Anorexia nervosa (AN) is a psychiatric syndrome characterised by distortion of body image and adoption of persistent and inappropriate eating behaviours for weight control, even if the person has a body weight below the recommended for age and sex(1). It consists of one of the most serious eating disorders and has the highest mortality rate among psychiatric diseases(Reference Herpertz-Dahlmann2,Reference Arcelus, Mitchell and Wales3) . Additionally, the results of studies indicate that individuals may develop behaviours that usually precede an eating disorder, such as eating restriction(Reference O’Neil, Quirk and Housden4,Reference Haynos, Field and Wilfley5) .
Restrained eating behaviour (REB) is considered a behavioural and cognitive dysfunctional strategy adopted by individuals to control body weight(Reference Bernardi, Cichelero and Vitolo6). A common characteristic among individuals with AN, such as those with REB, is concerned with weight-related eating(Reference Arcelus, Mitchell and Wales3,Reference Haynos, Field and Wilfley5) which can be expressed through the omission of meals, adoption of restrictive diets, fasting, episodes of self-induced vomiting and use of laxatives(Reference O’Neil, Quirk and Housden4,Reference Das, Salam and Thornburg7) and may compromise the state of health and nutrition throughout life(Reference Das, Salam and Thornburg7,Reference Barajas, Jáuregui and Laporta8) .
In childhood and adolescence, a period of greatest vulnerability, eating disorders and dysfunctional behaviours, in addition to food consumption, may be influenced by biological and psychosocial changes inherent to the phase, as well as by the interference of family, friends, media, personal and cultural beliefs(Reference Das, Salam and Thornburg7,Reference Barajas, Jáuregui and Laporta8) , especially when associated with dissatisfaction or distortion of body image(Reference Ribeiro-Silva, Fiaccone and Conceição-Machado9). Among the most frequent characteristics of food consumption in the course of AN and REB are low food intake in quantity and quality(Reference Morgan, Vecchiatti and Negrão10,Reference Schaumberg, Anderson and Anderson11) with a significant reduction in energy intake, carbohydrates, fats and dietary deficiencies of certain micronutrients(Reference Chiurazzi, Cioffi and De Caprio12,Reference Carvalho, Fonsêca and Priore13) .
Epidemiological studies have evaluated the association between AN/REB and food consumption(Reference Chiurazzi, Cioffi and De Caprio12,Reference Estecha Querol, Fernández Alvira and Mesana Graffe14,Reference Raatz, Jahns and Johnson15) ; however, the knowledge produced to date is not fully understood. This relationship imposes multiple interactions involving genetic and environmental factors, in addition to the complexity and challenges in the evaluation of food intake, especially in individuals with ED(Reference Gavrieli, Trichopoulou and Valsta16,Reference Schebendach, Porter and Wolper17) .
Individuals with AN/REB are usually less accurate when reporting on their food intake, although they seek to obtain more knowledge about specific aspects of food than the general population(Reference Raatz, Jahns and Johnson15,Reference Schebendach, Porter and Wolper17) . Nevertheless, the various methods of food surveys available have limitations, such as specific errors inherent to the interviewer, the individual, dietary measurement and data analysis that may favour bias in the results(Reference Gavrieli, Trichopoulou and Valsta16,Reference Fisberg, Marchioni and Colucci18,Reference Cade19) . However, in the analysis of dietary intake, some procedures can be used to minimise these errors, which include correction of coefficients and risk measures considering the intra-individual variability to the adjustment for energy intake in investigations of the association of food intake and health outcomes. In this sense, it is also important to observe the choice and application of robust and adequate tests to analyse both nutrients (e.g. probabilistic approach) and dietary patterns (e.g. factor analysis, cluster, structural equation modelling and latent classes)(Reference Pereira and Sichieri20).
Given the lack of evidence synthesis available in this field, this scope review may contribute to the advancement of knowledge in the area and to the development of strategies to prevent the development of AN/REB and nutritional deficiencies. Thus, the objective of this study is to systematically explore studies that evaluated the association between AN/REB and food consumption, as well as to identify the existing knowledge gaps from the following questions: (1) what is the extent and breadth of the existing literature on the relationship between AN/REB and food consumption in children and adolescents? and (2) what approaches and methods are used to identify food consumption in children and adolescents with AN/REB?
Methods
This scope review was reported according to the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis Extension for Scoping Reviews(Reference Matthew, Joanne and Patrick21).The checklist Preferred Reporting Items for Systematic Review and Meta-Analysis Extension for Scoping Reviews (Appendix) was applied. The protocol was developed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines(Reference Kamioka22) and the Joanna Briggs Institute manual(Reference Peters, Godfrey and McInerney23) with registration in the Open Science Framework (https://osf.io/wm79y).
Eligibility criteria
The research question and eligibility criteria were defined using the acronym PCC (Participant, Concept and Context). Experimental and observational studies (cross-sectional, cohort and case–control), systematic reviews and meta-analyses, conference abstracts, dissertations and theses were included in this scoping review. Studies with case reports or series, ecological studies, narrative reviews, communications, editorials, book chapters and study protocols were excluded. The inclusion and exclusion criteria for the selection of studies are described in Table 1.
Table 1. Eligibility criteria for the selected studies

ICD, international statistical classification of diseases and health-related problems; DSM, diagnostic and statistics manual for mental disorders.
Sources of information and search strategy
The search was performed on 14 November 2020. The articles were independently searched by two reviewers in the Medline/PubMed databases, Embase via Elsevier, Cochrane Library Databanks, Lilacs, Cumulative Index to Nursing and Allied Health Literature, Scopus, PsycINFO, PsyARTICLES, EPPI-Center database of health promotion research (BiblioMap), Epistemonikos, and in the grey literature by Index to Theses, ProQuest Dissertations and Theses Database and Psicodoc, OpenGrey and Google Scholar. The search strategies were performed in three stages. First, the following terms and their respective synonyms were selected in the Medical Subject Headings of PubMed: ‘food pattern’, ‘food consumption’, ‘food’, ‘food variety’, ‘dietary pattern’, ‘dietary intake’, ‘diet’, ‘diet variety’, ‘diet quality’, ‘dietary quality’, ‘dietary quality index’ and ‘dieting’, ‘dietary restriction’, ‘dietary restraint’, ‘dieting restrictive’, ‘feeding and eating disorder’, ‘disordered eating behaviour’, ‘eating disorder’, ‘eating disorder symptoms’, ‘anorexia nervosa’, ‘restrained eating’ and ‘restrained eating behaviour’. Next, to identify other terms not obtained in the first step, a limited search was performed in Medline/PubMed. At this stage, the terms ‘nutrients’, ‘macronutrients’, ‘micronutrients’ and ‘energy’ were identified in the titles, abstracts and keywords of the retrieved documents. Finally, all terms and their synonyms were selected from Medical Subject Headings, Embase Subject Headings (Emtree) and Health Sciences Descriptors (DeSC), added to the search strategy and applied to all databases. The Boolean operators ‘AND’ and ‘OR’ were used. The reference lists of all selected studies were searched to identify additional studies not indexed in the databases but relevant for inclusion in this review.
