Agitation is often considered a symptom of distress in people with dementia, Reference Cummings, Mintzer, Brodaty, Sano, Banerjee and Devanand1,Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris and Patel2 leading to family distress and burden. Reference Okura and Langa3,Reference Okura, Plassman, Steffens, Llewellyn, Potter and Langa4 It accounts for about 12% of health and social care costs for people with dementia. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris and Patel2,Reference Okura and Langa3,Reference Morris, Patel, Baio, Kelly, Lewis-Holmes and Omar5 Agitation refers to a range of behaviours including restlessness, pacing, repetitive vocalisations and verbally or physically aggressive behaviour Reference Cummings, Mintzer, Brodaty, Sano, Banerjee and Devanand1,Reference Cohen-Mansfield6 It is the most common neuropsychiatric symptom, Reference van der Linde, Dening, Stephan, Prina, Evans and Brayne7–Reference Ballard, Margallo-Lana, Fossey, Reichelt, Myint and Potkins9 and it is more persistent when more severe. Reference Ryu, Katona, Rive and Livingston10 In community settings, its prevalence increases with dementia severity. Prevalence is around 10% in people with mild cognitive impairment, Reference Ryu, Ha, Park, Yu and Liyingston11 15% in people with dementia presenting to memory clinics Reference Brodaty, Connors, Xu, Woodward and Ames12 and 30% in those living in the community. Reference Borsje, Wetzels, Lucassen, Pot and Koopmans13,Reference Lyketsos, Lopez, Jones, Fitzpatrick, Breitner and DeKosky14
Many people with dementia and agitation are admitted to care homes, with the relationship between agitation and admission mediated by carer distress. Reference Okura, Plassman, Steffens, Llewellyn, Potter and Langa4,Reference Gaugler, Yu, Krichbaum and Wyman15 Although we may, therefore, expect many residents of care homes to be agitated, there have been no large, representative studies to determine whether this is the case, or how agitation levels relate to dementia severity in care homes. In a UK survey of 233 care home residents, agitation was the most common clinically significant neuropsychiatric symptom, with 40% of participants experiencing some symptoms. Reference Margallo-Lana, Swann, O'Brien, Fairbairn, Reichelt and Potkins16 In the largest care homes study of agitation to date, among 1322 people with dementia in 59 units in the Netherlands, 85% showed at least one symptom of agitation, most frequently general restlessness. Reference Margallo-Lana, Swann, O'Brien, Fairbairn, Reichelt and Potkins16,Reference Zuidema, Derksen, Verhey and Koopmans17 Physical aggression was more common in people with very severe dementia, and disinhibition, irritability and verbally agitated behaviours were more common in moderate dementia. Reference Zuidema, de Jonghe, Verhey and Koopmans18 Agitation has been associated with lower quality of life in small care home studies. Reference Hoe, Hancock, Livingston and Orrell19–Reference Beerens, Zwakhalen, Verbeek, Ruwaard and Hamers21
Symptoms of agitation are often conceptualised as arising from unmet need Reference Algase, Beck, Kolanowski, Whall, Berent and Richards22 in a person unable to identify, communicate and respond to their own needs, who also has brain pathology predisposing to disinhibition and repetitive behaviour. This model is supported by findings from small randomised controlled trials that activities, sensory interventions, structured music therapy and interventions to improve staff communication, prevent or reduce agitation. Reference Livingston, Kelly, Lewis-Holmes, Baio, Morris and Patel23 Nonetheless, there is no good evidence that care home residents with dementia are less agitated or have a higher quality of life, when they have access to more activity or social interaction (from family visits or a higher number of staff). This is particularly important given the need to provide ways to manage agitation alternative to antipsychotic prescribing, Reference Schneider, Dagerman and Insel24,Reference Schneider, Dagerman and Insel25 levels of which may now be steady, despite initiatives to reduce their use. Reference Szczepura, Wild, Khan, Owen, Palmer and Muhammad26,Reference Cooper, Lodwick, Walters, Raine, Manthorpe and Iliffe27
This is the largest study of residents with dementia in care homes to date. Our primary aim is to discover how common clinically significant agitation is and test our hypothesis that in residents with dementia, higher levels of agitation are associated with lower quality of life. Our secondary hypotheses from the literature above suggesting that agitation is caused by dementia and unfulfilled needs in terms of less social interaction, stimulation and activity are that agitation is associated with: (1) more severe dementia, (2) fewer staff numbers per resident, (3) fewer family visits and (4) lower care home activity levels. We will also explore the relationship of agitation to psychotropic medication prescription and care home environment.
Method
This study reports the Managing Agitation and Raising QUality of lifE in dementia (MARQUE) longitudinal care home study baseline findings. It received ethical approval from the London (Harrow) NRES Committee (14/LO/0034).
