Numerous studies of humans show that a low birth weight, indicative of poor fetal growth, is associated with an increase in the risk of metabolic and cardiovascular diseases later in life(Reference Barker1). Various markers of maternal folate status also show a strong positive relationship with infant birth weight(Reference Refsum2–Reference Scholl and Johnson5); suggesting that fetal growth may be constrained by the availability of folic acid and the metabolites associated with it. Indeed, there is overwhelming evidence to demonstrate a reduction in the incidence of neural tube defects in mothers given periconceptional folate supplements(Reference Tamura and Picciano6). Thus it is possible that an inadequate supply of folic acid in the mother's diet will limit one-carbon metabolism, restricting fetal growth, modifying metabolism and increasing the susceptibility of the offspring to metabolic and cardiovascular diseases. Further evidence suggesting that altered one-carbon metabolism is an integral part of the programming mechanism comes from studies of laboratory animals(Reference Burdge, Hanson, Slater-Jefferies and Lillycrop7). In rats, low-protein diets fed during gestation programme elevated blood pressure and altered metabolism in the offspring. The adverse effects can be reversed by the addition of a supplement of either folic acid or the related amino acid glycine to the low-protein diet(Reference Lillycrop, Phillips, Jackson, Hanson and Burdge8–Reference Jackson, Dunn, Marchand and Langley-Evans10). These findings suggest that limiting the availability of methyl donors in the diet of pregnant rats would programme a similar postnatal phenotype in their offspring, i.e. elevated blood pressure, insulin resistance or other metabolic disturbances and changes in the expression of key genes such as acetyl-CoA carboxylase (ACC)-1 or carnitine palmitoyl transferase (L-CPT)-1(Reference Maloney, Gosby, Phuyal, Denyer, Bryson and Caterson11).
Methylated derivatives of folic acid produced from glycine and serine are essential for one-carbon metabolism(Reference Rees, Wilson and Maloney12). Animals fed folate-deficient diets utilise the alternative methyl donors, choline and methionine, to maintain the methyl supply. Because of this nutrient substitution, the effects of folate deficiency cannot be considered in isolation. In the case of the pregnant rat, folate-deficient diets increase the concentrations of homocysteine, glycine, serine and threonine in the maternal and fetal circulation, as well as changing choline and lipid metabolism in the maternal liver(Reference Maloney, Hay and Rees13, Reference McNeil, Hay, Rucklidge, Reid, Duncan, Maloney and Rees14). If the maternal diet is additionally low in methionine and choline, there are further increases in plasma homocysteine, glycine, serine and threonine concentrations, and more extensive perturbations of choline and lipid metabolism(Reference Maloney, Hay and Rees13, Reference McNeil, Hay, Rucklidge, Reid, Duncan, Maloney and Rees14). The impact of these extensive metabolic changes on fetal growth and the subsequent development of the offspring is unknown.
In order to test the hypothesis that altered one-carbon metabolism modifies fetal growth and postnatal phenotype, we have studied pregnant rats fed diets that were deficient in folic acid and the related methyl donors methionine and choline. The postnatal phenotype of the offspring was assessed by measuring blood pressure, glucose tolerance and gene expression.
Methods
Animals
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. The experimental diets contained 90 g casein per kg diet, with a mixture of synthetic amino acids equivalent to an additional 90 g casein as described previously(Reference Maloney, Hay and Rees13). Folic acid was omitted from all folate-deficient ( − F) diets. No additional methionine was added to the low-methionine (LM) diets (total methionine concentration 2·3 mg methionine/kg diet compared with 5·6 mg methionine/kg diet in the control) and choline chloride was reduced to 0·1 % (w/w) in the low-choline (LC) diets (compared with 0·2 % (w/w) in the control). Two experiments were conducted: the first to study fetal development, the second to examine the growth and phenotype of the offspring.
Experiment 1
Female rats of the Rowett hooded strain bred in the Institute were approximately aged 8–10 weeks at the start of the experiment. The animals were allocated to five groups of eight animals with a mean body weight of 208 g housed in solid-floored cages with sawdust bedding. Animals were fed control, folate-deficient ( − F), folate-deficient with low-methionine ( − F LM), folate-deficient with low-choline ( − F LC) or folate-deficient with low-methionine and low-choline ( − F LM LC) diets for a 2-week adaptation period and then were mated with males of the same strain. The day on which a vaginal plug was detected was denoted day 0. The female rats were maintained on their corresponding diets until they were killed on day 21 of gestation. The fetuses were rapidly removed, cooled on ice and killed by decapitation. All fetuses were weighed and organs from six fetuses chosen at random were carefully dissected and weighed.
