Risperidone is licensed by the European Medicines Agency for the short-term (up to 6 weeks) treatment of persistent aggression in patients with moderate to severe Alzheimer's dementia unresponsive to non-pharmacological approaches and when there is a risk of harm to self or others. 1 Meta-analysis of primary efficacy and safety data from four of the risperidone trials has been published, Reference Katz, de Deyn, Mintzer, Greenspan, Zhu and Brodaty2 together with an analysis of mortality in six studies. Reference Haupt, Cruz-Jentoft and Jeste3 As physicians who are frequently faced by decisions about treatment in this situation, R.H. and S.G.C. approached Janssen with two questions. First, given the known risks of risperidone treatment in this population, Reference Haupt, Cruz-Jentoft and Jeste3–Reference Maust, Kim, Seyfried, Chiang, Kavanagh and Schneider7 were there baseline characteristics of individual patients within the clinical trials database that could be used to identify those at higher (or lower) risk of death or cerebrovascular adverse event (CVAE) associated with risperidone treatment? Second, once treatment had been initiated, which treatment-emergent events were associated with risk of subsequent death or CVAE in patients treated with risperidone? We conducted a meta-analysis of all Janssen's double-blind randomised controlled trials (RCTs) of risperidone in dementia to address these questions, with a focus on identifying patient characteristics and treatment-emergent events that would result in differential risks of stroke and death between risperidone and placebo.
Method
Data from all six randomised, double-blind, placebo-controlled studies of risperidone in elderly patients with dementia conducted by Janssen were included in this analysis. The primary results of four of the studies – USA-63 (ClinicalTrials.gov registration NCT00253123), Reference Katz, Jeste, Mintzer, Clyde, Napolitano and Brecher8 INT-24 (NCT00249145), Reference De Deyn, Rabheru, Rasmussen, Bocksberger, Dautzenberg and Eriksson9 AUS-5 (NCT00249158) Reference Brodaty, Ames, Snowdon, Woodward, Kirwan and Clarnette10 and USA-232 (NCT00034762) Reference Mintzer, Greenspan, Caers, Van Hove, Kushner and Weiner11 – have been previously published. Our analysis also includes two studies that were not published owing to insufficient numbers of participants: a pilot study, BEL-14 (n = 39), and INT-83 (n = 18), which was terminated for (non-clinical) business reasons. Detailed study design characteristics are available in the primary publications. All included men and women aged 55 years or over with Alzheimer's, vascular or mixed dementia as classified by DSM-IV. 12 In four studies (USA-63, INT-24, AUS-5 and BEL-14), a Behavioral Pathology in Alzheimer's Disease (BEHAVE-AD) scale total score of 8 or above and a BEHAVE-AD global rating of 1 or more were inclusion criteria. Reference Reisberg, Borenstein, Salob, Ferris, Franssen and Georgotas13 In USA-232 and INT-83 a score of at least 2 on any item of the BEHAVE-AD psychosis subscale was an inclusion criterion. Treatment duration was 12 weeks for USA-63, INT-24 and AUS-5, 8 weeks for USA-232 and INT-83 and 4 weeks for BEL-14. The USA-63 study included three fixed-dose arms of risperidone (0.5 mg, 1 mg or 2 mg daily). Flexible dosing was employed in the other studies, with total daily dose ranges of 0.5–4 mg in INT-24, 0.5–2 mg in AUS-5, 1–4 mg in BEL-14 and 1–1.5 mg in USA-232 and INT-83. In our analysis all risperidone doses were combined into a single group.
Variables
The following baseline characteristics were examined for an association with CVAE or mortality: age (<80 years v. ⩾80 years), gender, ethnicity, diagnosis, body mass index (BMI), Mini-Mental State Examination (MMSE), Reference Folstein, Folstein and McHugh14 BEHAVE-AD delusion-related items, BEHAVE-AD global rating, creatinine clearance, diastolic blood pressure, pulse, blood levels of sodium and urea, cardiovascular, neurological or respiratory findings on medical history, and cardiovascular, neurological or respiratory findings on baseline physical examination. Treatment-emergent events examined for an association with CVAE or mortality included weight increase (⩾7%), weight decrease (⩾7%), creatinine clearance decrease (⩾10% and ⩾20%), diastolic blood pressure ⩾90 mmHg, sedation, malnutrition, dehydration, extrapyramidal symptoms, pulmonary condition, infection (urinary or pulmonary) and cardiovascular disease adverse events. A treatment-emergent event was considered ‘present’ only if the earliest occurrence of the event preceded the CVAE or death. In an additional analysis the treatment-emergent event was considered ‘present’ only if the earliest occurrence of the event preceded the CVAE or death by at least 7 days. Selected categories of concomitant medications, based on the World Health Organization Drug Dictionary Anatomic–Therapeutic–Chemical class, were also examined for their association with CVAE or mortality: potentially sedating medications, anti-inflammatory drugs, beta blockers, diuretics and laxatives. Concomitant medication use was defined in the same way as the presence of a treatment-emergent event, based on the earliest start date of the concomitant medication (medications with a missing start date were considered to have been present from baseline).