Setting and sampling
We recruited care homes across England. Our sampling frame comprised each provider type (voluntary, state and private), care provision (nursing, residential) and reflected English care home provision where people with dementia resided to ensure external validity and generalisability. We defined care home clusters as units within care homes in which staff and managers worked separately. If staff in units cross-covered each other we defined this as one cluster.
Procedures
We recruited through third sector partners, NHS trusts and clinicians, a Department of Health newsletter and the NIHR Clinical Research Network. We sought care home managers’ agreement for each home's inclusion. Each manager provided a staff list and identified residents with a known clinical dementia diagnosis. Care home staff completed the Noticeable Problems Checklist (NPC) Reference Levin28 for all residents without a known diagnosis of dementia, to identify those with probable undiagnosed dementia. The NPC is a six-item questionnaire covering memory, basic self-care, orientation, naming familiar people and ability to follow conversations, which has been used by non-clinicians and which has been validated against clinical diagnosis. Reference Levin28,Reference Moriarty and Webb29 Eligible participants were all residents with an existing dementia diagnosis or those who screened positive for dementia, and their family and care home staff.
Staff asked residents, whom they judged as having decisional competence for consent, if researchers could approach them. Residents who had decisional competence for consent were asked for written informed consent to the study. Consultees were asked to make this decision for those lacking capacity in line with the Mental Capacity Act (2005). For all other residents, the staff tried to contact the next-of-kin (to participate in family carer interviews) and asked if the researchers could contact them. Participating staff and relatives gave written informed consent. We asked a staff member working closely with each resident with dementia to rate proxy measures for the resident and then asked the relative to do the same. In addition, all consenting staff providing hands-on care completed questionnaires about themselves as did consenting relatives. All participants were recruited between 13 January 2014 and 12 November 2015.
Measures
Trained research assistants interviewed staff and residents in a private room at the care home. We interviewed family carers in their preferred location: in the care home, their own home or the researcher's office.
Care home measures
We recorded information about the care home, including whether it was a residential or nursing home, number of residents (in total and with dementia), staff numbers, and programmed activities with number of attendees.
We used the Therapeutic Environment Screening Survey for Nursing Homes and Residential Care (TESS-NH/RC), which has satisfactory psychometric properties, to rate the care home's physical environment. Reference Lawton, Weisman, Sloane, Norris-Baker, Calkins and Zimmerman30 The TESS sums 15 environmental items, each scoring from 0 to 2 (facility maintenance, cleanliness, handrails, call buttons, light intensity, light glare, light evenness, hallway length (shorter is better), homelikeness, room autonomy, the presence of telephones, tactile stimulation, visual stimulation, privacy and outdoor areas) into the Environmental Quality Score (EQS). Higher scores indicate better environmental quality. Reference Bicket, Samus, McNabney, Onyike, Mayer and Brandt31
Residents measures
We recorded demographic information and completed the following measures:
-
1 Agitation: our primary outcome was the Cohen-Mansfield Agitation Inventory (CMAI), a 29-item questionnaire with construct validity and reliability to measure agitation in people with dementia in care homes. Reference Cohen-Mansfield and Billig32,Reference Zuidema, Buursema, Gerritsen, Oosterwal, Smits and Koopmans33 The CMAI is an informant questionnaire and each item scores from 1 to 7, with 1 meaning ‘never’ and 7 ‘several times per hour’. The score sums individual items and ranges from 29 to 203. A score of >45 is usually regarded as clinically significant agitation. Reference Cohen-Mansfield, Marx and Rosenthal34
-
2 Quality of life: The DEMQOL and DEMQOL proxy are responsive, valid and reliable measures of quality of life in people with dementia. Reference Smith, Lamping, Banerjee, Harwood, Foley and Smith35,Reference Smith, Lamping, Banerjee, Harwood, Foley and Smith36 The DEMQOL-Proxy is a 31-item interviewer-administered questionnaire answered by a professional or family carer. The people with dementia who were able to were asked to complete the DEMQOL, a 28-item interviewer-administered questionnaire. Reference Smith, Lamping, Banerjee, Harwood, Foley and Smith36 As the DEMQOL has fewer questions than the DEMQOL proxy, the totals are not directly comparable.
-
3 Dementia severity: Staff gave information so the researcher could rate the severity of dementia by the Clinical Dementia Rating (CDR). Reference Hughes, Berg, Danziger, Coben and Martin37 This is a reliable and valid instrument for rating severity of dementia. Reference Morris38 It is used to rate performance in memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care, and this information was used to classify dementia severity of included residents into very mild, mild, moderate or severe.
-
4 Neuropsychiatric symptoms: the Neuropsychiatric Inventory (NPI) Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein39 is a validated instrument with 12 domains of neuropsychiatric symptoms, including agitation. Each domain scores between 0 and 12 with higher scores meaning increasing severity. A score of ≥4 on any domain is usually considered clinically significant severity. Reference Lyketsos, Lopez, Jones, Fitzpatrick, Breitner and DeKosky14 A summed scored can be calculated for total neuropsychiatric symptoms.