Selection of evidence sources and data extraction
The selection of studies was performed by a pair of independent reviewers. Data extraction was performed by a reviewer, and the researcher confirmed the accuracy of the information collected. Any disagreement in the selection of sources of evidence and data collected was discussed and resolved with a third reviewer. Endnote® was used to load all publications retained from the databases and to remove duplicates. During the screening, the titles and abstracts of the publications were read, followed by the complete reading and selection of eligible studies.
The data were extracted in a Microsoft Office ® Excel spreadsheet. The information extracted was article title, authors, date of publication, study site, study design, sample size, participant characteristics, inclusion and exclusion criteria, and data on dietary intake (statistical methods and techniques for assessing intake), tools used to measure feeding behaviour and AN. Missing information was requested by e-mail to the corresponding author.
Data synthesis
The collected data were presented in tables or graphs. The results were grouped according to the study design and evaluation of dietary intake and AN/REB.
Results
Flow of selection of evidence sources
The search strategy in the ten databases and in the grey literature retrieved 22·283 records, remaining after removal of duplicates, 18 856 for reading the title and abstract. Subsequently, sixty-one publications remained in the selection process for full reading. Of these, thirty-five articles were excluded because they did not meet the eligibility criteria. No additional articles were identified by searching the reference lists. A total of twenty-four studies(Reference Bischoff-Seals24–Reference Dunker and Philippi49) and, for two primary studies, two publications were retained in each study(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) (Fig. 1). It should be noted that in the study by Bischoff-Seals(Reference Bischoff-Seals24), only the abstract was identified, and the author was asked for the dissertation, but without feedback, so only the characterisation of the study was described in this review.

Fig. 1. Diagram of study selection.
Characteristics of the evidence sources
The selected studies were published from 1991 to 2020 and conducted in the USA (n 8)(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Bisset, Gauvin and Potvin28,Reference Woodruff, Hanning and Lambraki29,Reference Santiago, Zimmerman and Feinstein39,Reference Madhusmita, Tsai and Anderson42,Reference Weltzin, Fernstrom and Hansen44,Reference Grigolon, Dunker and Almeida45,Reference Dunker and Philippi48) , Brazil (n 3)(Reference Tsai, Chang and Lien31,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Mulvihill, Davies and Rogers37) , Spain (n 3)(Reference Aparicio, Canals and Pérez25–Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27), Canada (n 2)(Reference Bisset, Gauvin and Potvin28,Reference Woodruff, Hanning and Lambraki29) , Taiwan (n 2)(Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) , Australia (n 1)(Reference Allen, Mori and Beilin32), Ireland (n 1)(Reference Daly, O’Sullivan and Walton33), Sweden (n 1)(Reference Elfhag, Tholin and Rasmussen34), Japan (n 1)(Reference Kanayama, Sakai and Aoto35), Germany (n 1)(Reference Koch, Alexy, Diederichs and Buyken36) and England (n 1)(Reference Mulvihill, Davies and Rogers37) (Fig. 2).

Fig. 2. Distribution of study by country.
The sample size was 20–2·142 individuals aged between 9 and 20 years. Although the inclusion criterion had a maximum age of 19 years, we admitted three studies that had an age group between 12 and 20 years(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Weltzin, Fernstrom and Hansen44,Reference Grigolon, Dunker and Almeida45) . Thirteen studies involved female subjects(Reference Bischoff-Seals24–Reference Guevara, Urchaga and Cabaco26,Reference Bisset, Gauvin and Potvin28,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Mulvihill, Davies and Rogers37,Reference Santiago, Zimmerman and Feinstein39,Reference Baskaran, Carson and Campoverde Reyes40,Reference Madhusmita, Tsai and Anderson42–Reference Weltzin, Fernstrom and Hansen44,Reference Affenito, Dohm and Daniels46,Reference Dunker and Philippi48) and eleven recruited participants of both sexes(Reference Bischoff-Seals24–Reference Woodruff, Hanning and Lambraki29,Reference Daly, O’Sullivan and Walton33,Reference Elfhag, Tholin and Rasmussen34,Reference Koch, Alexy, Diederichs and Buyken36,Reference Caran, Santana and Monteiro38,Reference Santiago, Zimmerman and Feinstein39) . Few studies (n 4) reported the race/ethnicity of the participants(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Grigolon, Dunker and Almeida45) . Most studies (n 19) adopted a convenience sample without presenting the calculation of the sampling process(Reference Bischoff-Seals24–Reference Tsai, Chang and Lien31,Reference Daly, O’Sullivan and Walton33–Reference Koch, Alexy, Diederichs and Buyken36,Reference Caran, Santana and Monteiro38–Reference Madhusmita, Tsai and Anderson42,Reference Weltzin, Fernstrom and Hansen44–Reference Affenito, Dohm and Daniels46,Reference Dunker and Philippi48) (Table 2).
Table 2. Main characteristics of the selected studies

AN, anorexia nervosa; BN, bulimic nervosa; BED, binge eating disorder; PD, purging disorder.
Participants in nine studies had AN and were recruited in hospitals (n 5)(Reference Baskaran, Carson and Campoverde Reyes40–Reference Weltzin, Fernstrom and Hansen44), clinical centres (n 2)(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Grigolon, Dunker and Almeida45) and outpatient clinics (n 2)(Reference Allen, Mori and Beilin32,Reference Kanayama, Sakai and Aoto35) . The REB was evaluated in fifteen studies that collected samples in schools (n 13)(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Woodruff, Hanning and Lambraki29–Reference Koch, Alexy, Diederichs and Buyken36,Reference Caran, Santana and Monteiro38,Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Affenito, Dohm and Daniels46,Reference Striegel-Moore, Franko and Thompson47,Reference Dunker and Philippi49) , clinical centres (n 1)(Reference Grigolon, Dunker and Almeida45) and communities (n 1)(Reference Koch, Alexy, Diederichs and Buyken36) (Fig. 3).

Fig. 3. Distribution of the study by location: (a) anorexia nervosa; (b) restrictive eating behaviour.
Characteristic of anorexia nervosa and restrained eating behaviour
Seven studies evaluated the diagnosis of AN using the classification system of the Diagnostic Statistical Manual of Mental Disorders Third Edition (n 1)(Reference Weltzin, Fernstrom and Hansen44), Diagnostic Statistical Manual of Mental Disorders Fourth Edition (n 4)(Reference Allen, Mori and Beilin32,Reference Baskaran, Carson and Campoverde Reyes40,Reference Madhusmita, Tsai and Anderson42,Reference Nasserbakht, Brantner-Inthaler and Shih43) and Diagnostic Statistical Manual of Mental Disorders Fifth Edition (n 1)(Reference Santiago, Zimmerman and Feinstein39) and structured interviews based on the DMS-IV (Structured Clinical Interview for Axis I Disorders and the Eating Disorder Examination scale (n 1))(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25) . The study by Allen et al. (Reference Allen, Mori and Beilin32) conducted a two-phase study, first applying the Child Eating Disorder Examination and Eating Disorder Examination instruments – Questionnaire for screening and then the adolescents were evaluated for the presence of AN by the Diagnostic Statistical Manual of Mental Disorders Fourth Edition classification system. One study used diagnostic interviews(Reference Higgins, Hagman and Pan41) and another(Reference Kanayama, Sakai and Aoto35) reported that physicians experienced in the ED performed the diagnosis of AN (Table 3).