Statistical analysis
For analysis of differences between risperidone and placebo in individual studies, Fisher's exact test was used to compare the crude incidences of CVAE and mortality and Wald's method was used to compare exposure-adjusted incidence rates (EAIRs). Reference Liu, Wang, Liu and Snavely15 Analysis based on the combined studies used the Cochran–Mantel–Haenszel test for crude incidence and the Mantel–Haenszel method of Greenland & Robins for EAIRs. Reference Greenland and Robins16 The association between a CVAE or death and a baseline characteristic or treatment-emergent event (including concomitant medication use) was analysed by Fisher's exact test for crude incidence and Wald's method for EAIR. The placebo group only were included in these analyses, and the analysis was based on the cross-tabulation of the event of interest (CVAE or death) v. the characteristic (baseline or treatment-emergent). For treatment-emergent events an additional analysis by Cox proportional hazards regression was performed with the treatment-emergent event as a time-varying covariate. Analyses of treatment-emergent events were performed for both definitions of being present (earliest onset before the CVAE or death, and earliest onset ⩾7 days before the CVAE or death). In the Cox regression the treatment-emergent event was considered present from the time of its onset however defined until the onset of the CVAE or death, or until study discontinuation for participants who did not have a CVAE or die. In this way the treatment-emergent event is considered to become a participant characteristic at the time of its onset.
To evaluate how a baseline characteristic or treatment-emergent event modified the difference between risperidone and placebo, a Cox regression with factors of treatment group, baseline characteristic or treatment-emergent event, and the interaction of treatment and baseline characteristic or treatment-emergent event, was performed. Treatment-emergent events (including concomitant medications) were included as time-varying covariates as described above. The significance of the interaction term was based on likelihood ratio statistics comparing the models with and without that term. Hazard ratios (HRs) and 95% confidence intervals comparing risperidone and placebo were estimated from the full model at both levels of the baseline characteristic or treatment-emergent event. All statistical analyses were performed using SAS version 9.2. 17 Figures were generated using the R lattice package. Reference Sarkar18 Nominal P-values are presented throughout; there was no adjustment for multiplicity.
Results
In total, 1009 participants treated with risperidone and 712 participants given placebo were included in the combined database. Demographic features of participants are reported in Table 1. There was a statistically significant difference in the crude incidence of all CVAEs across all studies (Table 2): risperidone 4.9%, placebo 1.5%; P<0.001, Cochran–Mantel–Haenszel test stratified by study. Crude incidence of mortality was numerically higher in the risperidone group (4%, n = 40) than in the placebo group (3%, n = 22), but the between-group difference was not statistically significant (P = 0.527, Cochran–Mantel–Haenszel test stratified by study). The risperidone group had a statistically significantly higher exposure-adjusted incidence of any CVAE across all studies compared with placebo (P<0.001). Exposure-adjusted incidence of mortality was higher in the risperidone group compared with the placebo group, but this was not statistically significant (P = 0.466).