Staff self-rated measures
Staff working in the care home provided their demographic details and working patterns.
Family carer measures
We asked relatives visiting residents at least monthly to complete the DEMQOL-proxy Reference Smith, Lamping, Banerjee, Harwood, Foley and Smith36 and tell us how often they visited. We recorded their gender and relationship to the person with dementia.
Analysis
We used Stata version 14 for all analyses. Reference StataCorp40 Characteristics of care homes and people with dementia, including CMAI scores and presence of significant agitation, are summarised by frequency (%) mean (standard deviation (s.d.)) or median (interquartile range (IQR)) as appropriate. To obtain values more relevant to the types of care home in England, we weighted estimates, using population information about the distribution of care home types (nursing or residential and private sector or voluntary, local authority (LA) and National Health Service (NHS)). Probability weights were based on available figures in England from the Care Quality Commission (CQC) from 31 December 2012. At this time, 73% of the total 17 592 care homes were residential homes. The remaining 27% were either nursing homes or both nursing and residential. Seventy five per cent of care homes in England were private whereas 25% were ‘voluntary’ (non-profit sector), LA or NHS. In calculating the weights, we assumed that the percentage of residential and nursing homes was the same within the private sector and voluntary, LA and NHS.
To investigate our primary and secondary hypotheses, we used random effects models to account for care home or unit clustering and adjusted for residents’ age, gender, CDR dementia severity and care home type (residential or nursing or both, dementia specialist, dementia registered). For the primary hypothesis, we also fitted a three-level model accounting for clustering by staff member, as some provided information about multiple residents in the home. We carried out analyses with CMAI as a continuous score. As we found some skewness in model residuals for this outcome, we checked results in sensitivity analyses based on generalised linear models with a gamma distribution. In further sensitivity analyses, we fitted models with CMAI as two groups defined by presence of clinically significant agitation (CMAI>45). Again we controlled for residents’ age, gender, dementia severity, care home type (residential, nursing or both, dementia specialist, dementia registered). We also carried out an additional sensitivity analysis with significant agitation defined by the NPI agitation domain score (significant agitation is a score ≥4) in place of CMAI.
Results
Study participation
Out of the 114 care homes we contacted, 86 (75.4%) participated. Of the 28 who did not participate, 21 were nursing or mixed nursing and residential and 7 were residential only. Among the 28 care homes, 22 did not wish to participate, 5 were too busy or had a new manager and 1 was excluded as in another research project. We therefore recruited 86 care homes: 7 homes were divided into >1 cluster, totalling 18 clusters. The sample, therefore, was 97 clusters.
Our flow diagram (Fig. 1) shows residents’ recruitment to the study. After considering pre-existing clinical dementia diagnosis and those who screened positive on the NPC, 3053 (86.2%) residents within the care homes had probable dementia and thus were eligible. Out of the 2825 residents who were approached, 1489 (52.7%) participated. The common reasons for non-participation were refusal (27.3%) and staff being unable to contact the family consultee (17.6%). Of the participating residents, 300 (20.1%) had capacity to consent to the study and we used a consultee for the remainder. We have no data for six residents for whom we had consent, as five died and one moved before data were collected, so our analyses are based on 1483 people. Out of 1483, 1281 (86.4%) had a pre-existing clinical diagnosis of dementia and the remainder scored positive on the NPC. The number of recruited residents per cluster ranged from 2 to 55 (median 14).

Fig 1 MARQUE baseline flow diagram of recruited residents.
In total, 1281 (86.4%) of consenting residents had an identified family member who agreed to participate. A total of 1701 care home staff consented. Numbers of staff per cluster ranged from 3 to 54 (median 15). Care home and staff characteristics are summarised in Table 1.