Table 3. General results of articles that studied the relationship between anorexia nervosa and food intake in children and adolescents
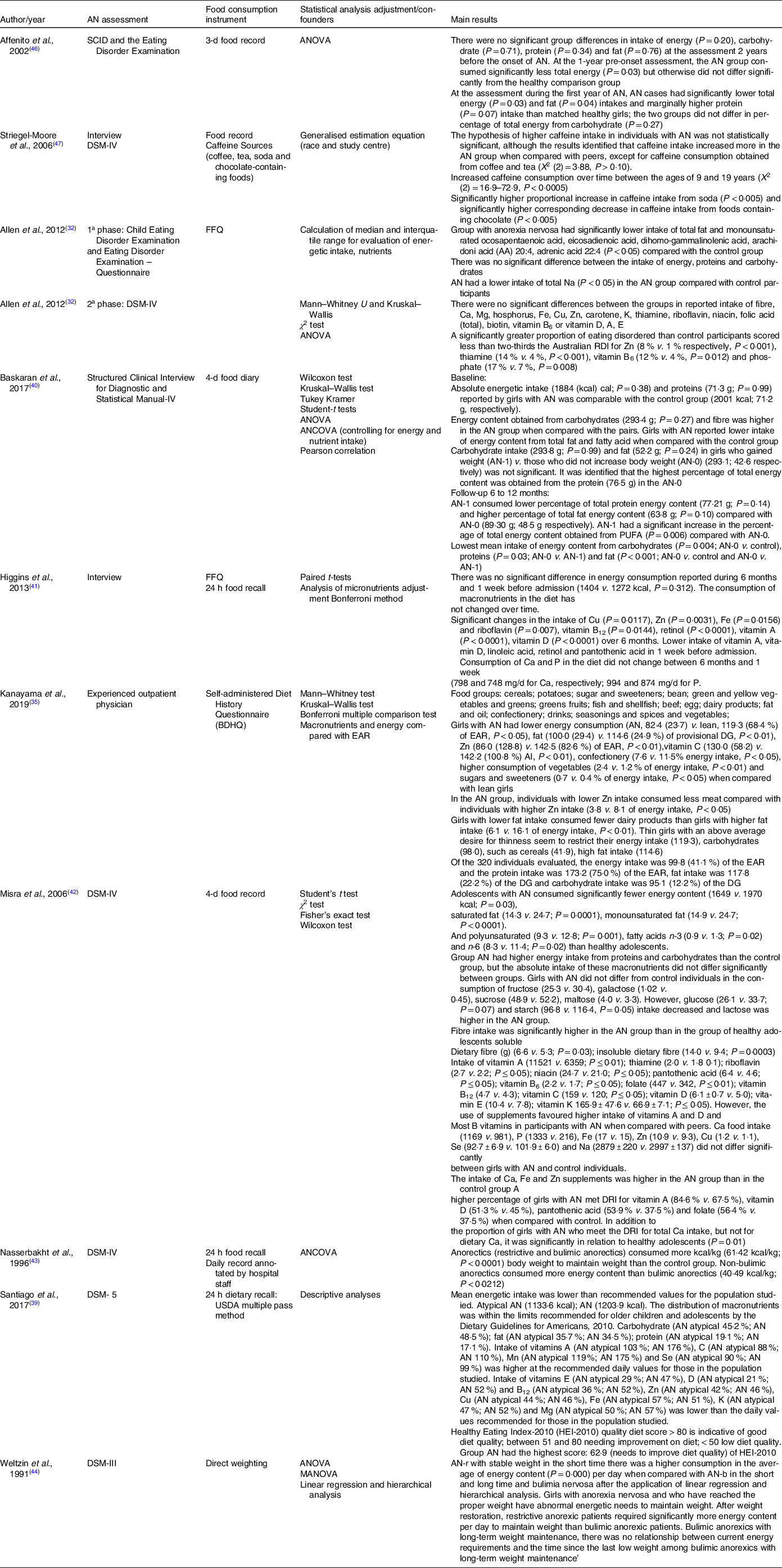
DSM, diagnostic statistical manual of mental disorders; DG, dietary goal for preventing life-style related diseases; MANOVA, multivariate analysis of variance; SCID, Structured Clinical Interview for Axis I Disorders.
The following scales were used to evaluate the REB: Eating Attitudes Test-26 (n 4)(Reference Tsai, Chang and Lien31,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Affenito, Dohm and Daniels46) ; Dutch Eating Behaviour Questionnaire (n 5)(Reference Bisset, Gauvin and Potvin28,Reference Daly, O’Sullivan and Walton33,Reference Elfhag, Tholin and Rasmussen34,Reference Koch, Alexy, Diederichs and Buyken36,Reference Mulvihill, Davies and Rogers37) ; Eating Attitudes Test-40 (n 2)(Reference Aparicio, Canals and Pérez25,Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27) ; Modified Three-Factor Eating Questionnaire (n 1)(Reference Bischoff-Seals24). Mulvihill et al.(Reference Mulvihill, Davies and Rogers37), in addition to using the DEBQ, also applied the Three-Factor Eating Questionnaire. One study used subscale food restriction of the Child-Eating Disorders Examination (n 1)(Reference Grigolon, Dunker and Almeida45). Nevertheless, two other studies adopted questions that investigated the adoption of a restrictive diet(Reference Guevara, Urchaga and Cabaco26,Reference Woodruff, Hanning and Lambraki29) . It should be noted that the studies by Bisset et al.(Reference Bisset, Gauvin and Potvin28), Koch et al.(Reference Koch, Alexy, Diederichs and Buyken36) and Mulvihill et al. (Reference Mulvihill, Davies and Rogers37) stratified the sample by tertiles of food restriction into low, medium and high (Table 4).
Table 4. General results of articles that studied the relationship between restrictive eating behaviour and food intake in children and adolescents

Dietary evaluation
Different dietary survey methods were used to obtain data on the participants food intake. For people with AN and those with REB, the studies adopted the following instruments: 24-h recall (R-24 h) from 1 to 3 d (n 9)(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Woodruff, Hanning and Lambraki29,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Santiago, Zimmerman and Feinstein39,Reference Weltzin, Fernstrom and Hansen44–Reference Affenito, Dohm and Daniels46,Reference Dunker and Philippi49) ; record or food diary (1–4 d) (n 5)(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Bisset, Gauvin and Potvin28,Reference Tsai, Chang and Lien31,Reference Higgins, Hagman and Pan41,Reference Madhusmita, Tsai and Anderson42) ; FFQ (n 5)(Reference Bisset, Gauvin and Potvin28,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Higgins, Hagman and Pan41,Reference Grigolon, Dunker and Almeida45) ; direct weighing (n 4)(Reference Bisset, Gauvin and Potvin28,Reference Daly, O’Sullivan and Walton33,Reference Mulvihill, Davies and Rogers37,Reference Weltzin, Fernstrom and Hansen44) . The self-administered diet history questionnaire was used by only one study in adolescents with AN(Reference Kanayama, Sakai and Aoto35). Questions regarding the food consumption of the instruments Health Behaviour in School-Aged Children (n 1)(Reference Guevara, Urchaga and Cabaco26) and Inventory of Health Behaviour in Scholars (n 1)(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27) were used in participants with REB. Mulvihill et al. (Reference Mulvihill, Davies and Rogers37) and Nasserbakh et al.(Reference Nasserbakht, Brantner-Inthaler and Shih43) also applied the daily record (Tables 3 and 4).
