There is a long tradition of psychological therapies for people with dementia, but rarely have they been rigorously evaluated, making it difficult for commissioners and providers to plan services from a solid evidence base, and also making it difficult to draw comparisons with pharmacological interventions (Reference Orrell and WoodsOrrell & Woods, 1996). Cognitive stimulation therapy (CST) for people with dementia was developed from the findings of two Cochrane reviews (Spector et al, Reference Spector, Woods and Davies1998a ,Reference Spector, Woods and Davies b ), incorporating aspects of psychological therapies found in scientifically rigorous trials, to improve cognition and behaviour significantly (Reference Spector, Orrell and DaviesSpector et al, 2001). A large-scale, single-blind randomised controlled trial demonstrated that CST significantly improves cognitive abilities and quality of life (Reference Spector, Thorgrimsen and WoodsSpector et al, 2003), but we do not know whether CST is cost-effective.
METHOD
Design
Recruitment of centres
Support for the study was received from health and social care organisations in London, Essex and Hertfordshire. Approval was obtained from the multiresearch ethics committee and local research ethics committees for the relevant areas. A total of 169 possible care homes and day centres (hereafter both are referred to as ‘centres’) were contacted and, if appropriate, a meeting was arranged with the manager. Centres required a minimum of 8 potential participants to be included. Many centres were excluded because they were unable to generate sufficient numbers of participants.
Selection of participants
The inclusion criteria were as follows:
-
(a) meeting DSM–IV (American Psychiatric Association, 1994) criteria for dementia;
-
(b) scoring between 10 and 24 on the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975);
-
(c) some ability to communicate and understand communication;
-
(d) being able to see and hear well enough to participate in a meaningful assessment;
-
(e) not displaying behaviour that would make interview impossible, such as constant wandering, shouting, or aggression;
-
(f) not having a diagnosis of learning disability or current clinical depression which would make reliable assessment difficult.
In the meeting with the manager of the centre, the inclusion criteria were used to select possible participants, who were then approached by their keyworker with information about the project. Before screening, informed consent was obtained from participants. After an explanation of the study, those who agreed to participate were asked to sign the consent form in the presence of a witness (usually a member of staff). If staff felt that an older person was too impaired to understand the nature of the study, that individual was excluded. Subsequently, possible participants were screened, using the MMSE (Reference Folstein, Folstein and McHughFolstein et al, 1975). A day was then agreed to conduct the full assessments in each centre.
Randomisation
The researcher conducting the assessments in a centre (‘the assessor’) generated a list of the participants in that centre. The assessor then ordered them alphabetically, assigned a number of 1 to 10 according to their sequence, and gave the list to the researcher conducting the group in this centre (‘the therapist’). The therapist was masked to all assessment outcomes. The therapist then drew numbers on identical discs from a counter selector. From the first five numbers to be drawn, a list of names was generated for participation in the experimental group, with no restrictions. The remaining three to five people were allocated to the control group who continued with usual activities for the duration of the groups. For most care homes ‘usual activities’ consisted of doing nothing. For other centres (care homes and day centres), usual activities included games such as bingo, music and singing, arts and crafts, and activity groups. The assessor remained masked to this allocation until after the trial was completed in the centre. From the pilot study (Reference Spector, Orrell and DaviesSpector et al, 2001), we estimated that a sample size of 64 in each group was required to achieve 80% power to detect a difference in means of two points (MMSE). This assumed that the common standard deviation was 4.0, using a two-group t-test with a 0.05 (two-sided) significance level.
Intervention
Procedure
The groups commenced on the week after the baseline assessment had been completed. They ran for 7 weeks, twice weekly for 45 min, in the same room at the same times. Each group consisted of the five participants, the therapist, and one designated member of the care staff team of the centre where the group was conducted. Informed consent was again obtained on the day of the full assessment, and an interview was subsequently conducted, lasting approximately 45 min.