Table 1 Care home or unit and staff characteristics (numbers are frequency (%) unless stated otherwise)
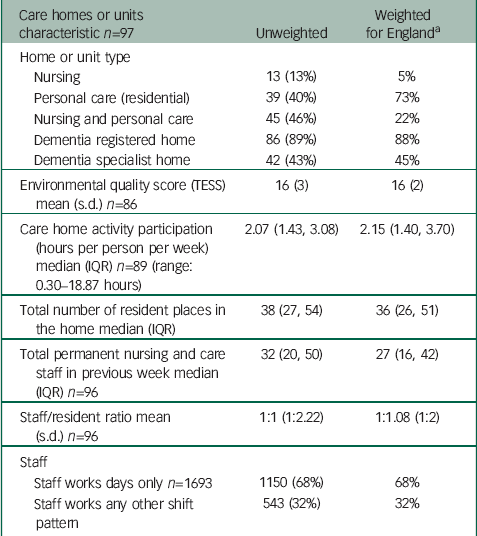
| Care homes or units characteristic n=97 | Unweighted | Weighted for England a | ||
|---|---|---|---|---|
| Home or unit type | ||||
| Nursing | 13 (13%) | 5% | ||
| Personal care (residential) | 39 (40%) | 73% | ||
| Nursing and personal care | 45 (46%) | 22% | ||
| Dementia registered home | 86 (89%) | 88% | ||
| Dementia specialist home | 42 (43%) | 45% | ||
| Environmental quality score (TESS) mean (s.d.) n=86 | 16 (3) | 16 (2) | ||
| Care home activity participation (hours per person per week) median (IQR) n=89 (range: 0.30–18.87 hours) | 2.07 (1.43, 3.08) | 2.15 (1.40, 3.70) | ||
| Total number of resident places in the home median (IQR) | 38 (27, 54) | 36 (26, 51) | ||
| Total permanent nursing and care staff in previous week median (IQR) n=96 | 32 (20, 50) | 27 (16, 42) | ||
| Staff/resident ratio mean (s.d.) n=96 | 1:1 (1:2.22) | 1:1.08 (1:2) | ||
| Staff | ||||
| Staff works days only n=1693 | 1150 (68%) | 68% | ||
| Staff works any other shift pattern | 543 (32%) | 32% | ||
IQR, interquartile range.
a For the MARQUE cohort, 28 care homes were private residential, 50 private nursing or nursing and residential, 11 non-private residential and 8 non-private nursing or nursing and residential. Probability weights were calculated as number of care homes in the given category in England or number of care homes in the same category in MARQUE.
Sample characteristics
Table 2 shows recruited residents’ and relatives’ demographic characteristics. Approximately equal proportions of residents were classified as having severe, moderate, or mild or very mild dementia. Around two-thirds of identified family members were women and a similar proportion were sons or daughters. The median number of visits residents received from their main family carer was six each month.
Table 2 Sociodemographic characteristics of baseline MARQUE cohort (Numbers are frequency (%) unless otherwise stated)
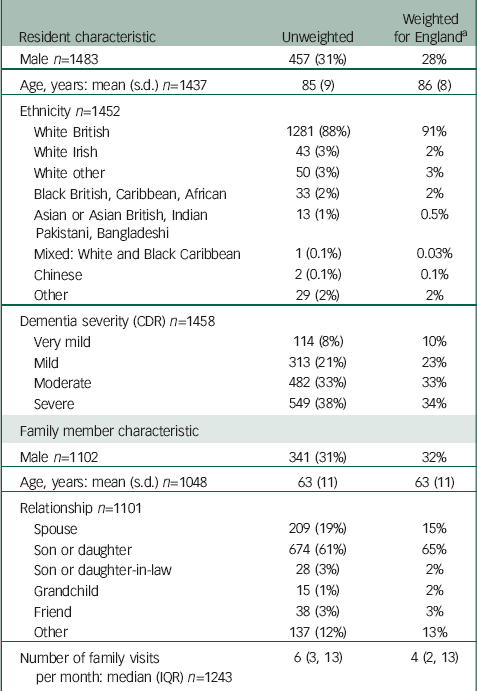
| Resident characteristic | Unweighted | Weighted for England a |
|---|---|---|
| Male n=1483 | 457 (31%) | 28% |
| Age, years: mean (s.d.) n=1437 | 85 (9) | 86 (8) |
| Ethnicity n=1452 | ||
| White British | 1281 (88%) | 91% |
| White Irish | 43 (3%) | 2% |
| White other | 50 (3%) | 3% |
| Black British, Caribbean, African | 33 (2%) | 2% |
| Asian or Asian British, Indian Pakistani, Bangladeshi | 13 (1%) | 0.5% |
| Mixed: White and Black Caribbean | 1 (0.1%) | 0.03% |
| Chinese | 2 (0.1%) | 0.1% |
| Other | 29 (2%) | 2% |
| Dementia severity (CDR) n=1458 | ||
| Very mild | 114 (8%) | 10% |
| Mild | 313 (21%) | 23% |
| Moderate | 482 (33%) | 33% |
| Severe | 549 (38%) | 34% |
| Family member characteristic | ||
| Male n=1102 | 341 (31%) | 32% |
| Age, years: mean (s.d.) n=1048 | 63 (11) | 63 (11) |
| Relationship n=1101 | ||
| Spouse | 209 (19%) | 15% |
| Son or daughter | 674 (61%) | 65% |
| Son or daughter-in-law | 28 (3%) | 2% |
| Grandchild | 15 (1%) | 2% |
| Friend | 38 (3%) | 3% |
| Other | 137 (12%) | 13% |
| Number of family visits per month: median (IQR) n=1243 | 6 (3, 13) | 4 (2, 13) |
CDR, Clinical Dementia Rating; IQR, interquartile range.
a For the MARQUE cohort, 28 care homes were private residential, 50 private nursing or nursing and residential, 11 non-private residential and 8 non-private nursing or nursing and residential. Probability weights were calculated as number of care homes in the given category in England or number of care homes in the same category in MARQUE.
