Parkinson disease (PD) is a neurodegenerative disorder of unknown aetiology that causes motor and non-motor symptoms (NMS) and signs. Motor symptoms of PD have long been the main focus in the diagnosis and the management of patients. However, most PD patients experience other symptoms including depression, anxiety, sleep disorders, bowel and/or bladder problems, other autonomic disturbances, and sensory complaints. Cognitive difficulties also occur and are associated with adverse outcomes such as nursing home placement and increased mortality. These symptoms have been termed NMS by Chaudhuri and others.Reference Chaudhuri, Healy and Schapira 1 The NMS Questionnaire (NMS Quest) was developedReference Chaudhuri, Martinez-Martin and Schapira 2 and validatedReference Savica, Rocca and Ahlskog 3 to screen for the presence of NMS.Reference Chaudhuri and Martinez-Martin 4 , Reference Chaudhuri and Naidu 5 The NMS Quest is a 30-item screening questionnaire to be used by the patient/caregiver as a screening tool. It contains ten domains to cover gastrointestinal symptoms, urinary tract symptoms, sexual functions, cardiovascular issues, apathy/attention/memory concerns, hallucinations/delusions, depression/anxiety, sleep problems/fatigue, pain, and miscellaneous complaints such as diplopia and weight loss.Reference Chaudhuri, Martinez-Martin and Schapira 2 In addition, effort has been made to update the Unified PD Rating Scale (UPDRS)Reference Goetz, Tilley and Shaftman 6 to better address non-motor features of PD.Reference Goetz, Fahn and Martinez-Martin 7 Individual rating instruments and scales are available for specific domains. NMS have been underrecognized, undertreated, and are implicated in functional disability experienced by patients with PD.Reference Chaudhuri and Martinez-Martin 4 , Reference Chaudhuri and Naidu 5 The onset of these symptoms may precede the onset of the motor symptoms.Reference Savica, Rocca and Ahlskog 3 This phenomenon is consistent with the Braak staging of the pathology of PD, and possibly related to widespread Lewy body and Lewy neurite deposits, including the olfactory and autonomic systems as well as in other non-dopaminergic nuclei early in the disease process.Reference Braak and Del Tredici 8 , Reference Braak, Rub, Jansen Steur, Del Tredici and de Vos 9
NMS are very common in PD patients. Barone et alReference Barone, Antonini and Colosimo 10 have shown that 98.6% of patients with PD reported NMS in a large collaborative study. The frequency of NMS increased with the disease duration and severity. As the disease advances, NMS can become the most troublesome feature,Reference Hely, Reid, Adena, Halliday and Morris 11 with a high impact on patients’ prognosis and decreased quality of life.Reference Kasten, Kertelge and Tadic 12
Motor phenotypes in PD can be divided into tremor dominant (TD) and postural instability gait difficulty (PIGD) types.Reference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 PIGD patients have predominant axial symptoms. Loss of postural reflexes is one of the characteristic features in the PIGD phenotype, along with freezing of gait. PIGD patients are particularly disabled in comparison to those who have predominantly limb manifestations such as tremor.Reference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 , Reference van Rooden, Visser, Verbaan, Marinus and van Hilten 14 Bulbar dysfunction is also more common in the PIGD group,Reference van Rooden, Visser, Verbaan, Marinus and van Hilten 14 raising the concern that NMS in general might be prominent in the PIGD subtype.
In spite of growing literature on NMS of PD, there are few data on the association between NMS and motor phenotypes of PD, and these usually focused only on specific domains of NMS, such as cognition, mood/anxiety issues, or sleep disorders.Reference Burn, Landau, Hindle, Samuel, Wilson and Hurt 15 - Reference Reijnders, Ehrt, Lousberg, Aarsland and Leentjens 18 A recent study by Herman et alReference Herman, Weiss, Brozgol, Wilf-Yarkoni, Giladi and Hausdorff 19 demonstrated the relationship between NMS and PD motor subtypes. The study revealed non-demented patients from the PIGD subtype experienced more NMS and poorer quality of life compared with the TD subtype.