Programme
The Cochrane systematic reviews on reality orientation and reminiscence therapy for dementia (Spector et al, Reference Spector, Woods and Davies1998a ,Reference Spector, Woods and Davies b ) were used to develop a group programme of evidence-based CST (Reference Spector, Orrell and DaviesSpector et al, 2001). Pilot studies in four centres found worthwhile improvements in cognition and depression compared with the control group (Reference Spector, Orrell and DaviesSpector et al, 2001). The sessions focused on themes (such as childhood and food), with an additional focus on the current day, and encouraged the use of information processing and implicit memory rather than factual knowledge and explicit memory. More than one sense (e.g. hearing plus vision) was involved whenever possible, and a choice of activities for each session enabled the facilitator to adapt the session to the group's abilities, interests and gender mix. Spector et al (Reference Spector, Orrell and Davies2001) provide a detailed description of the programme.
Outcome measures
Cognitive function was measured using the MMSE, a brief and well established 11-item test of cognitive function, with scores potentially ranging from 0 to 30. Good reliability and validity have been demonstrated for this scale. The MMSE was applied at baseline and again at the 8-week follow-up point.
Although other clinical measures were used, including the Alzheimer's Disease Assessment Scale – Cognitive (ADAS–Cog) (Reference Rosen, Mohs and DaviesRosen et al, 1984) and Qualify of Life in Alzheimer's Disease (QoL–AD; Reference Logsdon, Gibbons and McCurryLogsdon et al, 1999), the main cost-effectiveness analysis focused on changes in the MMSE (relative to cost) because this widely employed scale had been chosen as the primary outcome at the trial design stage, and had been used to power the study. However, care professionals and policy makers are very interested in quality of life, and so a secondary cost-effectiveness analysis examined cost differences between CST and treatment as usual relative to the incremental difference in QoL–AD score. Scores on the QoL–AD can range from 13 to 52.
Client Service Receipt Inventory
The CSRI (Reference Beecham, Knapp, Thornicroft, Brewin and WingBeecham & Knapp, 1992) was adapted for the study. It was completed by the manager of each centre, or the person's closest relative if living in the community, before the intervention, and once more 8 weeks later, at the end of the intervention period. The CSRI has been extensively involved in studies of mental health care. The inventory requires information about the service user's background, and comprehensively gathers data on accommodation, medication profile and services accepted.
Unit costs
All unit costs were based on national figures for England that could be taken as good approximations of long-run marginal opportunity costs. Unless otherwise stated, costs came from the Personal Social Services Research Unit compendium for the year during which most data were collected (Reference Netten, Rees and HarrisonNetten et al, 2001), or were inflated to 2001 price levels using the health service and personal social services inflators given in that volume. Medication prices were taken from the British National Formulary (British Medical Association & Royal Pharmaceutical Society of Great Britain).
Costing of CST intervention
The cost of preparing the CST intervention was borne by the research project and involved numerous people over a period of time. The two researchers were already skilled in group work techniques and received no particular training in providing the intervention. The cost estimate for CST therefore includes researchers’ time, travel expenses, care assistant time and equipment. A broad estimate of a 15 mile return journey per therapy session was assumed, based on calculations averaged across all centres where CST was delivered. Researchers’ time was averaged at 4 h per session, including preparation and travel. As the control group received no intervention, no additional costs were included for them. A care assistant from the centre or home prepared and attended the sessions. This was within normal work patterns, no replacement staff were employed, and 1 h of time was costed. Equipment, including two whiteboards and activity equipment, was also costed per session. This yielded a total cost of £90 per session (£73 researchers’ time, £11 care assistant's time, £5 travel and £1 equipment). With five people in each group, the cost of the intervention per person per week of the study was £31.50 at 2001 prices. Later we examine the sensitivity of the results to differences in group size.
Cost-effectiveness analysis
The economic evaluation was conducted from the perspective of health and personal social services. A cost-effectiveness analysis was conducted comparing these comprehensively measured service costs between the experimental and control treatments with changes in the primary outcome measure (cognition as measured by the MMSE) and the secondary outcome (quality of life as measured by QoL–AD). The incremental cost-effectiveness ratio was computed as the mean cost difference between CST and usual activities divided by the mean difference in change in MMSE (or QoL–AD). The CST would then be defined as more cost-effective than usual activities if:
-
(a) it is both less costly and more effective or;
-
(b) it is both more costly and more effective, and the decision-maker considers the additional cost to be justified by the improved outcomes or;
-
(c) it is both less costly and less effective and the decision-maker considers the savings achieved to warrant giving up some outcome difference.