Table 3 summarises agitation, quality of life scores and psychotropic medication. Staff and family members’ total quality of life proxy ratings were similar, however, the correlations between family- and staff-rated DEMQOL was low at 0.35. More than half the residents were prescribed psychotropic medication, most commonly antidepressants (40%).
Table 3 Resident agitation, quality of life and medication (numbers are frequency (%) or median (IQR))
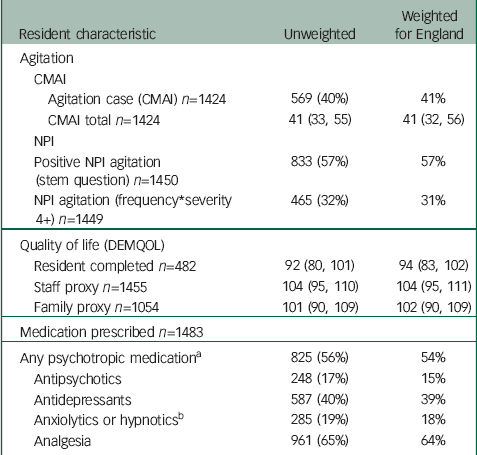
| Resident characteristic | Unweighted | Weighted for England | |
|---|---|---|---|
| Agitation | |||
| CMAI | |||
| Agitation case (CMAI) n=1424 | 569 (40%) | 41% | |
| CMAI total n=1424 | 41 (33, 55) | 41 (32, 56) | |
| NPI | |||
| Positive NPI agitation (stem question) n=1450 | 833 (57%) | 57% | |
| NPI agitation (frequency*severity 4+) n=1449 | 465 (32%) | 31% | |
| Quality of life (DEMQOL) | |||
| Resident completed n=482 | 92 (80, 101) | 94 (83, 102) | |
| Staff proxy n=1455 | 104 (95, 110) | 104 (95, 111) | |
| Family proxy n=1054 | 101 (90, 109) | 102 (90, 109) | |
| Medication prescribed n=1483 | |||
| Any psychotropic medication a | 825 (56%) | 54% | |
| Antipsychotics | 248 (17%) | 15% | |
| Antidepressants | 587 (40%) | 39% | |
| Anxiolytics or hypnotics b | 285 (19%) | 18% | |
| Analgesia | 961 (65%) | 64% | |
CMAI, Cohen-Mansfield Agitation Inventory; IQR, interquartile range.
a Any of antidepressants, antipsychotics, hypnotics or anxiolytics.
b Not including melatonin as not classed as a psychotropic drug (16 residents were prescribed melatonin).
Agitation levels and correlates
A total of 209 (14.7%) residents did not have symptoms of agitation, whereas 569 (40%) had clinically significant agitation according to CMAI and 465 (32%) on the NPI (Table 3). Fifteen (13%) of those with very mild dementia had clinically significant levels of agitation (CMAI cases). In comparison, the prevalence was higher in other CDR categories (mild dementia 102 (33%), moderate dementia 212 (45%), severe dementia 239 (45%)). A random effects logistic regression model adjusted for resident's age, gender, care home type (residential, nursing or both, dementia specialist, dementia registered) showed significantly greater odds of CMAI caseness in participants with mild, moderate and severe compared with very mild dementia. (odds ratios (ORs): mild dementia 4.49, 95% confidence interval (CI)=2.30 to 8.74; moderate dementia 6.95, 95% CI=3.63 to 13.31; and severe dementia 6.23, 95% CI=3.25 to 11.94).
Average CMAI score in those with very mild dementia was 37.0, s.d.=10.4, which was lower than other CDR categories (mild 43.5, s.d.=15.6; moderate 48.7, s.d.=19.0; severe 48.3, s.d.=19.7). A random effects model adjusted for resident's age, gender, care home type (residential, nursing or both, dementia specialist, dementia registered) indicated significant CMAI differences between very mild and other CDR categories (mild dementia coefficient 7.35, 95% CI=3.55 to 11.44; moderate dementia 11.04, 95% CI=7.34 to 14.71; severe dementia 9.70, 95% CI=6.01 to 13.39).
Higher agitation levels were significantly associated with lower staff and family ratings of the resident's quality of life, and with prescription of antipsychotics and hypnotics but not analgesics or antidepressants (Table 4). Agitation levels were not associated with frequency of family visits, time spent in activities per resident, staff ratios, number of residents or quality of the environment.