In our study, we aimed to explore the relationship between motor subtypes and NMS. We performed a retrospective cross-sectional chart review to clarify the relationship between NMS and PD phenotypes. The two subscores of UPDRS-III, subscore A, which largely correlates with dopamine-responsive features and manifests as nonaxial impairment, and subscore B, which largely correlates to dopamine nonresponsive dysfunction,Reference Levy, Tang, Cote, Louis, Alfaro and Mejia 20 were analyzed in relation to the PD subtypes and clinical features to determine whether subscore A or B or both subscores were independently associated with the NMS of PD. In addition, specific domains of NMS were examined in relation to PD subtypes and medication classes.
Methods
In this cross-sectional study, charts of all PD patients seen in the University of Alberta Movement Disorders Program in Edmonton between January 2009 and the end of 2012 were reviewed. The Movement Disorders Program is the only specialized PD clinic in northern Alberta. All patients were assessed and diagnosed by movement disorders neurologists. All patients with an established diagnosis of PD were given the NMS Quest form to fill out. The NMS Quest was prospectively completed at each clinic visit. When necessary, patients were instructed by a Movement Disorders Program staff member about how to complete the questionnaire before assessment by a movement disorders neurologist.
The diagnosis of PD was based on UK Brain Bank Criteria.Reference Daniel and Lees 21 Age of onset was defined as the age of the first motor symptom of PD. Duration of PD was defined as the period between the first motor symptom of PD and the clinical evaluation by a movement disorders neurologist. Cognitive function was assessed with the Mini-Mental State Examination (MMSE) during the same clinic visit. Motor function was evaluated by a movement disorders neurologist, using the UPDRS-III score.Reference Fahn and Elton 22 The motor phenotype was divided into TD, indeterminate. or PIGD typesReference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 based on the UPDRS. The TD group was defined by a ratio of mean tremor score/mean PIGD score ≥1.5 and the PIGD group was defined by a ratio of ≤1, as described previously.Reference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 If the ratio was between 1 and 1.5, the patient was placed in the indeterminate type group. PIGD score is composed of falling, freezing, walking, gait, and postural stability representing axial symptoms from UPDRS-II and -III. Individual medication and dosage were systematically recorded, and dosage of PD medications was adjusted to levodopa equivalent dose (LED) by using a conversion formula, as previously reported.Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke 23
Differences in demographic characteristics, disease severity, disease duration, and NMS severity were first analyzed with one-way analysis of variance (ANOVA). To determine whether NMS burden varied according to PD subgroup, we compared the TD, indeterminate, and PIGD groups. The primary outcome measure of interest was the relationship between NMS and PD motor subtype. Groups were compared using one-way ANOVA for continuous variables and chi-square tests for categorical variables. Analyses were carried out in a stepwise manner. Disease duration and disease severity were added as covariates in analyses involving group comparisons predicting NMS in analyses of covariance.
The contribution of disease severity to NMS was examined next. The correlation between NMS severity and UPDRS-III scores was evaluated using regression models. The UPDRS-III is divided into six motor domains (tremor, rigidity, bradykinesia, facial expression, speech, and axial impairment) and two subscores, subscore A (tremor, rigidity, bradykinesia, and facial expression), and subscore B (speech, arising from chair, gait, and posture stability),Reference Levy, Tang, Cote, Louis, Alfaro and Mejia 20 thus allowing for analysis of the entire cohort. Patients with more axial symptoms tend to be the PIGD type, with a higher subscore B. Analyses were performed to determine whether subscore A or B or both subscores were independently associated with the NMS of PD in the overall group. Subgroup analysis for relationship between the PD motor subtype and the NMS was then further analyzed using linear regression models. Standardized estimated regression coefficients (β) and coefficients of multiple determination (R 2) were calculated, with p<0.01 chosen to be significant. The lower p value was determined to correct for increased type 1 error rate because there were multiple comparisons in the linear regression model. The prevalence of each NMS among different PD motor subtypes was analyzed using chi-square test. The difference was then adjusted for disease severity (UPDRS-III) and disease duration in binary logistic regression models. All analyses were performed with GraphPad Prism6 and SPSS, version 21.