If (a) holds (usually described as ‘dominance’), CST would not only improve the well-being of service users (measured in this case by cognition or quality of life) but would simultaneously save money. The decision-maker would be very attracted to this therapy. For the purposes of analysis one would need to be absolutely sure that CST dominated usual activities. Study limitations, lack of statistical power or specific assumptions made during the evaluation could introduce an element of uncertainty.
If (c) holds, there would need to be careful consideration of whether it would be acceptable politically, professionally and to patients to introduce a treatment that led to worse health or well-being than current practice. Even quite substantial savings might make this a hard option to sell, although of course it needs to be considered seriously (Reference DowieDowie, 2004).
Cost-effectiveness acceptability curves
The other possibility (b) also raises difficult trade-off questions. It requires the decision-maker to consider whether it is worth incurring the higher costs in order to achieve the improved outcomes. Now the trade-off concerns a wider set of options, because spending more on (say) CST will necessarily mean spending less on something else, given that most decisions have to be taken within fixed budgets.
The approach taken to reveal the nature of these trade-offs – and to represent the inherent uncertainty in any evaluation – is to calculate net benefits and then to plot the cost-effectiveness acceptability curve (Reference van Hout, Al and Gordonvan Hout et al, 1994; Reference Fenwick, O'Brien and BriggsFenwick et al, 2004). This reveals to the decision-maker what the likelihood is of CST being cost-effective relative to usual care given different (implicit monetary) values placed on incremental outcome improvements. In the present study, for each different value attached to a one-point improvement in MMSE we calculated the probability that CST is viewed as more cost-effective than usual activities. The cost-effectiveness acceptability curve also represents the uncertainty in the estimation of the incremental cost-effectiveness ratio, including in circumstances where lack of statistical power limits the significance testing (Reference BriggsBriggs, 2000) and where one wants to understand the sensitivity of the results to key assumptions made in the analysis. The cost-effectiveness acceptability curve also provides a visually appealing and more generalised tool for taking the results from one trial and comparing them with results from other trials that use the same outcome measure and the same breadth of cost measure. Like any well-conducted economic evaluation, a study which fits a cost-effectiveness acceptability curve allows – indeed encourages – external comparisons to be made, beyond the confines of a two-armed trial, although only if there are comparable evaluative data on other treatment options using identical outcome indicators and measuring costs in a consistent fashion.
Net benefit (NB) was calculated for each individual using the usual formula:
where E is effectiveness (change in either MMSE or QoL–AD for the individual), C is cost and λ is the willingness to pay for one additional unit of outcome (effectiveness). A series of net benefits were calculated for each individual for a range of λ values between £0 and £200 (increments of £20). Then coefficients of differences in net benefits between groups were obtained through a series of bootstrapped linear regressions (1000 repetitions) of group upon net benefit. The resulting coefficients were examined to calculate the proportion of times that the treatment group had a greater net benefit than the control group for each value of λ. These proportions were plotted to generate cost-effectiveness acceptability curves. Bootstrap analysis is frequently used to address the problem of possible skewness in the distribution of a variable, as is often the case with measures of cost (Reference Barber and ThompsonBarber & Thompson 2002; Reference Dunn, Mirandola and AmaddeoDunn et al, 2003).
Analyses
An intention-to-treat analysis was conducted to preserve the unbiased distribution of factors (on average) in the groups produced by randomisation. This involved including all the people who were randomised, whether or not they took part in the whole programme.
RESULTS
Response rate and attrition
In all, 23 centres (18 care homes and 5 day centres) were included. Of 292 people screened, 201 were included in the study. The reasons for exclusion of individuals were: having MMSE score of less than 10 or communication difficulties (44); hearing being too impaired (10); vision being too impaired (7); not having dementia (15); having a learning disability (3); becoming distressed or aggressive during assessment (10); or dying between screening and full assessment (2).