Experiment 2
Animals with a mean body weight of 216·0 g were randomly allocated into three groups of ten animals fed the control, − F and − F LM LC diets. The experiment followed the protocol used in experiment 1, except that the animals were allowed to deliver naturally. Litters were culled to eight pups/dam on postnatal day 1, retaining four males and four females where possible. When they were available, surplus pups were fostered to litters of less than eight; however, these offspring were not studied further. After giving birth the dams were fed a stock diet (CRM breeder and grower diet 801772; Special Diet Services, Witham, Essex, UK) ad libitum until weaning was complete. The offspring were weaned onto the same stock diet, which was fed ad libitum for the remainder of the experiment. All animals were weighed twice weekly to monitor growth. When the offspring had reached age 4 weeks, one male and one female pup were selected at random from each litter. These animals were studied up to the age of 24 weeks. The remaining offspring were killed at age 4 weeks.
Folate analysis
Folic acid was estimated using the SimulTRAC* Radioassay Kit (catalogue no. 06B262226 MP; Biomedicals, Basingstoke, Hants, UK) as described previously(Reference Maloney, Hay and Rees13).
Blood pressure measurements
The systolic blood pressures of the offspring were measured at ages 10 and 20 weeks by tail-vein plesynthomography (model 229; Life Science Instruments, Woodland Hills, CA, USA). Before analysis, rats were placed in a thermostatically controlled chamber warmed to 28°C in order to dilate the tail vein. The animals were lightly restrained during the procedure, which was carried out three times within each week to calculate the average value.
Glucose tolerance tests
Glucose tolerance measurements were carried out using the protocol described previously(Reference Rees, Hay, Cruickshank, Reusens, Remacle, Antipatis and Grant15). Animals were fasted overnight and after a baseline blood sample was taken, the animals were given a single dose (200 mg/100 g body weight) of 30 % d-glucose solution by oral administration. Blood samples were collected in a heparin-treated tube for the measurement of plasma glucose and insulin.
Real-time polymerase chain reaction
Total RNA was extracted using the TRIzol reagent (Sigma, Poole, Dorset, UK) as described previously(Reference Maloney, Lilley, Cruickshank, McKinnon, Hay and Rees16). Samples of 50 ng total RNA were reverse transcribed using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Warrington, Cheshire, UK) primed with random hexamers and then amplified using SYBR Green PCR Master Mix (Applied Biosystems) using the primers for ACC-1, L-CPT and 18S ribosomal RNA described previously(Reference Maloney, Lilley, Cruickshank, McKinnon, Hay and Rees16). Relative target quantity was calculated from a standard curve and the results expressed as the ratio of the product relative to the product from the 18S rRNA.
Pancreatic insulin content
Either the whole pancreas (experiment 1) or a portion from the head of the pancreas (experiment 2) was weighed and dispersed in 0·17 m-HCl in 70 % (v/v) ethanol (about 100 mg tissue/ml) by sonicating for 2 × 15 s on ice. After centrifugation to remove debris, the extract was diluted twenty times with 0·17 m-HCl in 70 % ethanol and then further diluted ten times with zero standard (Mercodia AB, Uppsala, Sweden) and the insulin content measured by Mercodia High Range Rat Insulin ELISA (Mercodia AB, Uppsala, Sweden).
Statistics
All data are presented as mean values with their standard errors. Data were analysed by one-way ANOVA followed by Fisher's multiple comparison test (Genstat 7 statistical package; Lawes Agricultural Trust, Rothamsted Experimental Station, Harpenden, Herts, UK). Litter size was included as a covariate where appropriate. The block structure of the data took the dam into account in the analysis of fetuses and offspring killed at age 4 weeks. The Genstat programme was used to estimate the Gompertz parameters for each individual animal and these were then compared by ANOVA. The area under the curve for glucose and insulin was calculated using the PK2 add-in for Microsoft Excel (Joel Usansky and Atul Desai, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA, USA).