Table 1 Demographic characteristics of the participants
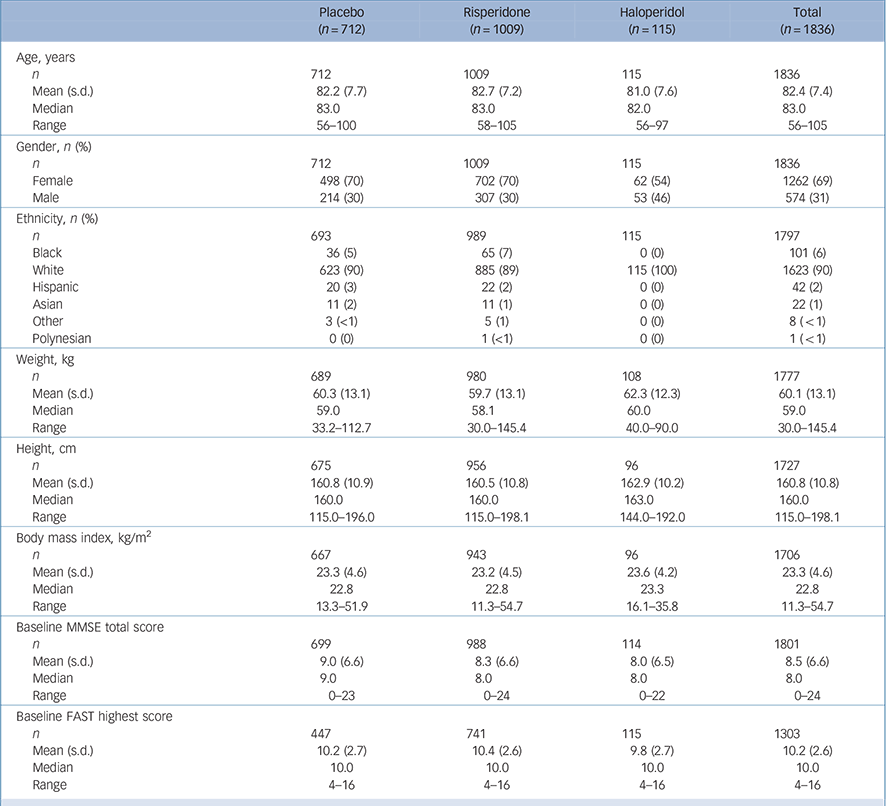
| Placebo (n = 712) |
Risperidone (n = 1009) |
Haloperidol (n = 115) |
Total (n = 1836) |
|
|---|---|---|---|---|
| Age, years | ||||
| n | 712 | 1009 | 115 | 1836 |
| Mean (s.d.) | 82.2 (7.7) | 82.7 (7.2) | 81.0 (7.6) | 82.4 (7.4) |
| Median | 83.0 | 83.0 | 82.0 | 83.0 |
| Range | 56–100 | 58–105 | 56–97 | 56–105 |
| Gender, n (%) | ||||
| n | 712 | 1009 | 115 | 1836 |
| Female | 498 (70) | 702 (70) | 62 (54) | 1262 (69) |
| Male | 214 (30) | 307 (30) | 53 (46) | 574 (31) |
| Ethnicity, n (%) | ||||
| n | 693 | 989 | 115 | 1797 |
| Black | 36 (5) | 65 (7) | 0 (0) | 101 (6) |
| White | 623 (90) | 885 (89) | 115 (100) | 1623 (90) |
| Hispanic | 20 (3) | 22 (2) | 0 (0) | 42 (2) |
| Asian | 11 (2) | 11 (1) | 0 (0) | 22 (1) |
| Other | 3 (<1) | 5 (1) | 0 (0) | 8 (<1) |
| Polynesian | 0 (0) | 1 (<1) | 0 (0) | 1 (<1) |
| Weight, kg | ||||
| n | 689 | 980 | 108 | 1777 |
| Mean (s.d.) | 60.3 (13.1) | 59.7 (13.1) | 62.3 (12.3) | 60.1 (13.1) |
| Median | 59.0 | 58.1 | 60.0 | 59.0 |
| Range | 33.2–112.7 | 30.0–145.4 | 40.0–90.0 | 30.0–145.4 |
| Height, cm | ||||
| n | 675 | 956 | 96 | 1727 |
| Mean (s.d.) | 160.8 (10.9) | 160.5 (10.8) | 162.9 (10.2) | 160.8 (10.8) |
| Median | 160.0 | 160.0 | 163.0 | 160.0 |
| Range | 115.0–196.0 | 115.0–198.1 | 144.0–192.0 | 115.0–198.1 |
| Body mass index, kg/m2 | ||||
| n | 667 | 943 | 96 | 1706 |
| Mean (s.d.) | 23.3 (4.6) | 23.2 (4.5) | 23.6 (4.2) | 23.3 (4.6) |
| Median | 22.8 | 22.8 | 23.3 | 22.8 |
| Range | 13.3–51.9 | 11.3–54.7 | 16.1–35.8 | 11.3–54.7 |
| Baseline MMSE total score | ||||
| n | 699 | 988 | 114 | 1801 |
| Mean (s.d.) | 9.0 (6.6) | 8.3 (6.6) | 8.0 (6.5) | 8.5 (6.6) |
| Median | 9.0 | 8.0 | 8.0 | 8.0 |
| Range | 0–23 | 0–24 | 0–22 | 0–24 |
| Baseline FAST highest score | ||||
| n | 447 | 741 | 115 | 1303 |
| Mean (s.d.) | 10.2 (2.7) | 10.4 (2.6) | 9.8 (2.7) | 10.2 (2.6) |
| Median | 10.0 | 10.0 | 10.0 | 10.0 |
| Range | 4–16 | 4–16 | 4–16 | 4–16 |
FAST, Functional Assessment Staging of Alzheimer's Disease scale; MMSE, Mini-Mental State Examination.