Of the 201 people, 40 did not have the CSRI completed; some managers found the CSRI too time-consuming to complete, and others perceived this information as inappropriate to disclose (even though such problems have rarely been encountered in other studies). At baseline there were 91 participants in the intervention group for whom we could calculate costs, and 70 in the control group. In the intervention group, 3 people died, 2 became ill during the study and 1 refused follow-up assessment; whereas in the control group, 1 person died, 1 became ill, 2 refused assessment and 1 moved out of the area. Thus 11 patients were not followed up.
There were no differences in baseline characteristics on measures for which we had the necessary data between those included and excluded from the economic evaluation.
Characteristics of participants
The characteristics of the participants included in the study are described in detail by Spector et al (Reference Spector, Thorgrimsen and Woods2003). For the full sample, at baseline mean age was slightly higher for participants in the intervention group (85.7 years) relative to controls (84.7 years). The control group had a somewhat higher female:male ratio (80% ν. 75% females). The mean MMSE for the intervention group at baseline was 14.2 (s.d.=3.9) compared with 14.8 (s.d.=3.8) for the control group. For QoL–AD, the baseline scores were 33.2 (s.d.=5.9) and 33.3 (s.d.=5.7), respectively. On none of the clinical measures used in the study was there a difference between the intervention and control groups at baseline (details in Reference Spector, Thorgrimsen and WoodsSpector et al, 2003).
CST programme
Mean attendance at the CST programme was 11.6 sessions (s.d.=3.2; range 2–14), with most people attending seven or more sessions.
Outcomes
For the full sample of people included in the trial, Spector et al (Reference Spector, Thorgrimsen and Woods2003) found a significant improvement at follow-up for people in the intervention group relative to controls on the MMSE (+1.14, s.d.=0.09, P<0.05), the ADAS–Cog (–2.37, s.d.=0.87, P<0.01), and the QoL–AD (+1.64, s.d.=0.78, P<0.05). These outcome differences were slightly different for the sample of people for whom we had cost data (details given below when describing cost-effectiveness).
Service use
The services used by participants in the 8 weeks before the intervention started (baseline) and during the 8-week intervention period (follow-up) are grouped and summarised in Table 1. Service use levels were generally very modest, and remained relatively stable over time; 78 participants (86%) in the intervention group and 61 controls (87%) lived in care homes at baseline, and these people used few other health or social care services. There were no accommodation moves during the research period.
Table 1 Service use
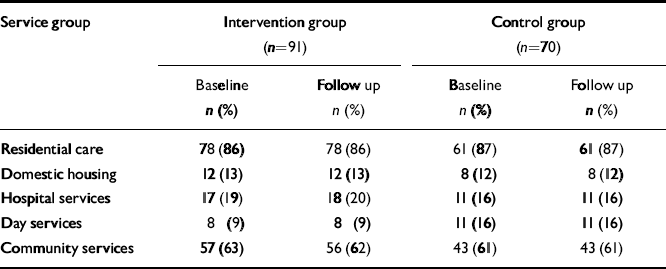
| Service group | Intervention group (n=91) | Control group (n=70) | ||
|---|---|---|---|---|
| Baseline n (%) | Follow up n (%) | Baselin n (%) | Follow up n (%) | |
| Residential care | 78 (86) | 78 (86) | 61 (87) | 61 (87) |
| Domestic housing | 12 (13) | 12 (13) | 8 (12) | 8 (12) |
| Hospital services | 17 (19) | 18 (20) | 11 (16) | 11 (16) |
| Day services | 8 (9) | 8 (9) | 11 (16) | 11 (16) |
| Community services | 57 (63) | 56 (62) | 43 (61) | 43 (61) |
The mean difference in use of medication – not included in Table 1 – was 0.14 fewer medications per person (s.d.=0.8) at follow-up compared with baseline for the CST group, and 0.16 fewer medications per person for the control group. Medication use appeared slightly higher for the control group compared with the experimental group. None of the differences in service use or medication use over time or between the groups approached statistical significance. No study participant was taking a cholinesterase inhibitor.