Results
A total of 398 charts were reviewed and 274 were analyzed. Charts were excluded due to missing components of the NMS Quest, MMSE, or UPDRS. Demographic data are shown in Table 1. There were more males than females. Young onset PD (age of onset, ≤45 years) composed 11.3% of the sample. Among the cohort, 145 patients were TD type (52.9%) and 104 were PIGD type (38.0%), with the remaining 25 patients (9.1%) falling into the indeterminate group. The average age of disease onset was 59.7±10.9 years, and average age at the time of assessment was 65.4±10.0 years. The majority of the patients were in the early to mid-stage of the disease with an average Hoehn and Yahr (H&Y) stage of 2.1±0.5. For the 124 charts that were excluded, disease duration, average age at disease onset, gender distribution, MMSE, H&Y staging, total UPDRS-III, and the LED daily dose were did not differ significantly from the patients included in the analysis (data not shown).
Table 1 Demographic and clinical characteristics of patients

H&Y=Hoehn and Yahr; LOPD=late onset PD; PD=Parkinson disease; UPDRS=Unified Parkinson’s Disease Rating Scale; YOPD=young onset PD.
* Subscore A: tremor, rigidity, bradykinesia, and facial expression.
† Subscore B: speech and axial impairment.
Comparing the TD, indeterminate, and PIGD groups (Table 2), PIGD patients had longer disease duration (p=0.0006), more advanced disease (p<0.0001 for H&Y staging; p=0.0001 for UPDRS-III total score), higher subscore B (p<0.0001), worse NMS profile (p=0.0006), and a higher dose of PD medications (p=0.0007). In the analyses of covariance, the covariates, disease duration, and severity were significantly related to the NMS profile in PD patients (F=4.56, p<0.001 and F=4.90, p<0.001, respectively). The total NMS Quest score was significantly higher in the PIGD group when compared with the TD and indeterminate groups after controlling for disease duration and severity (UPDRS-III).
Table 2 Demographic characteristics of TD, indeterminate, and PIGD groups

*Statistically significant.
H&Y=Hoehn and Yahr; LED=levodopa equivalent dose, NMS=non-motor symptoms; PIGD=postural instability gait difficulty, PD=Parkinson disease, SD=standard deviation, TD=tremor dominant, UPDRS=Unified Parkinson’s Disease Rating Scale.
Subscore A: tremor, rigidity, bradykinesia, and facial expression.
Subscore B: speech and axial impairment.
To determine whether the NMS in PD were affected by age, disease duration, severity of PD, cognitive status, or the impact of levodopa treatment, univariate analyses (Table 3) were performed in the overall group. The univariate analyses showed that age, gender, and MMSE did not correlate with NMS. In contrast, disease duration and disease severity (UPDRS-III) were significant predictors of NMS (p=0.009 and p=0.0001, respectively). LED dose (p=0.009, R 2=0.07) also correlated with a higher NMS.
Table 3 Correlation between multiple determinants with NMS and motor measures of pd using univariate linear regression models in the overall PD group
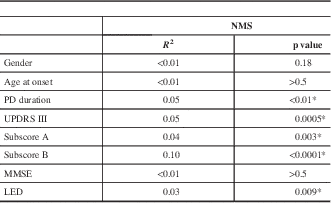
*Statistically significant.
LED=levodopa equivalent dose; MMSE=Mini-Mental State Examination; NMS=non-motor symptoms, PD=Parkinson’s disease, UPDRS=Unified Parkinson’s Disease Rating Scale.
Subscore A: tremor, rigidity, bradykinesia, and facial expression.
Subscore B: speech and axial impairment.