Table 4 Regression analysis of relationship of quality of life and other factors to agitation score (CMAI)
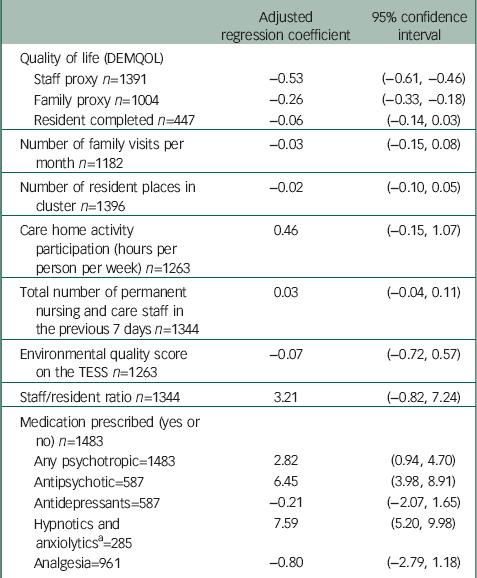
| Adjusted regression coefficient | 95% confidence interval | |
|---|---|---|
| Quality of life (DEMQOL) | ||
| Staff proxy n=1391 | −0.53 | (−0.61, −0.46) |
| Family proxy n=1004 | −0.26 | (−0.33, −0.18) |
| Resident completed n=447 | −0.06 | (−0.14, 0.03) |
| Number of family visits per month n=1182 | −0.03 | (−0.15, 0.08) |
| Number of resident places in cluster n=1396 | −0.02 | (−0.10, 0.05) |
| Care home activity participation (hours per person per week) n=1263 | 0.46 | (−0.15, 1.07) |
| Total number of permanent nursing and care staff in the previous 7 days n=1344 | 0.03 | (−0.04, 0.11) |
| Environmental quality score on the TESS n=1263 | −0.07 | (−0.72, 0.57) |
| Staff/resident ratio n=1344 | 3.21 | (−0.82, 7.24) |
| Medication prescribed (yes or no) n=1483 | ||
| Any psychotropic=1483 | 2.82 | (0.94, 4.70) |
| Antipsychotic=587 | 6.45 | (3.98, 8.91) |
| Antidepressants=587 | −0.21 | (−2.07, 1.65) |
| Hypnotics and anxiolytics a =285 | 7.59 | (5.20, 9.98) |
| Analgesia=961 | −0.80 | (−2.79, 1.18) |
CMAI, Cohen-Mansfield Agitation Inventory; TESS, Therapeutic Environment Screening Survey.
a Not including melatonin as not classed as a psychotropic drug (16 residents were prescribed melatonin).
Sensitivity analyses
Sensitivity analyses based on agitation caseness showed exactly the same pattern (Table 5) as did analyses with models based on a gamma distribution. Analyses with caseness based on NPI agitation scores also showed similar associations except increased staff numbers and staff:resident ratios were associated with increased resident agitation (adjusted analysis for higher staff numbers to NPI agitation caseness, OR=1.010 (95% CI 1.003, 1.017)) (Table 6). The significant relationship with staff-rated quality of life was also maintained in a three-level model incorporating clustering by staff member (adjusted regression coefficient −0.53, (95% CI: −0.61 to −0.46)). Information about the number of family visits each month was missing for 15% of people. A sensitivity analysis assuming this equated to no visits did not change the results.
Table 5 Relationship of quality of life and other factors to CMAI caseness
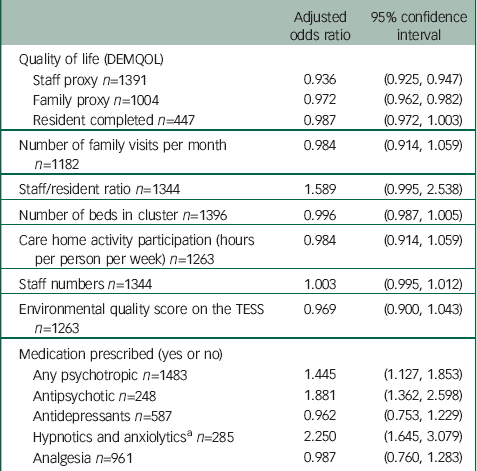
| Adjusted odds ratio | 95% confidence interval | |
|---|---|---|
| Quality of life (DEMQOL) | ||
| Staff proxy n=1391 | 0.936 | (0.925, 0.947) |
| Family proxy n=1004 | 0.972 | (0.962, 0.982) |
| Resident completed n=447 | 0.987 | (0.972, 1.003) |
| Number of family visits per month n=1182 | 0.984 | (0.914, 1.059) |
| Staff/resident ratio n=1344 | 1.589 | (0.995, 2.538) |
| Number of beds in cluster n=1396 | 0.996 | (0.987, 1.005) |
| Care home activity participation (hours per person per week) n=1263 | 0.984 | (0.914, 1.059) |
| Staff numbers n=1344 | 1.003 | (0.995, 1.012) |
| Environmental quality score on the TESS n=1263 | 0.969 | (0.900, 1.043) |
| Medication prescribed (yes or no) | ||
| Any psychotropic n=1483 | 1.445 | (1.127, 1.853) |
| Antipsychotic n=248 | 1.881 | (1.362, 2.598) |
| Antidepressants n=587 | 0.962 | (0.753, 1.229) |
| Hypnotics and anxiolytics a n=285 | 2.250 | (1.645, 3.079) |
| Analgesia n=961 | 0.987 | (0.760, 1.283) |
CMAI, Cohen-Mansfield agitation inventory; TESS, Therapeutic Environment Screening Survey.
a Not including melatonin as not classed as a psychotropic drug (16 residents were prescribed melatonin).