Results
Maternal food intake and growth: experiment 1
The food intakes and the corresponding body weights of the animals are shown in Table 1. Before mating there were no differences in the food intake between the diet groups. The intakes of all animals fed folate-deficient diets fell transiently during week 1 of gestation and this was more pronounced in the animals fed the low-methionine diets. The weight gain of animals fed the − F diet or the − F LC diet during the pre-mating period was not different from that of animals receiving the control diet, unlike the animals in the groups fed low-methionine ( − F LM and − F LM LC) diets which were approximately 15 % lower than the other groups at mating (data not shown). A similar pattern of growth was observed during gestation; animals in the − F or − F LC groups were not different from the animals receiving a complete diet, whereas the animals fed the low-methionine diets ( − F LM and − F LM LC) gained less weight (82 and 86 % respectively; P < 0·05).
Table 1 Food intakes and weight gain (experiment 1)*
(Mean values with their standard errors)

− F, folate-deficient; LM, low-methionine; LC, low-choline; NS, P >0·1.
a,b,c,d Mean values with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by one-way ANOVA followed post hoc by Fisher's unprotected test.
Fetal growth: experiment 1
Fetal and placental weights at day 21 of gestation are shown in Table 2. The number of fetuses was not affected by the maternal diet, although there was a negative correlation between litter size and the mean weights of the fetus, placenta and major organs (except for the brain). The average weights of fetuses from dams fed the folate-deficient diet ( − F) were approximately 18 % higher than those from the dams fed the control diet (P < 0·05), whereas average placental weights were similar to the control, leading to a consequent reduction in the ratio of fetal to placental weight in this group. The fetuses of dams fed the − F LM and − F LM LC diets were smaller (by approximately 14 and 9 % respectively; P < 0·05) than those in the control group, with a corresponding decrease in the weight of the placenta, maintaining the fetus:placenta ratio. The weights of fetuses from animals fed the − F LC diet were similar to the controls.
Table 2 Fetal weights at day 21 of gestation*
(Mean values with their standard errors)

− F, folate-deficient; LM, low-methionine; LC, low-choline; NS, P >0·1.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by one-way ANOVA, blocked for dam with number of fetuses included as a covariate. Data were analysed post hoc by Fisher's unprotected test. Organ weights are the mean of six fetuses chosen at random from each dam.
The liver and lungs decreased as a proportion of body weight compared with the control group in the fetuses of dams fed the − F diet (Table 2). Other organs were unchanged. In contrast, the fetuses of dams fed the − F LM and − F LM LC diets had proportionately smaller liver and kidneys and a larger brain (Table 2). The proportions of the major organs in the fetuses of animals fed the − F LC diet were not different from the control.
Postnatal growth: experiment 2
The protocol was similar to the first experiment except that dams were fed either the − F or − F LM LC diets in addition to the control. At mating, following the 2-week adaptation period, the average body weight of the animals was: control, 263·1 (sem 5·5) g (n 8); − F, 261·2 (sem 4·8) g (n 7); − F LM LC, 256·3 (sem 5·9) g (n 9). During the first 2 weeks of gestation there were no differences in weight gain (Fig. 1). However, by the end of week 1 the animals in the groups fed the − F LM LC diet tended to have a lower average body weight and during the final week of gestation these animals gained less weight (P < 0·05) than the other two groups. After the dams had given birth the weight of those fed the − F LM LC diet was less than the other two groups.

Fig. 1 Body weight of dams whose offspring were subsequently included in the study (experiment 2). Animals were fed a control diet (–♦–; n 7), a folate-deficient diet (-■-; n 7) or a folate-, choline- and methionine-deficient diet (–▲–; n 7). The day of birth is day 0. Values are means, with standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05).
The animals fed the − F LM LC diet gave birth to smaller litters, ranging from four to fourteen pups per dam, compared with twelve to seventeen pups per dam in the control and − F groups (Table 3). Hepatic folate concentrations in the surplus pups killed on day 1 were decreased to 28 % of the control in the pups from dams fed the − F diet (P < 0·001; n 6). Folate concentrations in the offspring of dams fed the − F LM LC diet were 40 % of the control (n 2). By age 4 weeks, hepatic folate concentrations were not significantly different from the control, suggesting that all of the offspring were fully recuperated by weaning (data not shown).