UPDRS-III total score and H&Y staging correlated with a higher NMS Quest score (p=0.0001, and p<0.0001, respectively). Subscores A and B also correlated with a higher NMS burden (Table 3; p=0.003 and p<0.0001, respectively). In multiple regression analyses, only disease duration, subscores A and B, and LED were included; age at disease onset, gender, and MMSE were not included because univariate analysis showed no correlations. Two models were used. Model 1 analyzed the correlations between the subscore A, subscore B, and LED with NMS severity; this model was significant with an R 2 of 0.142. Subscore A did not show any correlation, but subscore B (p<0.001) and LED (p<0.001) were both significantly associated with higher NMS burden. Model 2 examined the subscore A, subscore B, and duration of disease in relation to NMS severity. The model was significant (R 2=0.152). Duration of disease and subscore A did not correlate with NMS. Subscore B was associated with a higher NMS score in the multiple regression analysis (p=0.001).
Further subgroup analyses showed that in the TD and indeterminate groups, neither the total tremor score nor the ratio of mean tremor score/mean PIGD score correlated with NMS. In contrast, in the PIDG type, the total PIGD score was a significant predictor of worse NMS profile (p<0.0001, R 2=0.064), and the ratio of mean tremor score/mean PIGD score also correlated with a higher NMS quest score (p=0.01, R 2=0.055). In addition, in the TD and indeterminate groups, no significant correlation was observed between NMS Quest score and UPDRS-III total score, subscore A, or subscore B. In the PIGD group, there was significant correlation between NMS Quest score and UPDRS-III total score (p=0.0022), subscore A (p=0.0034), and subscore B (p=0.0012) in linear regression models.
Concerning the prevalence of NMS in each motor subtype group, the percentage of patients presenting with each symptom of NMS is summarized in Table 4. The PIGD group had higher proportions of patients endorsing all NMS on the NMS-Quest than TD group, except for nausea, fecal incontinence, weight loss, and leg swelling after adjustment for disease severity and duration (Table 4).
Table 4 Chi-square analysis of patient proportions endorsing each of the NMS symptoms in different PD motor subtypes

* Statistically significant.
NMS=non-motor symptoms; OH=orthostatic hypotension; PD=Parkinson disease; PIGD=posture instability gait difficulty type; REMBD=rapid eye movement behavior disorders; RLS=restless leg symptoms; TD=tremor dominant type.
PD medication use is summarized in Table 5 for each PD subtype. The relationships between NMS and different PD medications were analyzed because some PD medications can produce and exacerbate many of the NMS. Levodopa (including the CR formulation and Stalevo, with dose conversion to LED) correlated with total NMS burden (p=0.003) and with gastrointestinal symptoms (p=0.001), urinary symptoms (p<0.001), sensory complaints (p=0.004), neuropsychiatric issues (p=0.01), sexual dysfunction (p=0.005), orthostatic hypotension (p=0.004), and sleep difficulties (p=0.001). Dopamine agonists (adjusted and converted to LED) also correlated to the severity of NMS (p=0.007). Among all domains of symptoms, dopamine agonist usage correlated with neuropsychiatric symptoms (p=0.003), orthostatic symptoms (p=0.006), sexual dysfunction (p=0.01), and pain (p=0.005). The use of amantadine and anticholinergic agents, however, did not show any correlations to NMS severity in any group (data not shown), likely because of the very low percentage of patients on these agents.
Table 5 PD medication use in the motor subtypes
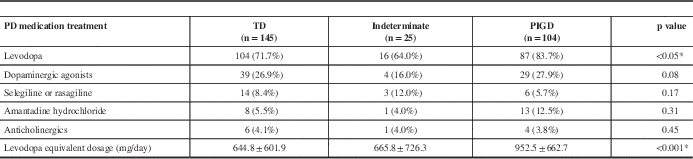
* Statistically significant.
NMS=non-motor symptoms; PD=Parkinson’s disease; PIGD=posture instability gait difficulty type; TD=tremor dominant type.
Discussion
NMS are common in PD. These symptoms often contribute to disability and impact negatively on quality of life even in early-stage disease.Reference Duncan, Khoo, Yarnall, O’Brien, Coleman and Brooks 24 They are frequently underrecognized and undertreated. These symptoms are diverse and may reflect dysfunction in non-dopaminergic systems, though dopaminergic transmission dysfunction may play a role. In spite of growing literature focusing on NMS of PD in the past few decades, there are limited data on the association between NMS and motor phenotypes of PD. Identifying patient groups at risk might be of clinical value because treating such patients may have a positive impact on quality of life.