Table 2 Crude incidence of mortality and cerebrovascular adverse event in risperidone studies
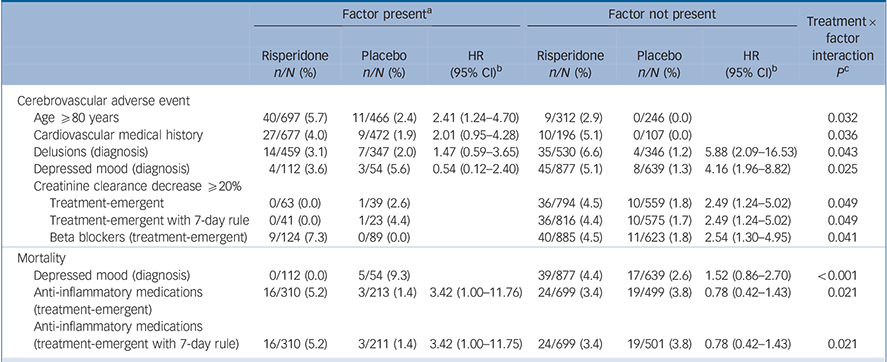
| Mortality | Any CVAE | |||||
|---|---|---|---|---|---|---|
| Study | n/N | % | P a | n/N | % | P a |
| All studies | ||||||
| Risperidone | 40/1009 | 4.0 | 0.527 | 49/1009 | 4.9 | < 0.001 |
| Placebo | 22/712 | 3.1 | 11/712 | 1.5 | ||
| AUS-5 | ||||||
| Risperidone | 6/167 | 3.6 | 0.769 | 18/167 | 10.8 | 0.002 |
| Placebo | 5/170 | 2.9 | 4/170 | 2.4 | ||
| BEL-14 | ||||||
| Risperidone | 1/20 | 5.0 | 1.000 | 0/20 | 0.0 | |
| Placebo | 0/19 | 0.0 | 0/19 | 0.0 | ||
| INT-24 | ||||||
| Risperidone | 1/115 | 0.9 | 0.119 | 12/115 | 10.4 | 0.010 |
| Placebo | 5/114 | 4.4 | 2/114 | 1.8 | ||
| INT-83 | ||||||
| Risperidone | 0/10 | 0.0 | 0.444 | 1/10 | 10.0 | 1.000 |
| Placebo | 1/8 | 12.5 | 0/8 | 0.0 | ||
| USA-232 | ||||||
| Risperidone | 9/235 | 3.8 | 0.445 | 5/235 | 2.1 | 0.283 |
| Placebo | 6/238 | 2.5 | 2/238 | 0.8 | ||
| USA-63 | ||||||
| Risperidone | 23/462 | 5.0 | 0.383 | 13/462 | 2.8 | 0.773 |
| Placebo | 5/163 | 3.1 | 3/163 | 1.8 | ||
CVAE, cerebrovascular adverse event.
a. Cochran-Mantel-Haenszel test stratified by study for ‘all studies’; Fisher's exact test for individual studies.
Placebo group risk factors
Patient characteristics
Crude incidence analysis. Figure 1 summarises the crude incidence of CVAEs and death in participants in the placebo group with and without the identified risk factors. Participants over 80 years old (P = 0.020) and with baseline depressed mood (P = 0.047) were at increased risk of CVAE. Analysis of potential baseline risk factors for mortality indicated that severe impairment of creatinine clearance (P = 0.024) and depressed mood (P = 0.023) were significantly associated with increased mortality, whereas male gender (P = 0.056) and severe dementia (MMSE score <9) (P = 0.087) failed to reach accepted significance levels.

Fig. 1 Incidence of cerebrovascular adverse events (CVAE) and death categorised by baseline risk factor in placebo group participants. BEHAVE, Behavioral Pathology in Alzheimer's Disease; BMI, body mass index; CrCl, creatinine clearance; DBP, diastolic blood pressure; MMSE, Mini-Mental State Examination. *P<0.05.
Exposure-adjusted incidence rates. Analyses based on EAIR differences identified age over 80 years (P = 0.004) and BEHAVE-AD global rating less than severe (P = 0.045) as significant risk factors for CVAE, whereas cardiovascular medical history (P = 0.073) failed to reach accepted significance levels. No significant risk factor for mortality was identified, with age over 80 years (P = 0.096), severe impairment of creatinine clearance (P = 0.093), depressed mood (P = 0.074) and male gender (P = 0.081) failing to reach significance.
Treatment-emergent adverse events
Crude incidence analysis. No treatment-emergent adverse event risk factor was significantly associated with CVAE. Factors associated with increased mortality included dehydration (P<0.001), infection (P<0.001) and pulmonary conditions (P = 0.012), whereas cardiovascular disease (P = 0.058) and sedation (P = 0.068) failed to reach accepted significance levels (see Fig. 2).