Table 3 Organ weights of the offspring*
(Mean values with their standard errors)

− F, folate-deficient; LM, low-methionine; LC, low-choline; NS, P >0·1.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
* Data were analysed by one-way ANOVA followed post hoc by Fisher's unprotected test.
† Values for mean pup weight per dam before offspring were culled to eight pups/dam. Data include both male and female offspring.
‡ The data at 4 weeks of age are from two to four pups per dam depending on the size and sex distribution of the litters.
§ The differences in the absolute weights of the female offspring killed at 24 weeks were a consequence of the selection process at 4 weeks of age and unrelated to the maternal diet.
On day 1 of postnatal life the pups in the − F LM LC group were lighter than those in the control and − F groups (Table 3). Dams in all groups were fed the commercial stock diet during lactation and the small differences in body weight of the offspring rapidly disappeared. As a result of this catch-up growth there were no significant differences in weight at weaning. The growth of one male and one female pup selected at random from each litter was studied up to age 24 weeks. There was a linear relationship (P = 0·004) between the body weight at 4 weeks and 24 weeks and when the growth curves for each individual animal were fitted to the Gompertz equation there were no differences in the parameters (data not shown). This indicates that the maternal diet has no effect on postnatal growth rates after age 4 weeks.
The organ weights expressed as a percentage of body weight of the offspring killed at 24 weeks are shown in Table 3. The differences in the absolute weights of the female offspring killed at 24 weeks were a consequence of the selection process at 4 weeks of age and unrelated to the maternal diet. The kidneys of the male offspring of dams fed the diet with the combined deficiency ( − F LM LC) were proportionately smaller when compared with the offspring of dams fed the control and folate-deficient diets. The other organs were unaffected. The kidneys of the female offspring of dams were also changed at 24 weeks. The kidneys of female offspring of dams fed the − F diet were larger as a proportion of the total body weight when compared with the offspring of dams fed the complete diet. The hearts of the female offspring from both the − F and − F LM LC dams were proportionately larger than those of the controls. The weight of the adipose deposits as a proportion of the total body weight was not changed in either male or female offspring at age 24 weeks.
Blood pressure of the offspring
The mean blood pressure and systolic blood pressure of the male and female offspring at ages 10 and 20 weeks are shown in Table 4. The maternal diet did not produce any significant differences in either parameter. The values for the control animals are similar to those reported for the same strain of rat in previous studies(Reference Gambling, Dunford, Wallace, Zuur, Solanky, Srai and McArdle17, Reference Rees, Hay and Cruickshank18).
Table 4 Blood pressure (bp) of the offspring*
(Mean values with their standard errors)
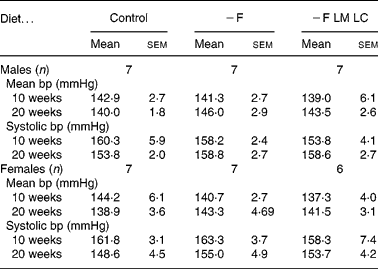
− F, folate-deficient; LM, low-methionine; LC, low-choline.
* There were no significant differences (P < 0·05) when data were analysed by one-way ANOVA.
Pancreatic insulin
The total insulin content of fetal pancreatic tissue (from experiment 1) was approximately 65 % higher in the fetuses of dams fed the − F diet compared with the control (Table 5). When the data were expressed per g of tissue the insulin content of the fetal pancreata from dams fed the − F and − F LM LC diets was approximately 20 % higher than the controls. Fetal pancreata from dams fed the − F LM diet gave intermediate values and those in the − F LC group were similar to the control. At 4 weeks of age (experiment 2) the insulin content of the pancreas expressed either as total insulin or on a per mg tissue basis tended to be approximately 23 % higher (P = 0·061) in the offspring of dams fed the folate-deficient diet ( − F).
Table 5 Pancreatic insulin content*
(Mean values with their standard errors)
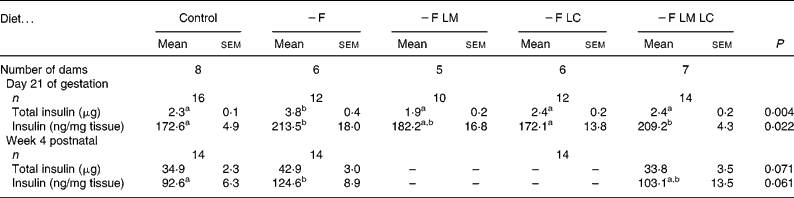
− F, folate-deficient; LM, low-methionine; LC, low-choline.
a,b Mean values with unlike superscript letters were significantly different (P < 0.05).