In the current study, we showed that PIGD patients had longer disease duration, more advanced disease, and a poorer NMS profile. After adjusting for disease duration and severity (UPDRS-III), this group of patients still had a significantly higher NMS Quest score, and the difference of NMS prevalence was evident in 26/30 domains. The only domains not differentially affected included nausea, fecal incontinence, weight loss, and leg swelling. In the overall group, disease duration, UPDRS-III score and total LED were significant predictors of NMS. These observations are in agreement with previous reports that long disease duration and more severe disease, as reflected by H&Y stage, are associated with a higher number of NMS.Reference Crosiers, Pickut, Theuns, Deyn, Van Broeckhoven and Martinez-Martin 25
Subscore A correlated with NMS in the whole cohort (Table 3); however, it was not significant in the two multiple regression models we examined. Subscore B, on the other hand, strongly correlated with a higher NMS burden and remained significant in the multiple regression model. These results suggest that in the PD population, those with more axial symptoms as demonstrated by a higher subscore B have a higher NMS burden.
We showed that NMS severity varies according to motor phenotype. Subscore B correlated with higher NMS burden in overall group and predicted worse NMS profile in the PIGD group, but not in the TD or indeterminate group. There was a significant correlation between PIGD score and higher NMS burden in the PIGD group, but no correlation between tremor score and NMS in the TD group. It is well-documented that the TD group has slower disease progression and a more favourable prognosis.Reference O’Suilleabhain 26 , Reference Josephs, Matsumoto and Ahlskog 27 For PIGD type, patients often present with more axial symptoms, or develop them over time, represented by a higher subscore B. This group usually responds poorly to dopaminergic treatment.Reference Muslimovic, Post, Speelman, Schmand and de Haan 28 Between the two major motor subtypes, PIGD patients usually develop more disability than those of TD subtype,Reference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 with more bulbar dysfunction and gait and balance difficulties, reducing functional independence and an overall poorer prognosis.Reference Jankovic, McDermott, Carter, Gauthier, Goetz and Golbe 13 , Reference Kompoliti, Wang, Goetz, Leurgans and Raman 29 This may be due, in part, to the poor responsiveness of subscore B signs to dopaminergic treatment. Our study is consistent with these observations, showing that the PIGD type, which has a higher subscore B, accumulates more NMS.
As PD advances, patients can change from TD type to PIGD type, and develop more NMS subsequently. This is consistent with the observation that higher H&Y staging and UPDRS-III score correlate with a poorer NMS profile. NMS are often more disabling in PD population than motor symptoms. Hely et alReference Hely, Morris, Reid and Trafficante 30 reported that 15 years after PD diagnosis, patients mainly suffered from non-dopaminergic–responsive features. The recent Profiling Parkinson’s disease (PROPARK) study, which is a longitudinal cohort study, showed PD patients with more axial symptoms and NMS were associated with a significantly increased risk of mortality.Reference de Lau, Verbaan, van Rooden, Marinus and van Hilten 31 Higginson and et alReference Higginson, Gao, Saleem, Chaudhuri, Burman and McCrone 32 highlighted, in a longitudinal study with 82 patients, that a complex mix of motor and NMS exist in patients with late-stage PD. The disability from NMS identifies palliative care needs and suggests that subjects need early palliative assessment and interventions. As demonstrated by the current observations that poor axial symptoms correlate with higher NMS scores, one can postulate that the axial symptoms in PIGD and some domains of NMS may share common pathophysiology.