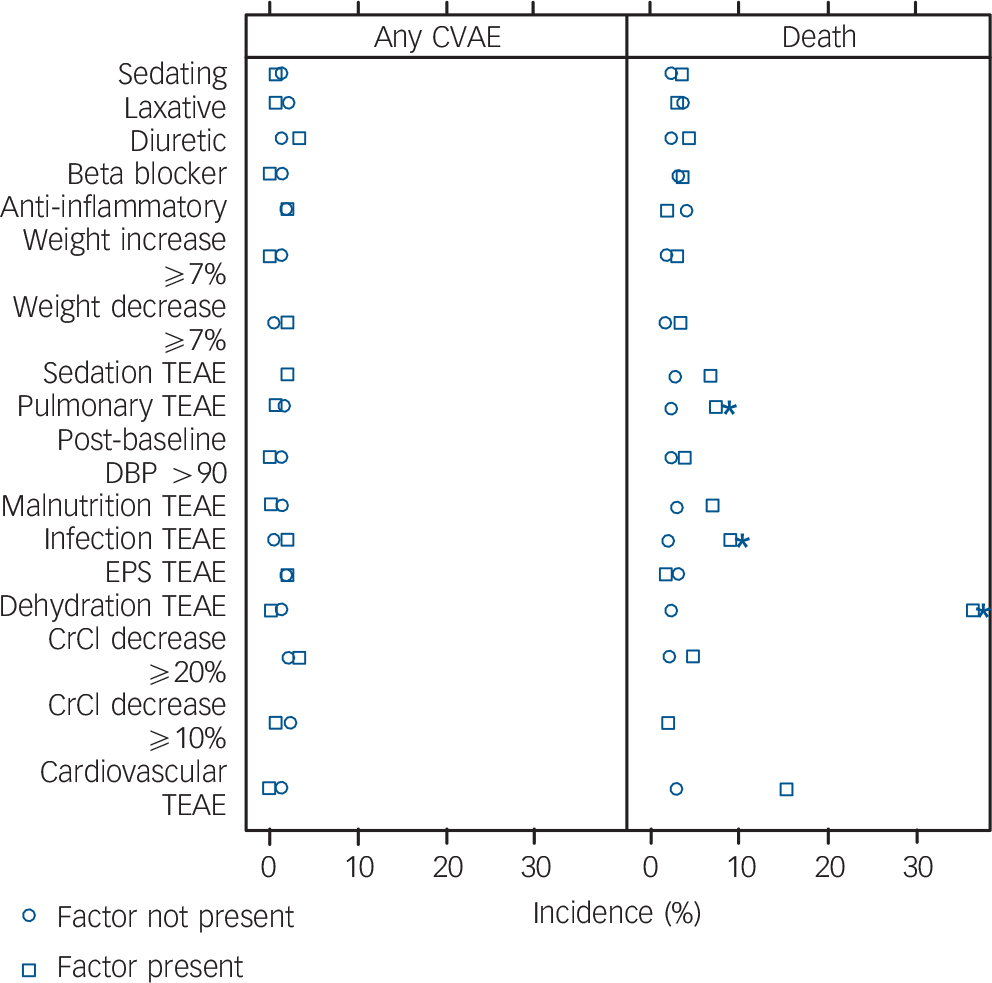
Fig. 2 Incidence of cerebrovascular adverse events (CVAE) and death categorised by treatment-emergent risk factor in placebo group participants. CrCl, creatinine clearance; DBP, diastolic blood pressure; EPS, extrapyramidal symptoms; TEAE, treatment-emergent adverse event. *P<0.05.
Exposure-adjusted incidence rates. For EAIR of CVAE, none of the examined risk factors was significantly associated. For mortality, dehydration (P = 0.042) and infection (P = 0.007) emerged as significant but pulmonary conditions (P = 0.056) failed to reach significance. Cox regression did not identify treatment-emergent risk factors significantly associated with CVAE. Risk factors for mortality confirmed as statistically significant by Cox regression included cardiovascular events (HR = 9.68, 95% CI 2.2–42.65; P = 0.022), creatinine clearance decrease >20% (HR = 9.86, 95% CI 1.98–49.07; P = 0.024), dehydration (HR = 31.61, 95% CI 10.49–95.25; P<0.001), infection (HR = 5.82, 95% CI 2.39–14.18; P<0.001) and pulmonary condition (HR = 5.22, 95% CI 2.07–13.12; P = 0.001). Weight decrease >7% (HR = 5.87, 95% CI 1.22–28.26; P = 0.065) and sedation (HR = 3.05, 95% CI 1.09–8.52; P = 0.054) failed to reach significance levels.