Costs of services
The costs of services used by participants before and after intervention are shown in Table 2. Within each group there were few apparent changes over time, and none was found to be statistically significant. Between the two groups there were no differences at baseline and almost no differences at follow-up. Hospital service costs appeared lower for the control group (P=0.051 after bootstrap correction). Aggregating costs across all health and social care services, the CST group was £45.18 more expensive per week (P=0.037). However, there was a difference of £28.53 at baseline between the two groups (P=0.241), and so we used analysis of covariance (ANCOVA) to adjust for the baseline cost difference between the groups; the effect of group on total follow-up costs was reduced (F 1,158=3.187; P=0.076).
Table 2 Mean weekly costs (£) of service use
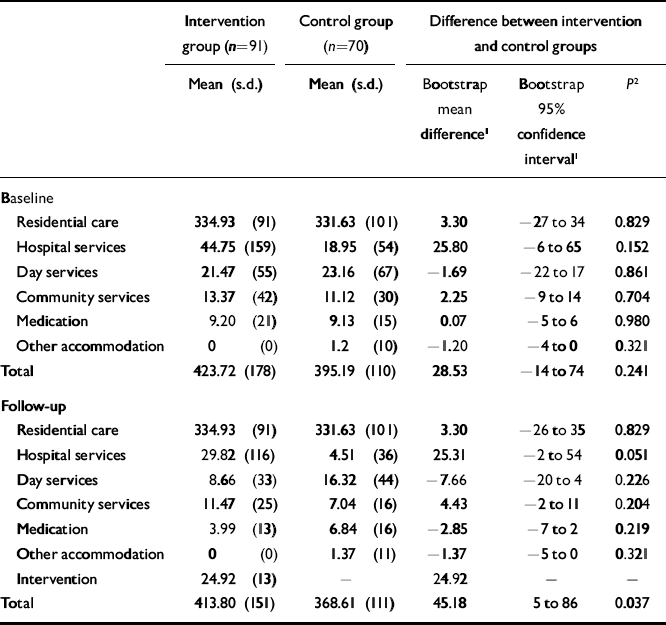
| Intervention group (n=91) | Control group (n=70) | Difference between intervention and control groups | |||
|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | Bootstrap mean difference1 | Bootstrap 95% confidence interval1 | P 2 | |
| Baseline | |||||
| Residential care | 334.93 (91) | 331.63 (101) | 3.30 | -27 to 34 | 0.829 |
| Hospital services | 44.75 (159) | 18.95 (54) | 25.80 | -6 to 65 | 0.152 |
| Day services | 21.47 (55) | 23.16 (67) | -1.69 | -22 to 17 | 0.861 |
| Community services | 13.37 (42) | 11.12 (30) | 2.25 | -9 to 14 | 0.704 |
| Medication | 9.20 (21) | 9.13 (15) | 0.07 | -5 to 6 | 0.980 |
| Other accommodation | 0 (0) | 1.2 (10) | -1.20 | -4 to 0 | 0.321 |
| Total | 423.72 (178) | 395.19 (110) | 28.53 | -14 to 74 | 0.241 |
| Follow-up | |||||
| Residential care | 334.93 (91) | 331.63 (101) | 3.30 | -26 to 35 | 0.829 |
| Hospital services | 29.82 (116) | 4.51 (36) | 25.31 | -2 to 54 | 0.051 |
| Day services | 8.66 (33) | 16.32 (44) | -7.66 | -20 to 4 | 0.226 |
| Community services | 11.47 (25) | 7.04 (16) | 4.43 | -2 to 11 | 0.204 |
| Medication | 3.99 (13) | 6.84 (16) | -2.85 | -7 to 2 | 0.219 |
| Other accommodation | 0 (0) | 1.37 (11) | -1.37 | -5 to 0 | 0.321 |
| Intervention | 24.92 (13) | — | 24.92 | — | — |
| Total | 413.80 (151) | 368.61 (111) | 45.18 | 5 to 86 | 0.037 |
Cost-effectiveness
The point estimate of the incremental cost-effectiveness ratio was £75.32 per additional point on the MMSE (mean cost difference £45.18; mean outcome difference 0.6). Calculation of the range of net benefit values and the use of bootstrap regressions (resampling 1000 times from the same core sample) allowed us to plot the cost-effectiveness acceptability curve for this cognitive outcome measure. The solid line in Fig. 1 shows the probability that each group is cost-effective for a range of values for decision-makers’ willingness to pay (denoted λ above) for an additional point improvement on the MMSE.