In studies with individuals with AN, the majority analysed dietary intake through the consumption of energy and macronutrients (n 9)(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Kanayama, Sakai and Aoto35,Reference Mulvihill, Davies and Rogers37,Reference Santiago, Zimmerman and Feinstein39,Reference Higgins, Hagman and Pan41–Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Affenito, Dohm and Daniels46) , followed by six studies that explored the intake of micronutrients(Reference Aparicio, Canals and Pérez25,Reference Kanayama, Sakai and Aoto35,Reference Mulvihill, Davies and Rogers37,Reference Santiago, Zimmerman and Feinstein39,Reference Higgins, Hagman and Pan41,Reference Madhusmita, Tsai and Anderson42) and two that recorded fibre intake(Reference Baskaran, Carson and Campoverde Reyes40,Reference Madhusmita, Tsai and Anderson42) . Striegel-Moore et al.(Reference Striegel-Moore, Franko and Thompson47) analysed caffeine consumption, and Kanayama et al.(Reference Kanayama, Sakai and Aoto35) identified fourteen food groups. The a priori dietary pattern was investigated in the study by Santiago et al.(Reference Santiago, Zimmerman and Feinstein39), which used the Healthy Eating Index-2010 (HEI-2010). Four studies compared dietary intake with references to quantitative estimates of nutrient intake based on the Australian recommendation(Reference Allen, Mori and Beilin32), in the dietary recommended intake (DRI)(Reference Kanayama, Sakai and Aoto35,Reference Higgins, Hagman and Pan41,Reference Madhusmita, Tsai and Anderson42) and average US dietary intake for girls aged 12–19 years(Reference Higgins, Hagman and Pan41) in the recommendations of the 2010 Dietary Guidelines, which is based on the recommendations of the DRI(Reference Santiago, Zimmerman and Feinstein39). Furthermore, Kanayama et al. (Reference Kanayama, Sakai and Aoto35) also used the dietary goal for preventing lifestyle-related diseases (DG) (Table 3).
In participants with REB, six studies evaluated energy intake(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Tsai, Chang and Lien31,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Higgins, Hagman and Pan41,Reference Affenito, Dohm and Daniels46) , five analysed macronutrient intake(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Tsai, Chang and Lien31,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Affenito, Dohm and Daniels46) and seven evaluated the intake of micronutrients(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25,Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31,Reference Mulvihill, Davies and Rogers37,Reference Caran, Santana and Monteiro38,Reference Dunker and Philippi48) . Koch et al.(Reference Koch, Alexy, Diederichs and Buyken36) evaluated the mean percentage of energetic intake; however, they considered the circadian, morning and evening dietary profiles. The study by Dunker and Philippi(Reference Dunker and Philippi48) calculated the mean values of the percentage of intake of macronutrients, Ca and Fe and compared them with the recommendations of the DRI and to evaluate the energy intake compared with the National Research Council. Two studies investigated the prevalence of inadequate energy intake(Reference Aparicio, Canals and Pérez25) and nutrients(Reference Aparicio, Canals and Pérez25,Reference Caran, Santana and Monteiro38) using the DRI probability created for the Spanish population(Reference Aparicio, Canals and Pérez25) and Institute of Medicine(Reference Caran, Santana and Monteiro38), and a study compared nutrient intake with reference nutrient intake (Reference Mulvihill, Davies and Rogers37). Two studies evaluated the fibre intake of individuals(Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) , four studies investigated the food group(Reference Bisset, Gauvin and Potvin28,Reference Mulvihill, Davies and Rogers37,Reference Grigolon, Dunker and Almeida45,Reference Dunker and Philippi48,Reference Dunker and Philippi49) and one identified dietary pattern a priori by the Healthy Eating Index – HEI-C(Reference Woodruff, Hanning and Lambraki29) (Table 4).
Results and summary of evidence sources
Anorexia nervosa and food intake
Nine studies that evaluated the relationship between AN and food consumption were retained, including four cohort studies(Reference Allen, Mori and Beilin32,Reference Baskaran, Carson and Campoverde Reyes40,Reference Higgins, Hagman and Pan41,Reference Weltzin, Fernstrom and Hansen44) , a case–control study (two generated articles)(Reference Bischoff-Seals24,Reference Aparicio, Canals and Pérez25) and four cross-sectional studies(Reference Kanayama, Sakai and Aoto35,Reference Santiago, Zimmerman and Feinstein39,Reference Madhusmita, Tsai and Anderson42,Reference Nasserbakht, Brantner-Inthaler and Shih43) (Fig. 4).

Fig. 4. Distribution by study design: (a) anorexia nervosa and food consumption; (b) restrictive eating behaviour and food consumption; (c) food consumption and restrictive eating behaviour.
Energy content, macronutrients, fibres, micronutrients and caffeine
Initially, the results of the cohort studies(Reference Allen, Mori and Beilin32,Reference Baskaran, Carson and Campoverde Reyes40,Reference Higgins, Hagman and Pan41,Reference Weltzin, Fernstrom and Hansen44) were obtained. Allen et al.(Reference Allen, Mori and Beilin32) used Mann–Whitney U and Kruskal–Wallis tests and found that the median total and monounsaturated fat intake was significantly lower (P < 0·05) among adolescents with AN. However, there was no significant difference in the intake of energy content, protein and carbohydrates between the groups. Baskaran et al.(Reference Baskaran, Carson and Campoverde Reyes40) evaluating the baseline data of the study with girls with AN, after applying the Wilcoxon, Kruskal–Wallis and Student’s t-tests, did not observe differences in the mean energetic (P = 0·38) and protein (P = 0·99). However, girls with NA had higher energy intake obtained from carbohydrates (P < 0·001) and lower energetic intake from fat (P = 0·0002), SFA (P < 0·001) and MUFA (P < 0·001), compared with the controls. In addition, when comparing macronutrient intake in girls with AN who gained body weight (AN-1) v. those with AN and who maintained weight (AN-0) by means of Pearson’s correlation analysis, it was found that in the AN group-0, there was a higher percentage contribution of energy content from protein intake (P = 0·046). Throughout 6–12 months, ANCOVA was used and it was identified that adolescents with AN-1 consumed a lower percentage of energy content from protein (P = 0·001) and a higher energetic percentage of fat and PUFA (P = 0·02) when compared with the AN-0 group. ANOVA and Tukey Kramer test were also used for multiple comparisons between groups, observing lower mean intake of energy content from carbohydrates (P = 0·004; AN-0 v. control), proteins (P = 0·03; AN-0 v. AN-1) and fats (P < 0·001; AN-0 v. control and AN-0 v. AN-1). Higgins et al.(Reference Higgins, Hagman and Pan41) did not observe significant differences in the mean intake of energy content (P = 0·312), carbohydrates, proteins and fats between the period of 6 months and 1 week before hospitalisation among the participants using the paired t test.