* Where possible, one male and one female fetus or pup were analysed from each litter. Data were analysed by ANOVA and post hoc by Fisher's unprotected test. There was no difference between the sexes and no interaction between sex and diet.
Oral glucose tolerance of the offspring: experiment 2
Fasting plasma concentrations of glucose and insulin were unaffected by the maternal diet in either the male or female offspring at age 24 weeks (data not shown). Following an oral glucose dose, plasma glucose concentrations increased by approximately 1·5- to 1·8-fold in both the males and females, reaching a peak within 15 min. The maternal diet did not change either the peak height or the total area under the curve in either male or female offspring. Following the glucose dose there was a rapid increase in plasma insulin concentrations. There were no significant differences in the peak height or the total area under the curve in the male offspring; however, the peak insulin concentration in the females tended (P = 0·092) to be higher in the offspring of dams fed the − F diet (37·56 (sem 3·77) μU/ml) compared with the control (29·72 (sem 4·92) μU/ml). The complete curves for the female offspring are shown in Fig. 2. The area under the curve also tended to be greater (P = 0·071) in the − F group (2572 (sem 239) μU/ml × min) compared with the control (2186 (sem 220) μU/ml × min) and − F LM LC (2246 (sem 145) μU/ml × min) groups.
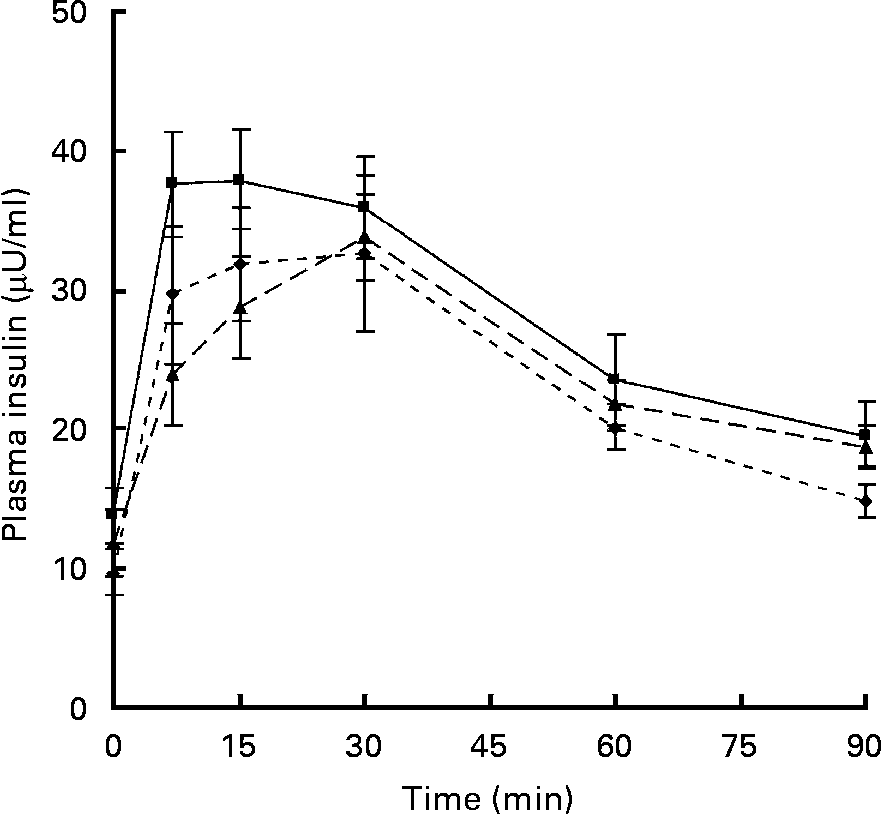
Fig. 2 Plasma insulin concentrations in female offspring at 24 weeks. Dams were fed a control diet (–♦–; n 8), a folate-deficient diet (-■-; n 7) or a folate-, choline- and methionine-deficient diet (–▲–; n 7). Values are means, with standard errors represented by vertical bars.