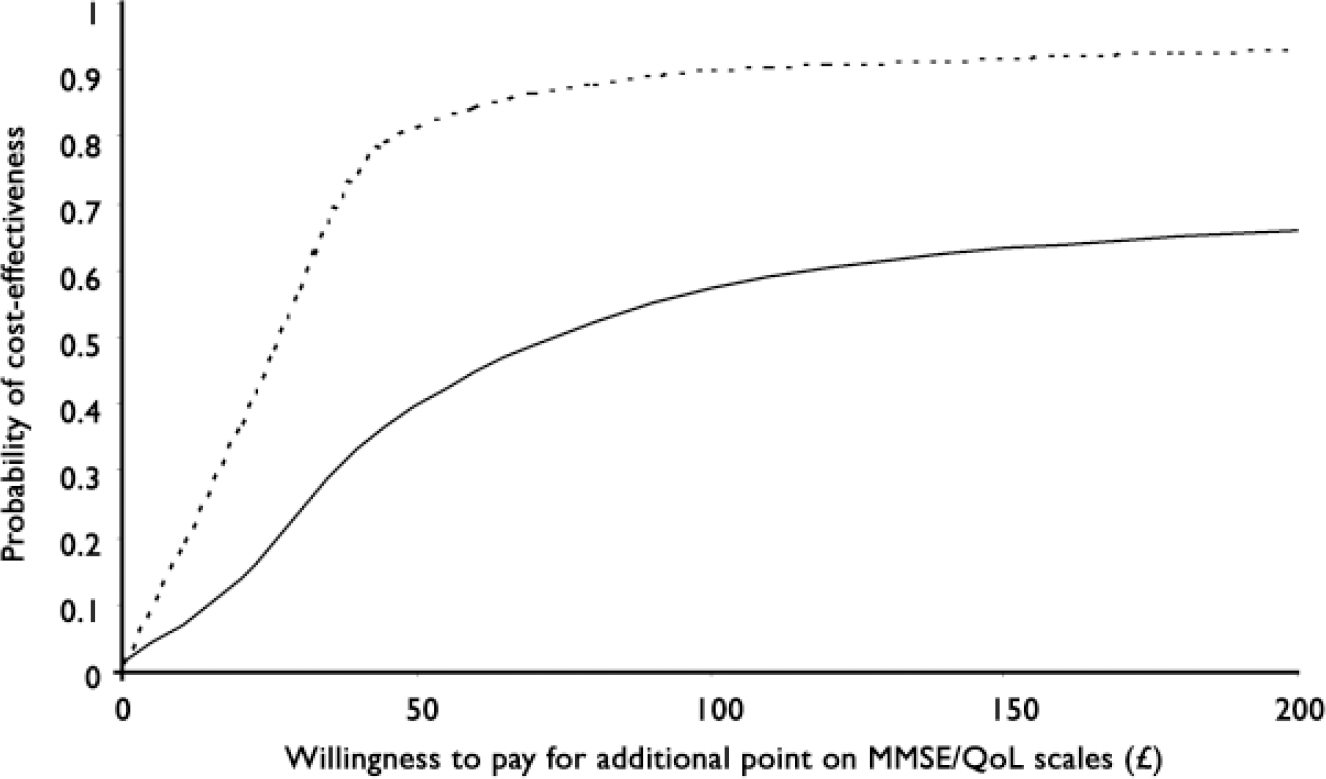
Fig. 1 Cost-effectiveness acceptability curve representing the probability that each group is cost effective for a range of values of decision-makers’ willingness to pay for additional point improvements on the MMSE and QoL–AD. Unbroken line, MMSE (Mini-Mental State Examination); broken line, QoL–AD (Quality of Life in Alzheimer's Disease).
