identifying the possible mechanism by which glioma formation from prior TBI occurs. Thus far, there have been several proposed mechanisms for oncogenic transformation. One possible mechanism suggests that the inflammatory response following structural TBI may predispose to tumor formation by cellular proliferation beyond normal repair; chronic inflammation is a predisposing factor in other solid tumors such as GI malignancies. Reference Tyagi, Theobald and Barger21 Another proposal is Knudson’s two-hit hypothesis, having inherited a loss of one copy of a tumor suppressor gene with TBI leading to the second “hit”. Reference Sabel, Felsberg, Messing-Jünger, Neuen-Jacob and Piek11
Another interesting consideration is the possible interpretation of these studies in the context of sports-related TBI, particularly as this has become an increasingly popular topic in the medical community and popular culture. Every published case report described a moderate to severe injury prior to glioma formation, in which there was evidence of skull fracture, contusion, hemorrhage, and/or penetrating injury. These severe injuries are rare occurrences in sports-related TBI, which is typically considered a “milder” form of TBI (i.e., concussion). Sports-related head injuries were investigated in two of the observational studies, both of which found no association. Reference Preston-Martin, Pogoda and Schlehofer25,Reference Inskip, Mellemkjaer, Gridley and Olsen34 While there are no case reports or observational studies to suggest a link between sports-related TBI and glioma formation, it is worth noting that repetitive “mild” sports-related TBI has been linked to a different neurologic process called chronic traumatic encephalopathy (CTE) that presents with neuropsychological symptoms. CTE is a progressive neurodegenerative disorder that is characterized by perivascular deposits of tau, proposed to occur in the setting of acceleration-deceleration mechanism leading to axonal injury, breakdown of the blood–brain barrier, neuroinflammation, and ultimately hyperphosphorylation of tau. Reference McKee, Stein, Kiernan and Alvarez47
Conclusions
Dr Harvey Cushing once commented, after struggling with a post-traumatic meningioma in General Leonard Wood, that brain tumors after head trauma occurred “too often to be ignored.” Reference Cushing48 While these sentiments were in regard to a nonmalignant brain tumor, review of the literature of post-traumatic glioma reveals numerous case reports of the development of glioma at the precise site of prior severe TBI in continuity with traumatic scar. Although there are conflicting positive and negative findings in regard to his association in the observational studies, these studies have significant limitations from which definitive conclusions cannot be made. Overall, we suggest that glioma formation after TBI is a rare occurrence, but is certainly possible. This is an important association to better elucidate as it relates to patient care and legal ramifications. Future directions could include investigating which patients may be at highest risk for glioma formation after TBI.
Statement of Authorship
MH contributed to the concept for the manuscript, the systematic search of articles, the review of the literature, the manuscript writing, and the final approval for submission. EP provided expert mentorship and contributed to critical review of the manuscript, the manuscript editing, and the final approval for submission.
Disclosures
MH has nothing to disclose. EP has nothing to disclose.