Table 6 Relationship between quality of life and other factors with NPI agitation caseness
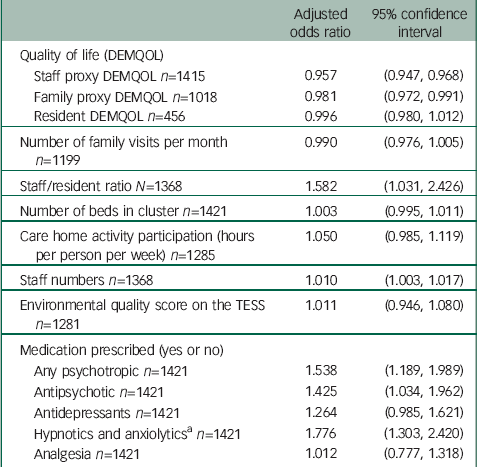
| Adjusted odds ratio | 95% confidence interval | |
|---|---|---|
| Quality of life (DEMQOL) | ||
| Staff proxy DEMQOL n=1415 | 0.957 | (0.947, 0.968) |
| Family proxy DEMQOL n=1018 | 0.981 | (0.972, 0.991) |
| Resident DEMQOL n=456 | 0.996 | (0.980, 1.012) |
| Number of family visits per month n=1199 | 0.990 | (0.976, 1.005) |
| Staff/resident ratio N=1368 | 1.582 | (1.031, 2.426) |
| Number of beds in cluster n=1421 | 1.003 | (0.995, 1.011) |
| Care home activity participation (hours per person per week) n=1285 | 1.050 | (0.985, 1.119) |
| Staff numbers n=1368 | 1.010 | (1.003, 1.017) |
| Environmental quality score on the TESS n=1281 | 1.011 | (0.946, 1.080) |
| Medication prescribed (yes or no) | ||
| Any psychotropic n=1421 | 1.538 | (1.189, 1.989) |
| Antipsychotic n=1421 | 1.425 | (1.034, 1.962) |
| Antidepressants n=1421 | 1.264 | (0.985, 1.621) |
| Hypnotics and anxiolytics a n=1421 | 1.776 | (1.303, 2.420) |
| Analgesia n=1421 | 1.012 | (0.777, 1.318) |
CMAI, Cohen-Mansfield agitation inventory; TESS, Therapeutic Environment Screening Survey.
a Not including melatonin as not classed as a psychotropic drug (16 residents were prescribed melatonin).
Discussion
We found that 86% of care home residents had dementia. Of those, 40% had clinically significant agitation symptoms and 86% had some symptoms. Those who were agitated had a lower quality of life as rated by staff and family carers, confirming our hypothesis that agitation in care home residents with dementia is associated with lower quality of life. Earlier studies had suggested that there may be relationship but a recent systematic review found there was insufficient available evidence to draw conclusions. Reference Wetzels, Zuidema, de Jonghe, Verhey and Koopmans20,Reference Beerens, Zwakhalen, Verbeek, Ruwaard and Hamers21,Reference Ballard and Margallo-Lana41
Agitation in care home residents is, as in the community, associated with more severe (as opposed to very mild) dementia. This relationship is not linear, with 45% of those with both moderate and severe dementia having clinically significant agitation. This indicates that agitation is not wholly a symptom of worsening brain pathology or it would parallel cognition in its severity. The high prevalence of agitation in care homes probably relates to the greater likelihood of people with agitation moving to a care home and the high prevalence of moderate and more severe dementia within care homes, as well as a lack of effective strategies to manage it.
Improving the overall environment, good staffing levels and overall time spent in activities is desirable. Although these factors differed among the homes in the study, they were not associated with levels of agitation. Our analysis did not find that lower staff numbers in care homes were related to agitation. There was a non-significant trend towards higher staff/resident ratios and more agitation (Table 4). This might be because additional staff members were booked to manage the most agitated residents, and because the quality rather than quantity of interaction is important. The number of staff present does not capture what they do and the degree to which individual residents’ needs are met. In addition, there are laws about statutory minimum levels of staffing, so while there is some variation in staffing levels between homes, it is not huge. A recent intervention study of a variety of strategies taught to staff including social interaction and exercise found that none of the strategies reduced agitation. Reference Ballard, Orrell, YongZhong, Moniz-Cook, Stafford and Whittaker42 It may be that staff also require the skills to communicate with residents who have difficulty knowing or conveying what they need, and to identify the needs of individual agitated residents.