Acetyl-CoA carboxylase and carnitine palmitoyl transferase-1 expression
Gene expression was studied in the female offspring since they are comparable with the offspring of dams fed low-protein diets studied previously(Reference Maloney, Lilley, Czopek, Hay and Rees19). Table 6 shows the levels of the mRNA for ACC-1 and L-CPT-1 relative to the ribosomal 18S RNA in the livers of female offspring 3 h after the administration of the glucose dose given as part of the glucose tolerance test. There were no significant differences in the levels of either mRNA.
Table 6 Relative gene expression of acetyl-CoA carboxylase 1 (ACC-1) and carnitine palmitoyl transferase (L-CPT) in the liver of female offspring at age 24 weeks*
(Mean values with their standard errors)

− F, folate-deficient; LM, low-methionine; LC, low-choline.
* The relative expression (arbitrary units) of ACC-1 and L-CPT-1 mRNA is expressed as the ratio of the threshold cycle value for each cDNA normalised with respect to the 18S ribosomal subunit. Six offspring, from different litters were chosen at random. There were no significant differences (P < 0·05) when data were analysed by one-way ANOVA.
Discussion
The results of the present study show that when the pregnant rat is fed a folate-deficient diet there was no change in adult blood pressure and only weak evidence for a small change in the insulin axis. This was despite a 3- to 5-fold elevation of homocysteine in the pregnant dams and extensive changes in amino acid and lipid metabolism(Reference Maloney, Hay and Rees13, Reference McNeil, Hay, Rucklidge, Reid, Duncan, Maloney and Rees14). Indeed, rather surprisingly, folate deficiency produced a transient increase in the weight of the fetuses of the dams fed the − F diet. For the majority of fetal organs, growth was proportionate with no long-lasting effect; the only exceptions were in the female offspring where the heart and kidneys were proportionately larger. Reducing the choline content of the diet had little effect on growth, unlike the low-methionine diet which restricted both maternal and fetal growth, producing a degree of growth restriction which was comparable with the 12 % reduction in fetal weight on day 21 of gestation seen in animals fed a diet containing 9 % protein(Reference Rees, Hay, Buchan, Antipatis and Palmer20). Both low-protein and the low-methionine diets decrease the growth of the liver and kidney while preserving the brain and heart(Reference Rees, Hay and Cruickshank18). Thus the low-methionine diets are in some ways similar to those with reduced protein content, except that fetal growth is restricted by the limited availability of a single obligatory amino acid as opposed to a global restriction in the N supply in the case of the low-protein diet.
Blood pressure in the offspring of dams fed the − F LM LC diet was not different from the controls despite a reduction in the relative sizes of the kidneys. This is in contrast to the increase in blood pressure seen in some studies of low-protein diets(Reference Jackson, Dunn, Marchand and Langley-Evans10). The offspring of animals protein restricted during gestation have smaller kidneys with fewer nephrons, and it has been proposed that the increase in blood pressure is due to a greater blood flow being required to maintain the same filtration rate(Reference Langley-Evans, Gardner and Jackson21). The larger heart in the − F and − F LM LC females may be a means of increasing the blood flow; however, there is no evidence for a corresponding increase in blood pressure in these animals. These findings are similar to our observations in the low-protein model, where despite changes in the proportions of the kidneys there was no change in blood pressure(Reference Rees, Hay and Cruickshank18). Thus fetal growth restriction caused by low methionine or low protein can modify renal structure but does not appear to be one of the primary mechanisms leading to hypertension(Reference Langley-Evans, Langley-Evans and Marchand22).
Poor folate status is associated with widespread changes in metabolism, which include an increase in the concentration of homocysteine in the plasma(Reference Maloney, Hay and Rees13). In humans there are inverse associations between plasma homocysteine concentrations and infant birth weight, suggesting that homocysteine itself may influence fetal growth and development(Reference Murphy, Scott, Arija, Molloy and Fernandez-Ballart23). However, despite a nearly five-fold increase in homocysteine in the maternal plasma in dams fed the − F diet there is no effect on adult blood pressure. The increased blood pressure in the low-protein rat model has been linked to a restriction in the glycine supply which causes changes in the vasculature(Reference Jackson, Dunn, Marchand and Langley-Evans10, Reference Brawley, Torrens, Anthony, Itoh, Wheeler, Jackson, Clough, Poston and Hanson24). Whilst plasma glycine concentrations are lower in animals fed the low-protein diets(Reference Rees, Hay, Buchan, Antipatis and Palmer20, Reference Rees, Hay and Antipatis25), plasma concentrations are increased in animals fed the − F and − F LM LC diets(Reference Maloney, Hay and Rees13). This improvement in the glycine supply may explain why the blood pressure of the offspring of dams fed the − F or − F LM LC diets is not increased, an observation that supports the suggestion that the hypertension is a consequence of glycine deficiency(Reference Jackson, Dunn, Marchand and Langley-Evans10).