When applying the ANOVA test, Affenito et al.(Reference Affenito, Dohm and Daniels46) did not find significant differences in the mean energy intake (P = 0·20) and in the percentage of energy obtained from carbohydrates (P = 0·71), proteins (P = 0·34) and fats (P = 0·76) 2 years before the onset of symptoms of AN. However, there was a significant reduction in total energy intake (P = 0·03) in the first year prior to diagnosis. Affenito et al. also found out that among girls with AN, there was a lower intake mean number of energy content (P = 0·03) and the percentage of as well as higher percentage protein intake energy content in the first year of disease. After 2 years of follow-up, there was no difference in the intake of energy content from carbohydrates (P = 0·27) in both girls with AN and in pairs. Striegel-Moore et al.(Reference Striegel-Moore, Franko and Thompson47) applied the model of generalised estimation equations to investigate the relationship between caffeine consumption and NA. The investigators identified an increase in caffeine consumption over time in the AN group. Lower consumption of this food component by means of chocolate (P < 0·005) and higher consumption of caffeine from soft drinks (P < 0·0005) were also recorded, but no significant difference was identified in the intake of caffeine from coffee and tea (P > 0·10).
Among the results of cross-sectional studies, Kanayama et al.(Reference Kanayama, Sakai and Aoto35) adopted the Mann–Whitney and Kruskal–Wallis tests and identified that, compared with lean individuals, the AN group had a significantly lower mean intake of energy content (P < 0·05) and fat (P < 0·01). A similar result was found in the study of Misra et al.(Reference Madhusmita, Tsai and Anderson42): adolescents with AN had lower mean energetic intake (P = 0·03) and lower percentage of energy intake from total fat (P < 0·0001), saturated fat (P = 0·0001), monounsaturated fat (P < 0·0001) and polyunsaturated fat (P = 0·001). Misra et al.(Reference Madhusmita, Tsai and Anderson42) also identified, higher intake of the percentage of energy content from protein (P < 0·0001) and carbohydrates (P = 0·0009); however, no difference was observed in the intake of animal or plant proteins, after using Student’s t-test. Santiago et al.(Reference Santiago, Zimmerman and Feinstein39) determined the frequency of macronutrient intake and suggested that adolescents with AN in the complete (AN-c) and atypical (AN-a) forms have higher intake of the mean percentage of energy distribution than individuals with bulimic nervosa (BN) and lower intake than those with avoidant restrictive food intake disorder. In addition, the mean daily energetic intake was below the values recommended for the study population. Higher carbohydrate intake was also identified in adolescents with AN-c, followed by participants with BN and atypical AN and lower intake than participants with avoidant restrictive food intake disorder. Regarding fat and protein intake, individuals with AN-a had a higher percentage intake of these macronutrients than the other participants, except for protein intake in individuals with BN. Furthermore, the distribution of the percentage intake of macronutrients was in accordance with the Dietary Guidelines for Americans, 2010(Reference Jin50) (Table 3).
Nasserbakht et al.(Reference Nasserbakht, Brantner-Inthaler and Shih43) applied the ANCOVA method and found that among the AN subtypes, adolescents with restrictive AN (AN-r) ingested more energy content than girls with bulimic AN (AN-b) (P < 0·0212). However, both NA subtypes had higher energy intake (P < 0·0001) than controls and BN. A similar result was identified in the study by Weltzin et al. (Reference Weltzin, Fernstrom and Hansen44), who observed that individuals with AN-r with short-term stable weight had higher average energy intake (P = 0·000) per day when compared with short- and long-term AN-b and BN after applying linear regression And hierarchical analysis. Furthermore, after restoring weight, it was observed that r-AN individuals required greater energy intake per day for weight maintenance than those with AN-b.
When assessing fibre intake, two studies found higher intake in people with AN than in peers without this syndrome(Reference Baskaran, Carson and Campoverde Reyes40,Reference Madhusmita, Tsai and Anderson42) . Misra et al.(Reference Madhusmita, Tsai and Anderson42) identified that the intake of soluble and insoluble fibres was higher 24·0 and 49·6 %, respectively, in the AN group than in healthy adolescents.
Micronutrients
Allen et al.(Reference Allen, Mori and Beilin32) identified a lower mean Na intake (P < 0·05) among people with AN but not for the mean intake of the other micronutrients studied. After using the χ 2 test, they indicated that a higher percentage of participants with AN had less than two-thirds of the intake recommended by Australian RDI for Zn (P < 0·001), thiamine (P < 0·001), vitamin B6 (P = 0·012) and phosphate (P = 0·008). Higgins et al.(Reference Higgins, Hagman and Pan41) observed lower average intakes of vitamins A and D, linoleic acid, pantothenic acid and retinol 1 week before hospitalisation among participants with AN. Over the course of 6 months, there was a reduction in the mean intake of Cu (P = 0·0117), Zn (P = 0·0031), Fe (P = 0·0156) and most of the B vitamins intake of these nutrients remained within the limits recommended by the DRI. Additionally, the total intake of Ca and P remained unchanged throughout the study, with values below those recommended by the DRI (Table 3).
The results of two cross-sectional studies differed when evaluating the intake of vitamin C and Zn(Reference Kanayama, Sakai and Aoto35,Reference Madhusmita, Tsai and Anderson42) . Kanayama et al.(Reference Kanayama, Sakai and Aoto35) reported lower mean vitamin C intake (P < 0·01) and Zn (P < 0·01), while Misra et al. (Reference Madhusmita, Tsai and Anderson42) described higher mean vitamin C intake (P ≤ 0·05) among AN individuals, but there was no difference in Zn intake among the participants. Furthermore, Misra et al.(Reference Madhusmita, Tsai and Anderson42) identified a higher mean intake of vitamins A (P ≤ 0·01), K (P ≤ 0·05) and B complex (except niacin). However, there was no difference in the intake of other vitamins and minerals evaluated by the study. In addition, the use of supplements favoured a greater intake of vitamins A and D and most of the B complex, Ca, Zn and Fe in the group with AN and a higher percentage of girls with NA met the recommendations of the DRI regarding the intake of vitamins A and D, pantothenic acid and folate and Ca (Table 3).
Santiago et al.(Reference Santiago, Zimmerman and Feinstein39) identified higher percentage intake of vitamin A and Mn in individuals with AN in complete form followed by participants with avoidant restrictive food intake disorder, atypical AN and BN, as well as lower intake of vitamin E, D, Se and K among individuals with AN-a. When comparing the daily values recommended by the 2010 Dietary Guidelines, higher intake of vitamins A and C and Mn and Se and lower intake of vitamins E, D and B12 were observed, as well as for Zn, Cu, Fe, K and Mg the participants.
Food groups and a priori dietary pattern
Kanayama et al.(Reference Kanayama, Sakai and Aoto35) reported a higher intake of vegetables (P < 0·01) and lower consumption of confectionery foods (P < 0·05) among adolescents with AN. In addition, thin girls without a diagnosis of AN but with a high desire for thinness usually restricted the intake of carbohydrate sources (e.g. cereals) but maintained the intake of high fat.