We repeated the analyses under two different assumptions about group size. Assuming a smaller CST group – three people per group, rather than five – increased the cost of the group per person per week from £31.50 to £52.50, and the cost difference between the samples widened to £61.20 (P=0.005 after bootstrap correction; and P=0.011 from the ANCOVA adjusting for the cost difference at baseline). The incremental cost-effectiveness ratio grew to £102.00 per incremental change in MMSE. Assuming a larger CST group – seven people per group – reduced the CST group cost per person per week to £22.50, and narrowed the cost difference between the samples to £38.32 (P=0.076; and P=0.150 from the ANCOVA adjusting for the cost difference at baseline). The incremental cost-effectiveness ratio was then £63.87 per incremental improvement in MMSE score. However, it needs to be remembered that the effectiveness findings from this study are based on the observed group size of five people, and the outcomes might have been different with smaller or larger groups.
For the quality of life outcome, the point estimate for the incremental cost-effectiveness ratio was £22.82 per additional point on the QoL–AD (mean cost difference £45.18; mean outcome difference 1.98). The cost-effectiveness acceptability curve for the quality of life measure is plotted as the dotted line in Fig. 1. Repetition of these analyses for different assumed group sizes produced the same pattern as for the MMSE.
No differences in the results were found between care homes and day centres (data not shown).
DISCUSSION
Resources for healthcare are almost always scarce relative to needs or wants, and lack of available resources has commonly been used as an argument against implementing novel interventions to address the needs of people with dementia. In fact, there may be good economic and social arguments for increasing expenditure on dementia services in circumstances where the benefits in terms of improved health status and quality of life are substantial (Reference Knapp, Wigglesworth, Wimo, Jönsson and KarlssonKnapp & Wigglesworth, 1998). Therefore, this study investigated the resource implications and cost-effectiveness of a CST programme delivered in care homes and day centres.
Service use
Study participants, the majority of whom were resident in care homes, had relatively low service use compared with rates reported for people with dementia living in the community (Reference Livingston, Manela and KatonaLivingston et al, 1997; Reference Kavanagh and KnappKavanagh & Knapp, 1999). During the 8 weeks of the study, one in five participants used some kind of hospital service. One in eight attended day care (although none of the people living in care homes used any day service outside their place of residence), and two-thirds used some kind of community service, the most common being chiropody and primary care. Participants had a mean of 2.1 different drugs prescribed to them. There may have been substitution of the CST for some other services, but the short duration of the study did not make it possible to observe any such effects.
Costs of services
Accommodation cost remained stable throughout the short study, which is not altogether surprising (cf. Reference Wolstenholme, Fenn and GrayWolstenholme et al, 2002), but the costs of other services fell somewhat. However, none of the between-group differences was significant, either before or after adding the cost of the intervention.
Cost-effectiveness
The recent development of cost-effectiveness acceptability curves reminds us that the absence of a statistical difference (in either costs or effects) does not necessarily mean that two treatments cannot in fact be distinguished. Lack of statistical power is a common problem in economic evaluations (Reference BriggsBriggs, 2000) especially in mental health (Reference Sturm, Unutzer and KatonSturm et al, 1999). Moreover, in a decision-making context it could be argued that it would be perverse to reject an intervention with the highest probability of being cost-effective because of the limitations of conventional hypothesis-testing (Reference Claxton, Sculpher and DrummondClaxton et al, 2002).
The study found that CST has benefits for cognition and quality of life in dementia (Reference Spector, Thorgrimsen and WoodsSpector et al, 2003; Reference Thorgrimsen, Spector and WoodsThorgrimsen et al, 2006). Costs were not significantly higher for the CST group if adjustment was made for the baseline difference in costs between the two samples. On average, the cost of achieving an incremental improvement in MMSE score was £75.32 higher for the CST group than for the group receiving usual care. Because of uncertainty and the need to cast these findings in a decision-making framework, we plotted the cost-effectiveness acceptability curve. Looking at the cognition outcome, under reasonable assumptions there appears to be a high probability that CST is more cost-effective than treatment as usual, although we are not aware of evidence on society's or health system decision makers’ (or indeed patients’) actual willingness to pay for such cognitive improvements.