There is no evidence for changes in the postnatal growth of the offspring as a result of changes in epigenetic programming. This has been shown in other species where, for example, there is fetal overgrowth accompanied by changes in the allometric relationships between liver, heart, kidneys and plantaris muscle following culture of ovine embryos in vitro (Reference Sinclair, Dunne, Maxfield, Maltin, Young, Wilmut, Robinson and Broadbent26). However, these changes are in the opposite direction to those found in the present study, i.e. the organs are disproportionately larger than in the controls(Reference Farin, Piedrahita and Farin27). Folate supplements also modify the methylation status of regulators such as PPAR-α which modify lipid metabolism in the offspring(Reference Lillycrop, Phillips, Jackson, Hanson and Burdge8). However, the accretion of fat, as determined by the fat pad weights, was not changed in either the − F or − F LM LC offspring, and furthermore there were no changes in the expression of ACC-1 and L-CPT-1, two important genes regulating fatty acid synthesis and oxidation. In contrast, these two mRNA are regulated in the offspring of dams fed the low-protein diets(Reference Maloney, Gosby, Phuyal, Denyer, Bryson and Caterson11), suggesting that there are some important differences in the adult outcomes from the two models.
Folate deficiency in the maternal diet leads to a 25 % increase in the insulin content of the fetal pancreas, a change that appears to persist into early postnatal life. By the time that the female − F offspring have reached the age of 24 weeks there is a weak trend (P = 0·071) for glucose-stimulated insulin release to be higher. The lack of effect in the male offspring is similar to the sex-specific effects seen in the low-protein model, where females are affected to a greater extent than males. We believe that a larger experiment with greater statistical power would have resolved a modest change in insulin action and that the increase in the pancreatic insulin content at fetal stages has persisted into adult life. The preliminary results of a study in which larger groups of animals (n 16) were fed similar methyl-deficient diets for the first 5 d of gestation have demonstrated a modest change in insulin action and glucose tolerance in the offspring at age 6 months(Reference Maloney, Hay and Rees28). However, the overall effects of the folate-deficient diets are much smaller than the three-fold changes in the area under the curve for insulin observed in studies of the low-protein model(Reference Rees, Hay, Cruickshank, Reusens, Remacle, Antipatis and Grant15) and on which the power calculations for the present study were based.
In conclusion, despite widespread changes in maternal and fetal metabolism, the effects of a physiologically relevant folate deficiency on the offspring of the rat are quite modest. Because derivatives of folic acid are involved in a variety of important cellular reactions, including nucleotide synthesis and methylation reactions dependent on S-adenosyl methionine, extensive homeostatic mechanisms are in place to protect the fetus. In general, fetal metabolism appears to be less disturbed than maternal with the limited supply of folate being preferentially distributed to the fetus(Reference Maloney, Hay and Rees13). This additional folate may be sufficient to support more appropriate metabolic activity in the fetal compartment, minimising the effects of deficiency.
Acknowledgements
W. D. R. and C. A. M. designed the research; S. M. H., C. A. M. and W. D. R. performed the research; W. D. R., S. M. H. and C. A. M. analysed the data; C. A. M. and W. D. R. wrote the paper. The authors declare no conflict of interest. The present study was supported by the Environment and Rural Affairs Department of the Scottish Government as part of the core funding of the Rowett Research Institute. C. A. M. was supported by a cooperative agreement from the National Institutes of Health (U01 HD044638) as a component of the National Institute for Child Health and Human Development (NICHD) Cooperative Program on Female Health and Egg Quality. We wish to express our thanks to Dr F. Wilson and Ms C. Lilley for technical help, staff from the Bioresources Unit for animal care, and to Dr G. Holtrop (Biomathematics and Statistics, Scotland) for advice on the statistical analysis.