A study by Santiago et al.(Reference Santiago, Zimmerman and Feinstein39) reported that individuals with AN (full and partial AN) had the highest mean HEI-2010 score: 62·9, that is, it needs improvement when compared with participants with BN and avoidant restrictive food intake disorder after applying the Kruskal–Wallis test. However, the mean score of the HEI-2010 was below the value that indicates good diet quality in all groups.
Restrained eating behaviour and food intake
The relationship between REB and food consumption was investigated in thirteen studies(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Woodruff, Hanning and Lambraki29–Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34–Reference Caran, Santana and Monteiro38,Reference Higgins, Hagman and Pan41,Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Affenito, Dohm and Daniels46,Reference Striegel-Moore, Franko and Thompson47,Reference Dunker and Philippi49) , with twelve cross-sectional studies(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Woodruff, Hanning and Lambraki29–Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34–Reference Caran, Santana and Monteiro38,Reference Higgins, Hagman and Pan41,Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Affenito, Dohm and Daniels46,Reference Striegel-Moore, Franko and Thompson47) and one cohort(Reference Koch, Alexy, Diederichs and Buyken36) (Fig. 4). The following statistical tests were identified to assess the relationship between dietary intake and REB: χ 2 test(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Nasserbakht, Brantner-Inthaler and Shih43,Reference Dunker and Philippi49) , Student’s t (Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Affenito, Dohm and Daniels46,Reference Striegel-Moore, Franko and Thompson47) , Levene’s test(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27), ANOVA(Reference Aparicio, Canals and Pérez25,Reference Guevara, Urchaga and Cabaco26,Reference Mulvihill, Davies and Rogers37) , hierarchical linear modelling(Reference Bisset, Gauvin and Potvin28), linear regression(Reference Aparicio, Canals and Pérez25,Reference Elfhag, Tholin and Rasmussen34,Reference Caran, Santana and Monteiro38) , linear regression of mixed effects (fixed and random)(Reference Koch, Alexy, Diederichs and Buyken36) and multivariate logistic regression(Reference Tsai, Chang and Lien31) (Table 4).
Energy content, macronutrients and fibres
When evaluating food intake, Aparicio et al.(Reference Aparicio, Canals and Pérez25) identified a lower mean energy intake (P < 0·05). After the linear regression analysis, a lower energy intake was associated with REB, especially in girls. Three studies(Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35,Reference Affenito, Dohm and Daniels46) indicated lower mean energy intake among participants with REB; however, one study(Reference Caran, Santana and Monteiro38) did not observe a significant difference in the mean energy intake. In addition, Koch et al.(Reference Koch, Alexy, Diederichs and Buyken36) and Mulvihill et al.(Reference Mulvihill, Davies and Rogers37) suggested an inverse association between dietary restriction and energetic intake (P < 0·0001; P < 0·05, respectively). Koch et al.(Reference Koch, Alexy, Diederichs and Buyken36) also reported that among girls, greater dietary restriction was associated with higher morning energy intake (P = 0·03). The study excluded food records that did not have all morning and evening intake data during the 3-d period, noting that there was no change in the association between food restriction and morning energy intake. However, reduced intake at night was found among individuals with high dietary restriction (P = 0·06). Furthermore, over 4 years, the greatest dietary restriction was associated with lower nocturnal energy intake in both sexes and lower morning intake in girls. In addition, Aparicio et al. (Reference Aparicio, Canals and Pérez25) reported that among girls, a higher prevalence of inadequate energy intake was observed, that is, below 2/3 of the recommended intake for both the DRI for the Spanish population and the Institute of Medicine (Table 4).
When evaluating macronutrients, the studies indicated lower mean carbohydrate intake(Reference Aparicio, Canals and Pérez25,Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) , proteins(Reference Aparicio, Canals and Pérez25,Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) and fats(Reference Aparicio, Canals and Pérez25,Reference Tsai, Chang and Lien31) in individuals with REB. Furthermore, Aparicio et al.(Reference Aparicio, Canals and Pérez25) found an association between lower carbohydrate, protein and fat intake and REB. Similar results were reported by Mulvihill et al. (Reference Mulvihill, Davies and Rogers37) who identified a lower mean intake of these macronutrients, with significance only for fat intake among participants with high restriction, when compared with individuals with medium and low dietary restriction. On the other hand, a study(Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) reported a higher mean percentage of protein distribution in the REB group. Nevertheless, other studies did not observe differences in the mean carbohydrate intake(Reference Tsai, Chang and Lien31,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) , protein(Reference Caran, Santana and Monteiro38) and fat(Reference Tsai, Chang and Lien31,Reference Allen, Mori and Beilin32,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) . Dunker and Philippi(Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) also reported that, among the participants, the carbohydrate intake was adequate (closer to the lower limit), while the fat intake was above the recommended percentage. Fibre intake was evaluated in two studies(Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) that described higher mean intake among participants with REB compared with people without this condition (Table 4).
Micronutrients
Investigations involving micronutrient intake in adolescents with REB identified lower mean Fe intake(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27,Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) , Zn(Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) , vitamins B6 and B12 (Reference Aparicio, Canals and Pérez25,Reference Chang, Lin and Wong30,Reference Tsai, Chang and Lien31) . Aparicio et al.(Reference Aparicio, Canals and Pérez25) suggested a lower intake of certain micronutrients (Ca, vitamins A, E, C, D, B1, Na, K, hosphorus, pantothenic acid, folic acid) in both sexes and Mg only among girls(Reference Aparicio, Canals and Pérez25). The results of studies did not identify a significant difference in the mean intake of some micronutrients, namely vitamins A, B1, B2 (Reference Chang, Lin and Wong30) and Ca(Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) . Furthermore, Caran et al.(Reference Caran, Santana and Monteiro38) reported a higher mean intake of vitamin C, and Mulvihill et al.(Reference Mulvihill, Davies and Rogers37) did not find a reduction in the intake of the micronutrients studied among the food restriction groups; however, the intake of most vitamins and minerals (except for Ca, Fe and Zn) was in accordance with that recommended by the reference nutrient intake (Table 4).
When assessing the prevalence of inadequate micronutrient intake (vitamins E, A and C, thiamine, riboflavin, niacin and folic acid), Aparicio et al.(Reference Aparicio, Canals and Pérez25) reported that a higher percentage of girls with REB had more than 50 % risk of inadequate micronutrient intake. For Ca, Fe, Mg, hosphorus, vitamins D and B6, 60 % of the girls with REB had inadequate intake. In continuity, Caran et al.(Reference Caran, Santana and Monteiro38) identified that the lowest percentage of participants with REB had inadequate intake of vitamins C and E (Table 4).
Food group and a priori dietary pattern
Bisset et al.(Reference Bisset, Gauvin and Potvin28) performed hierarchical linear modelling and identified that, at the baseline of the study, high dietary restriction was associated with lower consumption of snacks of low nutritional quality (P < 0·02) than the group with moderate dietary restriction. After 5 years, having low food restriction was associated with a higher frequency of low-quality snacks (P < 0·001). The consumption of fast food decreased over time, except for individuals who reported low dietary restriction (P < 0·019). A reduction in the intake of fruits and vegetables at baseline and over time was also identified, but without association with dietary restriction (P < 0·025).