The same is true for the secondary analysis of cost-effectiveness, looking at quality of life as the outcome measure. At mean values, the cost per incremental improvement in QoL–AD was £22.82, and the plotted cost-effectiveness acceptability curve shows a quite high probability of CST being viewed as more cost-effective than treatment as usual for values of the willingness to pay parameter up to £100, although again it is difficult to find external reference values.
Very few studies have estimated the costs of delivering psychosocial therapies for people with dementia (Reference Ernst and HayErnst & Hay, 1997), or examined their cost-effectiveness (Reference Jönsson, Jönsson, Wimo, Maj and SartoriusJönsson et al, 2002), and none has fitted a cost-effectiveness acceptability curve. Nor have cost-effectiveness acceptability curves been plotted in many pharmacotherapy studies for dementia. We can, however, compare the findings from this analysis of CST with two recent English studies. Wolstenholme et al (Reference Wolstenholme, Fenn and Gray2002) investigated the relationship between disease progression and costs of care in dementia over a long period. They estimated that a one-point decrease in the MMSE would add £56 to direct direct health and social care costs over a 4-month period. The AD2000 Collaborative Group (2004) compared long-term donepezil treatment to placebo. The donepezil group cost £498 more (annually, at what appear to be 2000 prices) than the placebo group (excluding the costs of the medication and institutionalisation), with a treatment effect of 0.83 points on the MMSE over 114 weeks. This is equivalent to a considerably larger cost per incremental outcome gain than our estimate for CST in the present study.
Attribution of effectiveness
It is possible that the social interaction provided by the groups could have been of benefit in the centres which provided some activities, but our Cochrane Review of reality orientation (Reference Spector, Woods and DaviesSpector et al, 1998a ) found that in randomised controlled trials social groups appeared to be of no benefit to cognition. This suggests that the results are because of the specific effects of CST rather than the non-specific effects of attention or social interaction.
Limitations
The power calculation for this study was based on change in cognition as the primary outcome measure. The sample may not have been large enough to test the cost-effectiveness hypothesis, which is one reason for being attracted to a decision-making approach. Costs data were not collected for 40 participants involved in the study of this intervention, although these people were not found to be different from people for whom costs were measured.
The short follow-up period (8 weeks) means that we do not know the longer-term implications of delivering CST to older people with dementia.
Most study participants were resident in care homes, and future research should look at the economic consequences of running CST for people with dementia living in the community, particularly in view of government policy emphasis on the latter.
The CST was given by trained researchers in this study. It would be less expensive to deliver CST by training care home staff, home care workers or nurses, but we do not know whether this would generate different outcomes from those observed.
Most participants had mild to moderate dementia and some functional hearing and vision, and it is not possible to generalise to other groups from the present results.
Centres with fewer than eight eligible participants had to be excluded, although there is no reason to believe that this has biased the findings.
Recommendations
In this study we found that taking part in the evidence-based CST group programme made little difference to the costs for the participants relative to people receiving care as usual, but cognitive outcomes as measured by the MMSE and ADAS–Cog were improved, as was quality of life as measured by QoL–AD. The estimated cost-effectiveness acceptability curves for both cognitive improvement and quality of life change suggest that decision-makers would be likely to view CST as a comparatively cost-effective option, although costs and outcomes were measured over a relatively short period.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Conducting a cognitive stimulation therapy (CST) group programme made little difference to costs for its participants relative to controls receiving standard care.
-
▪ CST groups have been shown to have beneficial effects on cognition and quality of life for people with dementia.
-
▪ It is likely that decision-makers will see CST as a comparatively cost-effective treatment option.
LIMITATIONS
-
▪ Most participants in this study lived in residential care, and were people with mild to moderate dementia and some functional hearing and vision. Generalisation to other groups is not necessarily possible.
-
▪ Power calculations were based on cognition, and the test of the cost-effectiveness difference may be under-powered.
-
▪ The short follow-up period means that longer-term costs and outcomes remain unclear.
Acknowledgements
The authors thank Jennifer Beecham, University of Kent and Institute of Psychiatry, Renee Romeo and numerous reviewers whose comments certainly helped to improve the paper.
This study was supported by grants from North East London Mental Health NHS Trust and North Thames NHS Executive Funding Group.






eLetters
No eLetters have been published for this article.