Analysis with 7-day offset. For the crude incidence of CVAE, no treatment-emergent factor was statistically significant. For the crude incidence of mortality, significant factors identified included dehydration (P = 0.013), infection (P = 0.004) and pulmonary condition (P = 0.050), whereas weight decrease >7% (P = 0.081) did not reach statistical significance. Analyses based on EAIR differences between adverse event groups did not identify any new risk factors for CVAE compared with analysis of crude incidence, and only infection (P = 0.045) was significantly associated with mortality. Analyses based on the Cox regression identified the same risk factors for increased mortality as analysis without the 7-day offset, together with an additional risk factor, weight decrease >7% (HR = 6.68, 95% CI 1.4–31.88; P = 0.050).
Concomitant medication
There was a non-significant trend for anti-inflammatory drugs to be associated with a lower risk of death in the EAIR analyses (P = 0.081) and for sedating medications to be associated with increased mortality based on Cox regression (HR = 2.15, 95% CI 0.86–5.4; P = 0.094).
Risperidone group risk factors
This analysis investigated which factors conferred a difference in risk between patients treated with risperidone and those treated with placebo, as evidenced by a significant treatment × risk factor interaction in the Cox regression between the HR of risperidone and placebo in those with or without the risk factor. Results for baseline characteristics, treatment-emergent factors or concomitant medications that had a statistically significant treatment × factor interaction term are summarised in Table 3.
Table 3 Treatment × factor incidence of any cerebrovascular adverse event or death

| Factor present a | Factor not present | Treatment × factor interaction P c |
|||||
|---|---|---|---|---|---|---|---|
| Risperidone n/N (%) |
Placebo n/N (%) |
HR (95% CI) b |
Risperidone n/N (%) |
Placebo n/N (%) |
HR (95% CI) b |
||
| Cerebrovascular adverse event | |||||||
| Age ⩾80 years | 40/697 (5.7) | 11/466 (2.4) | 2.41 (1.24–4.70) | 9/312 (2.9) | 0/246 (0.0) | 0.032 | |
| Cardiovascular medical history | 27/677 (4.0) | 9/472 (1.9) | 2.01 (0.95–4.28) | 10/196 (5.1) | 0/107 (0.0) | 0.036 | |
| Delusions (diagnosis) | 14/459 (3.1) | 7/347 (2.0) | 1.47 (0.59–3.65) | 35/530 (6.6) | 4/346 (1.2) | 5.88 (2.09–16.53) | 0.043 |
| Depressed mood (diagnosis) | 4/112 (3.6) | 3/54 (5.6) | 0.54 (0.12–2.40) | 45/877 (5.1) | 8/639 (1.3) | 4.16 (1.96–8.82) | 0.025 |
| Creatinine clearance decrease ⩾20% | |||||||
| Treatment-emergent | 0/63 (0.0) | 1/39 (2.6) | 36/794 (4.5) | 10/559 (1.8) | 2.49 (1.24–5.02) | 0.049 | |
| Treatment-emergent with 7-day rule | 0/41 (0.0) | 1/23 (4.4) | 36/816 (4.4) | 10/575 (1.7) | 2.49 (1.24–5.02) | 0.049 | |
| Beta blockers (treatment-emergent) | 9/124 (7.3) | 0/89 (0.0) | 40/885 (4.5) | 11/623 (1.8) | 2.54 (1.30–4.95) | 0.041 | |
| Mortality | |||||||
| Depressed mood (diagnosis) | 0/112 (0.0) | 5/54 (9.3) | 39/877 (4.4) | 17/639 (2.6) | 1.52 (0.86–2.70) | <0.001 | |
| Anti-inflammatory medications (treatment-emergent) |
16/310 (5.2) | 3/213 (1.4) | 3.42 (1.00–11.76) | 24/699 (3.4) | 19/499 (3.8) | 0.78 (0.42–1.43) | 0.021 |
| Anti-inflammatory medications (treatment-emergent with 7-day rule) |
16/310 (5.2) | 3/211 (1.4) | 3.42 (1.00–11.75) | 24/699 (3.4) | 19/501 (3.8) | 0.78 (0.42–1.43) | 0.021 |
HR, hazard ratio.
a. Factors with treatment × factor interaction P<0.05 (Cox regression).
b. Risperidone v. placebo.
c. Likelihood ratio test of treatment × factor interaction term in Cox proportional hazards regression.