Although NMS have been thought to reflect dysfunction in non-dopaminergic systems, there is evidence in the literature to suggest that dopaminergic pathologies may be associated with NMS.Reference Chaudhuri and Schapira 33 Dopaminergic treatment has been shown to be beneficial for several NMS in previous studies.Reference Hyacinthe, Barraud, Tison, Bezard and Ghorayeb 34 - Reference Seppi, Weintraub, Coelho, Perez-Lloret, Fox and Katzenschlager 36 These included pramipexole for the treatment of depressive symptoms,Reference Seppi, Weintraub, Coelho, Perez-Lloret, Fox and Katzenschlager 36 and D1-receptor agonists for sleep-wake cycle-related symptoms.Reference Hyacinthe, Barraud, Tison, Bezard and Ghorayeb 34 In the current study, we demonstrated that levodopa dosage significantly correlated with a higher NMS Quest score, suggesting that NMS in general do not respond well to dopaminergic therapy. The correlation of levodopa dose with poorer NMS profile may reflect that patients at later stage PD or with more severe disease require higher dose of levodopa treatment and tend to have a higher NMS score.
There are some limitations with the current study. Many patient charts were excluded from the analysis, primarily because of missing components of the NMS Quest. We observed from the clinical history a tendency for patients without positive symptoms to leave the questions blank. There is no rationale to believe that differences exist between TD and PIGD patients when filling the NMS Quest. The parameters in the excluded patients showed no difference from those included in the analyses.
A previous report suggested that dementia was more likely to occur in the PIGD category of patient than in the TD type.Reference Burn, Rowan, Allan, Molloy, O’Brien and McKeith 37 In our current study, we failed to demonstrate a direct correlation between MMSE and NMS, although the PIGD group had a significant lower MMSE score than the TD group. This might be due to the poor correlation between MMSE and dementia. Future studies using a better cognitive measure, such as the Montreal Cognitive AssessmentReference Mocanu, Ganea, Siposova, Filippi, Demjen and Marek 38 might provide more information. In this study, the old version of the UPDRS-III was used to keep the consistency of data collection through the study period. In the future studies, application of MDS-UPDRS would be helpful addition to the current findings.
Strength of our study includes the systematic collection of NMS in a representative clinical population. The relatively large sample allowed an examination of motor subtype in relation to NMS.
In conclusion, our current study has shown that in the PD cohort, NMS severity increases in more advanced disease and varies according to motor phenotype. The PIGD but not the TD subtype shows correlations with a worse NMS score. Patients with a PIGD phenotype who have more axial involvement, associated with advanced disease and poor motor response, are at risk for a higher NMS burden. In addition, we observed NMS in the TD group were not related to disease severity (UPDRS-III total score) or subscore B, supporting our hypothesis that NMS have a lower prevalence in patients with the TD subtype.
Acknowledgements
This study was funded by CIHR Fellowship Program to Fang Ba, and King Fahad Medical City, Saudi Arabia to Mona Obaid.
Disclosures
FB has received a fellowship and research support from the Canadian Institutes of Health Research (CIHR). MO is employed at and receives a salary from King Fahad Medical City. MW is a researcher at and has received an operating grant and research support from CIHR, and is a clinical trial leader and has received subcontract and research support from the National Institutes of Health (NIH; National Center for Complementary and Alternative Medicine NCCAAM) and the CHDI Foundation. RC has received funding from the CIHR and the University of Alberta Hospital Foundation as principal investigator and is a coinvestigator on studies funded by the Alberta Innovates Solutions, the Michael J Fox Foundation, CIHR, and the NIH; he also serves on the scientific advisory board of the Parkinson Society of Canada and as a scientific committee member for CIHR. WRWM has received a research grant from CIHR and clinical trial support from NIH and the CHDI Foundation; is an employee and has received salary support from the University of Alberta; is a researcher and has received an operating grant and research support from CIHR, NIH, and the CHDI Foundation.
Statement of Authorship
FB was involved in the manuscript preparation, writing of the first draft, and statistical analysis with design and execution. MO was involved in the initiation of the project, data collection, and review of the manuscript. MW was involved in organization and execution of the project, review and revision of the manuscript. RC was involved in the conception, planning and supervising the execution of the research project; planning and supervision of data analysis; and critical revision final review of the manuscript. WRWM was involved in the conception, planning and supervising the execution of the research project; and critical revision final review of the manuscript.