Dunker and Philippi(Reference Elfhag, Tholin and Rasmussen34,Reference Kanayama, Sakai and Aoto35) reported that adolescents with symptoms of AN have higher intake of fruits, vegetables, skimmed milk and peppermint drops and lower intake of bread, rice, type B milk, curd, orange juice, sugar, soft drinks, chocolate, pasta and potato chips when compared with pairs. Elfhag et al.(Reference Elfhag, Tholin and Rasmussen34) described that restrictive behaviour was correlated with lower consumption of sweets (both sexes) and soft drinks (only in boys). Mulvihill et al.(Reference Mulvihill, Davies and Rogers37) reported that beverage consumption (P < 0·05), sugars, meat and meat products and confectionery (P < 0·005) were inversely related to REB. In addition, energy consumption from bread, cereal products, fruits (P < 0·05) and milk and milk products (P < 0·005) was directly related to REB. In addition, other researchers reported a lower intake of sausages, other sausages and sweets (P < 0·001)(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27) and higher consumption of legumes (P < 0·005), nuts (P < 0·001)(Reference Guevara, Urchaga and Cabaco26) and confectionery (P < 0·001)(Reference Guevara, Urchaga and Cabaco26) in adolescents with REB. In a study(Reference Quiles-Marcos, Balaguer-Solá and Pamies-Aubalat27), girls with a high risk of AN symptoms had a lower consumption of unhealthy foods (P < 0·001) than those with a low risk; however, boys with a high risk of developing symptoms of AN had a higher consumption of healthy foods (P < 0·001) than boys with a low risk (Table 4).
Woodruff et al.(Reference Woodruff, Hanning and Lambraki29) applied the χ 2 test and observed that the mean HEI-C score in all groups was 69·0, that is, the quality of the diet of the participants ‘needs improvement’. Nevertheless, it was identified that adolescents who were concerned about weight and dieting (group 4) were more likely to have a lower HEI-C score (P = 0·001) than participants who were not concerned with weight and were not dieting (group 1) after ordinal logistic regression analysis (Table 4).
Food consumption and feeding restriction behaviour
Only two studies had as an outcome the behaviour of food restriction and adopted a cross-sectional design(Reference Daly, O’Sullivan and Walton33,Reference Grigolon, Dunker and Almeida45) (Fig. 4).
Energy and macronutrients
Daly et al.(Reference Daly, O’Sullivan and Walton33) calculated Pearson’s and Spearman’s correlation and showed that the mean total energy intake and mean fat intake in energy content were negatively correlated with the REB (−0·343; P < 0·001; −0·113; P = 0·02, respectively), while the energy intake from carbohydrates was positively correlated (0·100; P = 0·04) with the restriction behaviour. There was no significant difference in mean protein intake (−0·020; P = 0·683) between groups.
Food group
Grigolon et al.(Reference Grigolon, Dunker and Almeida45) using generalised linear models reported that the consumption of meat, poultry, fish and eggs was significantly correlated (P = 0·001) with the participants with the food restriction subscale. Lower consumption was also identified in the group of bread, cereals, rice and pasta and in the group of oils and fats, but there was no statistically significant association between individuals and the food restriction subscale (Table 4).
Discussion
This review identified twenty-four primary studies published from 1991 to 2020 that provided important information on the characteristics of food intake in children and adolescents with AN/REB. The results suggest a lower mean intake of energy content, macronutrients, especially, fat, and certain micronutrients (Na, K, Cu, Zn, Fe, Se, B complex, vitamins D and E) and a higher prevalence of inadequate energy intake and micronutrients. Additionally, the consumption of snacks, fast food, sweets, beverages, meat and meat-based products, carbohydrate and fat source foods and higher intake of caffeine, fibre, fruits, vegetables, legumes and nuts was identified. In addition, the intake of meat, poultry, fish and eggs groups was related to the participants with REB. When evaluating the dietary pattern by the HEC, the study participants had a ‘need to improve’ their diet.
There is consistent evidence that energy restriction is considered an essential factor related to AN/REB(1,Reference Schaumberg, Anderson and Anderson11) , mainly due to insufficient consumption of carbohydrates and fats(Reference Schebendach, Uniacke and Walsh51–Reference Mayer, Schebendach and Bodell53) and by the preference of intake of foods considered to have low energetic value, for example, fruits, vegetables and legumes, which have more fibre. It is also considered a common practice in individuals with AN/REB and higher caffeine intake due to the appetite inhibiting effect and for promoting weight reduction(54). In addition, the lower micronutrient intake and higher prevalence of nutrient inadequacy among adolescents identified in the studies may influence the development and satisfactory growth inherent to this phase, causing damage to health over time, which requires the adoption of specific nutritional intervention measures(Reference Chiurazzi, Cioffi and De Caprio12). Another important point is the need to improve the quality of the diet found in two studies. It is noteworthy that the study participants did not have sufficient energetic intake for adequate application of HEI. However, it favours the reflection of the risk gradient for the development of chronic diseases related to food among adolescents with AN/REB, which can be continued into adulthood(Reference Volp, Alfenas and Costa55).
Researchers suggest that biological, psychological and sociocultural factors may influence the food intake of individuals with AN/REB(Reference Tyszkiewicz-Nwafor, Rybakowski and Dmitrzak-Weglarz56–Reference Haines, Kleinman and Rifas-Shima58). In biological lines, studies show that nutrient deficiency for a prolonged period of time increases opioid activity in the brain, triggering feelings of pleasure that allow adherence to a restrictive diet(Reference Rask-Andersen, Olszewski and Levine59–Reference Ruiz-Prieto, Bolaños-Ríos and Jáuregui-Lobera61). Another hypothesis explored is the participation of brain-derived neurotrophic factor. Researchers note that individuals with AN have lower brain-derived neurotrophic factor plasma concentrations than individuals without AN(Reference Dalton, Campbell and Chung62,Reference Brandys, Kas and Van Elburg63) . Brain-derived neurotrophic factor regulates a series of physiological processes, including food intake and decreased serum concentration of this neuropeptide, favouring the restriction of food intake(Reference Tyszkiewicz-Nwafor, Rybakowski and Dmitrzak-Weglarz56). Furthermore, it has been reported that an increase in the level of the 5-hydroxytryptamine or serotonin receptor (5-HT2A) is associated with decreased energy metabolism, resulting in lower food intake, especially in the restrictive subtype(Reference Sarrassini, Santos and Ribeiro64). Continuously, individuals with AN have low plasma levels of leptin related to low weight, and this hormone may be causing an erroneous signal of satiety to the brain, even when the body weight is not yet fully restored(Reference Stroe-Kunold, Buckert and Friederich65), promoting reduced food intake and increased energy expenditure(Reference Morgan, Vecchiatti and Negrão10,Reference Bernardi, Harb and Levandovski66) , suggesting the participation of leptin in AN maintenance.