Patient characteristics
For CVAE, a significant interaction effect was observed for age 80 years or over, positive cardiovascular medical history, baseline delusions and baseline depressed mood. For baseline delusions and depressed mood, the risperidone v. placebo HR was lower in patients with the complication (HR = 1.47, 95% CI 0.59–3.65 for delusions; HR = 0.54, 95% CI 0.12–2.40 for depressed mood) compared with participants without the complication (HR = 5.88, 95% CI 2.09–16.53 for delusions; HR = 4.16, 95% CI 1.96–8.82 for depressed mood). For age 80 years or above and cardiovascular medical history, the significant interaction was probably the result of no one in the placebo group without the baseline characteristic having a CVAE. The relative risk of CVAE among people aged at least 80 years or with cardiovascular medical history is similar to the relative risk among all participants (see Table 2). For mortality, a significant interaction effect was observed for baseline depressed mood, with more deaths in the placebo group (9%, n = 5) than in the risperidone group (0%) among participants with baseline depressed mood, compared with more deaths in the risperidone group than in the placebo group among participants without depressed mood at baseline (HR = 1.52, 95% CI 0.86–2.70).
Treatment-emergent factors
Only a creatinine clearance decrease of more than 20% was associated with a modified hazard ratio for mortality (HR if risk factor present undefined as no CVAE in risperidone group; if risk factor not present, HR = 2.49, 95% CI 1.24–5.02; P = 0.049). There was only one event (in the placebo group, v. no event in the risperidone group) among those with the creatinine clearance decrease. No treatment-emergent factor was associated with an excess mortality risk in participants treated with risperidone when compared with the placebo group.
Concomitant medication
There was no concomitant medication whose use was associated with differential CVAE risk in the risperidone group compared with placebo. The treatment × risk factor interaction term was statistically significant for analyses of mortality for anti-inflammatory drug use, with a higher risperidone v. placebo hazard ratio in patients with use of these drugs (if risk factor present, HR = 3.42, 95% CI 1.00–11.76; if risk factor not present, HR = 0.78, 95% CI 0.42–1.43; P = 0.021). The presence or absence of the 7-day offset did not change this result.
Discussion
Meta-analyses of clinical trials in elderly patients with dementia indicate that individual antipsychotic drugs have different mortality risks, with quetiapine being associated with the lowest risk and haloperidol being associated with the highest risk of death. Reference Kales, Kim, Zivin, Valenstein, Seyfried and Chiang6,Reference Maust, Kim, Seyfried, Chiang, Kavanagh and Schneider7,Reference Huybrechts, Gerhard, Crystal, Olfson, Avorn and Levin19,Reference Gerhard, Huybrechts, Olfson, Schneeweiss, Bobo and Doraiswamy20 For a given drug there is a dose–response relationship with mortality. Reference Maust, Kim, Seyfried, Chiang, Kavanagh and Schneider7 A physician faced with a patient whose symptoms and behaviour are distressing and could place the patient and others in significant danger needs to evaluate the potential benefits balanced with the risks of treatment in an individual case. Reference Rabins and Lyketsos21 For example, depending on the atypical antipsychotic, the number of patients who would need to be treated to observe improvement in psychosis and aggression in a single patient ranges from 5 to 14, Reference Schneider, Dagerman and Insel5 with quetiapine having less convincing evidence of efficacy than risperidone and olanzapine. Reference Maust, Kim, Seyfried, Chiang, Kavanagh and Schneider7,Reference Maher, Maglione, Bagley, Suttorp, Hu and Ewing22 There is a significant chance that psychosis symptoms will return when treatment is stopped. Reference Devanand, Mintzer, Schultz, Andrews, Sultzer and de la Pena23 The most extreme contrasting risk of treatment is reflected by the number of patients needed to be treated to observe a single death, ranging from 27 to 100. Reference Schneider, Dagerman and Insel4,Reference Maust, Kim, Seyfried, Chiang, Kavanagh and Schneider7
Baseline factors
In terms of baseline patient factors associated with increased exposure-adjusted incidence of CVAE in placebo-treated patients, we confirmed the previously reported age over 80 years, as well as the novel finding of a BEHAVE-AD global rating less than severe; however, no significant risk factor for mortality was identified by this method. No treatment-emergent event was associated with increased exposure-adjusted incidence of CVAE in placebo-treated patients. Treatment-emergent events associated with increased exposure-adjusted incidence of mortality were dehydration, infection and pulmonary conditions. Baseline characteristics that modified the risk of CVAE in patients treated with risperidone compared with placebo included age 80 years or above and cardiovascular disease history. Presence of delusions or depressed mood at baseline was associated with a lower risperidone v. placebo hazard ratio for CVAE compared with not having those complications. Baseline depressed mood was also associated with a reduced risperidone v. placebo hazard ratio for mortality. None of the investigated treatment-emergent risk factors modified the differential risk for CVAE and mortality in patients treated with risperidone compared with placebo except for creatinine clearance decrease >20%, which was associated with a reduced hazard ratio for CVAE. This is hard to explain and may be a consequence of the small number of patients with an observed creatinine clearance decrease prior to a CVAE. There was only one CVAE in a placebo-treated patient v. none in the risperidone group among participants with the creatinine clearance decrease. We also found that concomitant use of anti-inflammatory drugs was associated with a greater risperidone v. placebo hazard ratio for mortality compared with no use of such drugs.