In a cross-sectional study, the number of family visits a month may reflect a resident's needs, with relatives visiting more when a resident is more unwell and, conversely, with relatives visiting those who are most impaired less frequently with few perceived opportunities for communication. Reference Gaugler43 Individuals whose relatives have been unable to manage are more likely to be admitted to care homes and their family members are often themselves distressed. Seeing family can trigger feelings of loss, particularly when they leave. Therefore some family carers may avoid visiting. We measured how often the main family carer visited and did not account for other visitors. Thus it may be too simplistic to expect that agitation may be related to this measure.
Perhaps more extensive participation in activities was not associated with reduced agitation because only less agitated residents are approached to take part, as they are more likely to agree to join in an activity, to remain there and be less disruptive. A recent ethnographic study found that residents with the highest levels of needs were not included in activities. Reference Backhouse, Killett, Penhale and Gray44
Previous estimates of the proportion of residents in care homes with dementia are similar to ours but they vary depending on the type of care home, with lower proportions of people with dementia in residential than nursing homes and the highest proportion in designated homes for elderly residents with mental illness. Reference Margallo-Lana, Swann, O'Brien, Fairbairn, Reichelt and Potkins16,Reference Prince, Knapp, Guerchet, McCrone, Prina and Comas-Herrera45 Unsurprisingly, residents with agitation are more likely to be taking antipsychotics and sedatives as medication is a frequently used management strategy for these behaviours. In contrast, residents with agitation are no more likely to be prescribed analgesics or antidepressants. This suggests that staff do not consider that agitation may be a manifestation of pain, despite some trial evidence that it can be. Reference Szczepura, Wild, Khan, Owen, Palmer and Muhammad26,Reference Husebo, Ballard, Sandvik, Nilsen and Aarsland46 Encouragingly, we reported lower antipsychotic use that appears to have been halved (to 15%) because an influential 2010 report recommended that they are used less. Reference Banerjee47 However, we report higher rates of antidepressants prescriptions compared with the 2010 report. Both these trends are consistent with international studies. Reference Kales, Zivin, Kim, Valenstein, Chiang and Ignacio48–Reference Olfson and Marcus50
We did not find a link between the quality of the environment (measured by the TESS in our study) and quality of life. Surprisingly, the one other study to investigate this found that quality of life was negatively associated with a good environment. Reference Fleming, Goodenough, Low, Chenoweth and Brodaty51 Thus a good environment may not be enough to improve quality of life. An improved environment may be of little benefit to some individuals, especially if they remain in one room of the care home and do not routinely access the better space, the outdoors and natural light.
The staff and family mean total proxy ratings of quality of life on the whole group of residents were similar and this is in line with a systematic review and meta-analysis of previous reported studies using other quality of life measures for people with dementia; Reference Robertson, Cooper, Hoe, Hamilton, Stringer and Livingston52 the correlation between the ratings regarding individuals was not high. This also showed that staff and families have previously taken into consideration different factors when considering quality of life and we will explore this further in this study to help us understand the role of different proxy raters.
This study is large and weighted for representativeness; it covered varied homes throughout England and was planned to ensure external generalisability. Sensitivity analyses found the same results. Most homes approached agreed to participate. It may, however, be that homes which feel more confident about being scrutinised are more likely to agree to research and those residents or their families who refused participation or who could not be contacted were more agitated or had more severe dementia. A slightly higher proportion of nursing homes refused to participate. We may, therefore, have underestimated the prevalence of agitation, although our figures are similar to those in previous studies. Reference Okura, Plassman, Steffens, Llewellyn, Potter and Langa8,Reference Zuidema, Derksen, Verhey and Koopmans17
We conclude that most residents in care homes have dementia and many are agitated with low quality of life. This indicates that new interventions are needed to reduce agitation. For those persons with dementia, agitation and a lowered quality of life, our findings from this survey suggest that investing in more of the current systems of care (increasing staff to resident ratios and activities within the care home and improving the environment) are unlikely to be sufficient to reduce agitation. We suggest that future research should focus on applying personalised approaches to managing agitation in residents with dementia, while also determining which specific individualised activities would be of greatest benefit. Tools should be provided for staff to understand, communicate with and engage individual residents to enable them to analyse the underlying reasons for agitation, which may include pain, discomfort, loneliness and boredom. This would enable care homes to deliver personalised interventions to reduce agitation and increase quality of life of their residents.
Acknowledgement
We thank all the care homes, the residents and their families and the staff. We also thank all the other UCL and research network researchers involved in the study, all members of the steering committee (chaired by Prof. Sube Banerjee) and all members of the Community of Interest (chaired by Matt Murray from the Alzheimer's Society).
Funding
This study was funded by a grant from the UK Economic and Social Research Council and the National Institute of Health Research Grant number NIHR/ESRC ES/L001780/1. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.










eLetters
No eLetters have been published for this article.