Among the psychological factors, studies suggest that individuals with AN/REB have dysfunctional beliefs and attitudes about food or nutrients, favouring energy restriction, mainly by reducing the consumption of foods with high fat content and inadequate intake of certain micronutrients. These dysfunctional beliefs and attitudes can become habitual behaviour driven by neural mechanisms related to the formation of habits that favour the maintenance of food restriction(Reference Grzelak, Dutkiewicz and Paszynska57,Reference Zambrowicz, Schebendach and Sysko67,Reference Steinglass, Foerde and Kostro68) . In addition, individuals with AN have greater pre-meal anxiety and reduced pleasure related to eating when compared with controls, especially when the meal has a higher energetic value(Reference Grzelak, Dutkiewicz and Paszynska57).
Additionally, the pressures and social stigmas for an ideal body may favour adolescents to be confronted with such images and experience dissatisfaction with body image and exaggerated concern with weight. This involves both the fear of gaining weight and frustration with the body, as well as disappointment for not achieving the ideal of beauty to which they do not belong(Reference Haines, Kleinman and Rifas-Shima58,Reference DeBraganza and Hausenblas69–Reference O’dea71) , adopting disordered eating behaviours involving inadequate methods for weight control, such as restrictive diet and exclusion of foods with high energetic density(Reference Haynos, Field and Wilfley5,Reference Haines and Neumark-Sztainer72) .
This review also identified different types of food survey instruments (food records, R-24 h, FFQ, food diaries, self-administered diet history questionnaires, direct weighing and specific questions about food consumption) to assess dietary intake. The choice of the food survey instrument to assess food consumption depends on the objective of the study. The use of R-24 h, daily or food record instruments is recommended to quantitatively investigate nutrient intake because they provide details regarding the types and amount of food consumed. The FFQ estimates the usual diet and can be used to investigate dietary patterns, food intake or specific nutrients and is widely used in epidemiological studies to verify the diet–disease relationship. Dietary history is suggested to obtain data on current and past eating habits. It should be added that the methods of investigation of food consumption have limitations that involve the time and memory of the interviewee, interference of sex, age, environment, passing through the educational level, cognitive skills and behavioural change of the interviewee (the individual knows that he/she is being evaluated) until the interviewer’s ability to establish good communication and avoid inducing responses. The main measurement errors of the food surveys reduce the accuracy of the results, which can lead to under- or overestimation of intake (Reference Fisberg, Marchioni and Colucci18). However, these errors can be minimised with the adoption of more than one R-24 h or food record to allow obtaining information on the daily variability in intraindividual food intake(Reference Dodd, Guenther and Freedman73).
In addition, most studies did not perform analysis of dietary data by adjusting intrapersonal variability or by energy. These approaches are important to reduce the total variance of distribution, which can influence the number of people with inadequate intake and the removal of intrapersonal variability, favouring that the results of the studies report only interpersonal variability(Reference Slater, Marchioni and Fisberg74). Only two studies(Reference Aparicio, Canals and Pérez25,Reference Caran, Santana and Monteiro38) estimated the prevalence of nutrient inadequacy, and this analysis is important to provide information on the proportion of individuals who have intake above or below a certain recommendation, which is essential for monitoring or interventions of health actions(Reference Slater, Marchioni and Fisberg74).
Only two studies evaluated the dietary pattern a priori (Reference Woodruff, Hanning and Lambraki29,Reference Santiago, Zimmerman and Feinstein39) from more robust statistical methods that take into account the interrelationships (correlations) between foods. Evaluating dietary patterns, rather than their individual components (such as macro- and micronutrients), has become increasingly important in epidemiological studies to identify the relationship between diet and diseases, especially due to the complex interaction and correlation between nutrients and other nutrients, Food components(Reference Kant75,Reference Slattery76) . The assessment of dietary pattern is more comprehensive than the assessment of nutrients or isolated foods.
In addition, many studies used only bivariate statistical methods between exposure and outcome to identify the relationship between food consumption and AN/REB, and Student’s t-test and χ 2 test were used squares most frequently. The ANOVA test was also applied, which allows identifying if there is a difference and where is the difference between the groups but does not quantify it and is therefore considered an initial step in the analysis of factors that affect a given dataset(Reference Freedman77) and should be followed by regression models. In addition, of the longitudinal studies identified in this review (n 5), only two(Reference Koch, Alexy, Diederichs and Buyken36,Reference Striegel-Moore, Franko and Thompson47) used appropriate statistical models that take into account temporal variation and inter- and intra-individual variability, such as generalised estimation equation and mixed-effects modelling.
This review has some limitations. First, the quality of the studies was not evaluated, although it was not considered a mandatory step in a scope review. Second, most of the studies included in this review have a cross-sectional design and do not suggest causality. Third, it refers to the instruments for evaluating food consumption that have diversified among the publications, which could explain, at least partially, the divergence between the results. Finally, the various statistical techniques to evaluate the associations between food consumption and AN/REB may have affected the quality of the results identified by the different authors, as well as their interpretations of this binomial.
Strengths were identified in this review. To our knowledge, this is the first review to assess food intake in children and adolescents with AN/REB. A review methodology was used according to Preferred Reporting Items for Systematic Review and Meta-Analysis Extension for Scoping Reviews, and the protocol was registered to obtain greater scientific rigor. In addition, large databases were used, including grey literature and a list of references of the selected studies, which allowed for greater selection of studies according to the objective of this review. Additionally, a wide diversity of studies was identified in different populations that included European, Asian, American and Australian countries.
Conclusion
In conclusion, most studies identified that adolescents with AN/REB have a lower intake of energy content, fat and certain micronutrients and a higher prevalence of inadequate energy intake and micronutrients. A lower intake of foods or a group of foods with a high content of carbohydrates and fats, sweets, low-quality snacks, fast food and a higher intake of caffeine, fruits, legumes, nuts and fibre was also found. The ‘need for improvement’ of the diet was observed according to quality assessment indices. The correlation between food intake and REB was recorded for meat, poultry, fish and egg intake. Thus, it is important to consider the general set of scientific evidence to make dietary recommendations for individuals, since food consumption is influenced by several biopsychosocial factors and is reflected in health over time. The identification of restrictive dietary characteristics is expected to be recognised early and to assist in the prevention of diseases, thus promoting higher life expectancy of future adults. It is noteworthy that healthy eating not only contributes to an adequate body weight but also brings psychosocial benefits by favouring the consumption of different foods from different cultures, origins and various food preparations(78).
Thus, the future development of a systematic review on the subject will be beneficial for the increase of knowledge in this field of research to enable understanding, counselling and deeper nutritional treatment of the AN/REB relationship and food consumption.
Acknowledgements
This work was financially supported by the Foundation for Research Support in Bahia – FAPESB, through the granting of the doctoral scholarship (BOL292/2018).
E. M. P. performed the selection of the records, data extraction, data synthesis and writing of the manuscript. K. B. B. D. S. participated in the selection of records and assistance in writing the manuscript. P. R. D. F. C. synthesised the data and wrote the manuscript. L. E. M. D. S. performed the synthesis of the data and revision of the manuscript. C. M. M. N. performed the selection of the records and revision of the manuscript. H. B. M. D. S. performed data extraction and data checking. É. D. S. S. participated in the selection of records, data extraction and data checking. C. D. M. C. participated in the writing and supervision of the manuscript. M. L. P. D. S. participated in the writing and supervision of the manuscript. We declare that we have approved the final article.
The authors report no declaration of interest.