Our data show some overlap with the results of the analysis of the integrated olanzapine database, Reference Kryzhanovskaya, Jeste, Young, Polzer, Roddy and Jansen24 which identified age over 80 years, concomitant benzodiazepine use, treatment-emergent sedation and pulmonary conditions to be associated with an increased risk of mortality, and found that age over 80 years and diagnosis of vascular or mixed dementia were associated with an increased risk of CVAE. However, this analysis identified risk factors in a combined sample of patients treated with olanzapine or placebo. Their results would therefore reflect a combination of background risk factors for CVAE and death in a general elderly population (from placebo patients), with specific risk factors for an adverse outcome in patients treated with olanzapine. By examining for characteristics that modified risk in patients treated with risperidone compared with placebo, rather than simply in the combined trial population, we were able to identify patient-related factors that could potentially inform a more individualised benefit–risk analysis in the initiation of antipsychotic treatment.
Psychiatric symptoms
The association of delusions or depression with reduced CVAE risk and depression with reduced mortality in risperidone-treated compared with placebo-treated patients may indicate that specific psychiatric symptoms – rather than clusters of behaviours such as agitation – mark patients for whom atypical antipsychotic treatment carries less risk. In the Clinical Antipsychotic Trials of Intervention Effectiveness – Alzheimer's Disease (CATIE-AD) study, Reference Schneider, Tariot, Dagerman, Davis, Hsiao and Ismail25 specific symptoms including paranoid ideas were more likely to improve with atypical antipsychotic treatment. Reference Sulzer, Davies, Tariot, Dagerman, Lebowitz and Lyketsos26 Also, in a meta-analysis of placebo-controlled risperidone trials, Reference Katz, de Deyn, Mintzer, Greenspan, Zhu and Brodaty2 ‘people are stealing things’ or infidelity delusions were more likely to respond to treatment than other symptoms. A meta-analysis of atypical trials in dementia, however, reported smaller treatment effect sizes for patients selected on the basis of psychosis symptoms, Reference Schneider, Dagerman and Insel27 so the literature is not consistent. Our data add to evidence that delusions may represent a target symptom in Alzheimer's disease where potential treatment benefits are significant and risks of CVAE smaller. This finding will need to be replicated in an independent sample before it can form the basis for advice to guide clinicians when making decisions about stratifying risk in this treatment indication. However, our data add to an emerging story that the presence of particular individual psychiatric symptoms in patients with dementia is associated with both the potential benefits and risks of antipsychotic treatment.
Although anti-inflammatory drugs are associated with increased cardiovascular events and all-cause mortality in the general medical population, Reference Trelle, Reichenbach, Wandel, Hildebrand, Tschannen and Villiger28 these agents did not increase the risk of stroke or death in the placebo group in the trials examined here. The increased relative mortality in patients taking risperidone who were also treated with anti-inflammatory medications represents an easily modifiable risk factor in clinical practice.
Study limitations
A limitation of our findings is that, as in previous investigations of potential risk factors for stroke and death in people with dementia treated with an atypical antipsychotic, Reference Kryzhanovskaya, Jeste, Young, Polzer, Roddy and Jansen24 we did not make statistical correction of our results for the effects of multiple comparisons. We would acknowledge that some of our findings, with P-values in the 0.02–0.05 range, might have arisen by the effects of chance and the multiple statistical comparisons made in our analyses. We would argue that this is unlikely, and that the consistency of the findings with what we understand clinically and with each other argues against such a negative explanation.
Clinical implications
Clinicians will continue to consider antipsychotic short-term treatment for behavioural and psychiatric symptoms in dementia when those symptoms cause significant distress or carry risk of harm to the patient or others, and when alternative, non-pharmacological interventions are unavailable or have failed. Reference Rabins and Lyketsos21,Reference Steinberg and Lyketsos29 These symptoms are independently associated with more rapid progression to severe dementia and earlier death, Reference Peters, Schwartz, Han, Rabins, Steinberg and Tschanz30 and their treatment represents a potential opportunity to modify the clinical course of dementia. Our analysis has confirmed some of the previously established patient factors associated with increased risk of CVAE and mortality during antipsychotic treatment. Importantly, we have also reported that the presence of some psychiatric symptoms was associated with reduced risk of CVAE and mortality within the population of people with dementia treated with risperidone in the pivotal trials.
Funding
R.H. is supported by the NIHR UCLH BRC.
Acknowledgements
Janssen and Johnson & Johnson employees worked on the analyses and drafting of the manuscript but the companies did not provide any direct funding for the study.








eLetters
No eLetters have been published for this article.